The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis
Abstract
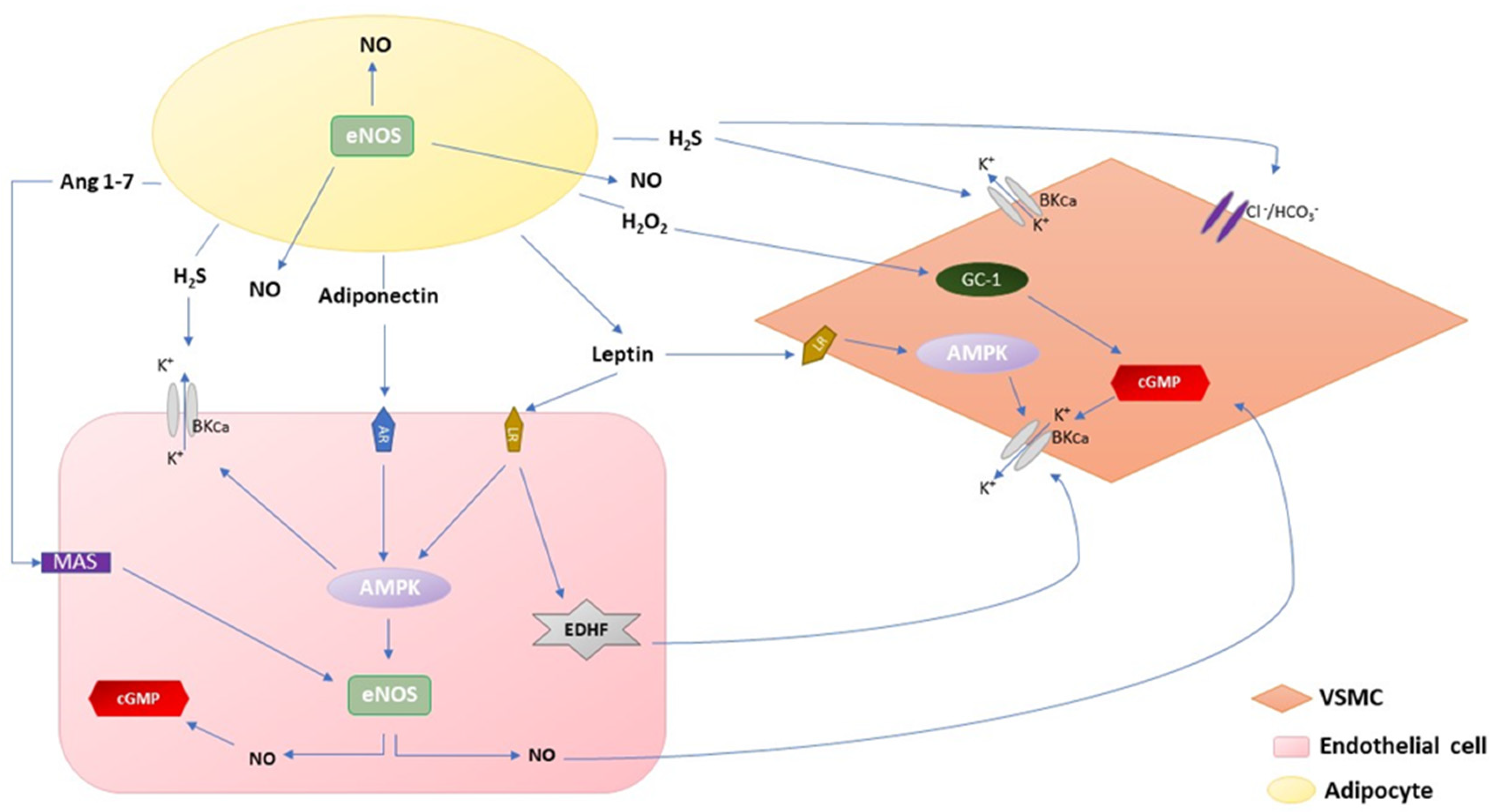
:1. Introduction
2. The Influence of PVAT-Derived Factors on Vascular Function
2.1. Adiponectin
2.2. Leptin
2.3. H2O2
2.4. H2S
2.5. Angiotensin 1–7 and Angiotensin II
2.6. NO
2.7. COX-Derived Factors
2.8. Noradrenaline
3. The Role of Inflammation, Oxidative Stress, and Hypoxia in Obesity
4. Dysregulation of Vascular Function Induced by PVAT Dysfunction in Obesity
4.1. The RAAS in Obesity
4.2. PVAT-Derived Factors Dysregulation with Effect on Vascular Tone
5. The Influence of Exercise among Obese on PVAT
6. The Potential Influence of Diet on PVAT-Derived Factors among Obese Patients
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Study 2015 (GBD 2015) Obesity and Overweight Prevalence 1980–2015 | GHDx. Available online: http://ghdx.healthdata.org/record/ihme-data/gbd-2015-obesity-and-overweight-prevalence-1980-2015 (accessed on 3 August 2021).
- Hales, C.M. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 8. [Google Scholar]
- Overweight and Obesity-BMI Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics (accessed on 6 September 2021).
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The Global Burden of Disease Attributable to High Body Mass Index in 195 Countries and Territories, 1990–2017: An Analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef] [PubMed]
- Kolotkin, R.L.; Andersen, J.R. A Systematic Review of Reviews: Exploring the Relationship between Obesity, Weight Loss and Health-related Quality of Life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef] [Green Version]
- Tremmel, M.; Gerdtham, U.-G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alfonso, M.S.; Gil-Ortega, M.; García-Prieto, C.F.; Aranguez, I.; Ruiz-Gayo, M.; Somoza, B. Mechanisms of Perivascular Adipose Tissue Dysfunction in Obesity. Int. J. Endocrinol. 2013, 2013, 402053. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology 2012, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, F.W.; Cohen, P.; Plutzky, J. Fifty Shades of Brown: Perivascular Fat, Thermogenesis, and Atherosclerosis. Circulation 2012, 126, 1012–1015. [Google Scholar] [CrossRef] [Green Version]
- Police, S.B.; Thatcher, S.E.; Charnigo, R.; Daugherty, A.; Cassis, L.A. Obesity Promotes Inflammation in Periaortic Adipose Tissue and Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arter. Thromb. Vasc. Biol. 2009, 29, 1458–1464. [Google Scholar] [CrossRef] [Green Version]
- Verlohren, S.; Dubrovska, G.; Tsang, S.-Y.; Essin, K.; Luft, F.C.; Huang, Y.; Gollasch, M. Visceral Periadventitial Adipose Tissue Regulates Arterial Tone of Mesenteric Arteries. Hypertension 2004, 44, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Yudkin, J.S.; Eringa, E.; Stehouwer, C.D.A. “Vasocrine” Signalling from Perivascular Fat: A Mechanism Linking Insulin Resistance to Vascular Disease. Lancet 2005, 365, 1817–1820. [Google Scholar] [CrossRef]
- Gao, Y.-J. Dual Modulation of Vascular Function by Perivascular Adipose Tissue and Its Potential Correlation with Adiposity/Lipoatrophy-Related Vascular Dysfunction. Curr. Pharm. Des. 2007, 13, 2185–2192. [Google Scholar] [CrossRef]
- Takaoka, M.; Suzuki, H.; Shioda, S.; Sekikawa, K.; Saito, Y.; Nagai, R.; Sata, M. Endovascular Injury Induces Rapid Phenotypic Changes in Perivascular Adipose Tissue. Arter. Thromb. Vasc. Biol. 2010, 30, 1576–1582. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Torsney, E.; Afzal, A.R.; Davison, F.; Metzler, B.; Xu, Q. Abundant Progenitor Cells in the Adventitia Contribute to Atherosclerosis of Vein Grafts in ApoE-Deficient Mice. J. Clin. Investig. 2004, 113, 1258–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular Adipose Tissue (PVAT) in Atherosclerosis: A Double-Edged Sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef] [Green Version]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernández-Alfonso, M.S. Regional Differences in Perivascular Adipose Tissue Impacting Vascular Homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lasar, D.; Julius, A.; Fromme, T.; Klingenspor, M. Browning Attenuates Murine White Adipose Tissue Expansion during Postnatal Development. Biochim. Biophys Acta 2013, 1831, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, W.; Xia, L.; Xia, P.; Yan, Y. Gene Expression Profiling Reveals Heterogeneity of Perivascular Adipose Tissues Surrounding Coronary and Internal Thoracic Arteries. Acta Biochim. Biophys. Sin. 2017, 49, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Britton, K.A.; Pedley, A.; Massaro, J.M.; Corsini, E.M.; Murabito, J.M.; Hoffmann, U.; Fox, C.S. Prevalence, Distribution, and Risk Factor Correlates of High Thoracic Periaortic Fat in the Framingham Heart Study. J. Am. Heart Assoc. 2012, 1, e004200. [Google Scholar] [CrossRef] [Green Version]
- El Khoudary, S.R.; Shields, K.J.; Janssen, I.; Hanley, C.; Budoff, M.J.; Barinas-Mitchell, E.; Everson-Rose, S.A.; Powell, L.H.; Matthews, K.A. Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J. Clin. Endocrinol. Metab. 2015, 100, 3304–3312. [Google Scholar] [CrossRef]
- Victorio, J.A.; da Costa, R.M.; Tostes, R.C.; Davel, A.P. Modulation of Vascular Function by Perivascular Adipose Tissue: Sex Differences. Curr. Pharm. Des. 2020, 26, 3768–3777. [Google Scholar] [CrossRef]
- Marchesi, C.; Ebrahimian, T.; Angulo, O.; Paradis, P.; Schiffrin, E.L. Endothelial Nitric Oxide Synthase Uncoupling and Perivascular Adipose Oxidative Stress and Inflammation Contribute to Vascular Dysfunction in a Rodent Model of Metabolic Syndrome. Hypertension 2009, 54, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Sowka, A.; Dobrzyn, P. Role of Perivascular Adipose Tissue-Derived Adiponectin in Vascular Homeostasis. Cells 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Villacorta, L.; Li, R.; Hamblin, M.; Xu, W.; Dou, C.; Zhang, J.; Wu, J.; Zeng, R.; Chen, Y.E. Loss of Perivascular Adipose Tissue on Peroxisome Proliferator-Activated Receptor-γ Deletion in Smooth Muscle Cells Impairs Intravascular Thermoregulation and Enhances Atherosclerosis. Circulation 2012, 126, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Ozen, G.; Daci, A.; Norel, X.; Topal, G. Human perivascular adipose tissue dysfunction as a cause of vascular disease: Focus on vascular tone and wall remodeling. Eur. J. Pharm. 2015, 766, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.-Y.; Li, Z.-Y. The Role of Perivascular Adipose Tissue in Vascular Smooth Muscle Cell Growth. Br. J. Pharm. 2012, 165, 643–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollasch, M. Vasodilator Signals from Perivascular Adipose Tissue. Br. J. Pharmacol. 2012, 165, 633. [Google Scholar] [CrossRef] [Green Version]
- Szasz, T.; Webb, R.C. Perivascular Adipose Tissue: More than Just Structural Support. Clin. Sci. 2012, 122, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gálvez-Prieto, B.; Somoza, B.; Gil-Ortega, M.; García-Prieto, C.F.; de las Heras, A.I.; González, M.C.; Arribas, S.; Aranguez, I.; Bolbrinker, J.; Kreutz, R.; et al. Anticontractile Effect of Perivascular Adipose Tissue and Leptin Are Reduced in Hypertension. Front. Pharm. 2012, 3, 103. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-C.; Chang, H.-H.; Chiang, C.-L.; Liu, C.-H.; Yeh, J.-I.; Chen, M.-F.; Chen, P.-Y.; Kuo, J.-S.; Lee, T.J.F. Role of Perivascular Adipose Tissue-Derived Methyl Palmitate in Vascular Tone Regulation and Pathogenesis of Hypertension. Circulation 2011, 124, 1160–1171. [Google Scholar] [CrossRef] [Green Version]
- Gálvez-Prieto, B.; Bolbrinker, J.; Stucchi, P.; de Las Heras, A.I.; Merino, B.; Arribas, S.; Ruiz-Gayo, M.; Huber, M.; Wehland, M.; Kreutz, R.; et al. Comparative Expression Analysis of the Renin-Angiotensin System Components between White and Brown Perivascular Adipose Tissue. J. Endocrinol. 2008, 197, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Takemori, K.; Su, L.; An, W.; Lu, C.; Sharma, A.; Lee, R. Perivascular Adipose Tissue Promotes Vasoconstriction: The Role of Superoxide Anion. Cardiovasc. Res. 2006, 71, 363–373. [Google Scholar] [CrossRef]
- Cheng, C.K.; Bakar, H.A.; Gollasch, M.; Huang, Y. Perivascular Adipose Tissue: The Sixth Man of the Cardiovascular System. Cardiovasc. Drugs 2018, 32, 481–502. [Google Scholar] [CrossRef]
- Weston, A.H.; Egner, I.; Dong, Y.; Porter, E.L.; Heagerty, A.M.; Edwards, G. Stimulated Release of a Hyperpolarizing Factor (ADHF) from Mesenteric Artery Perivascular Adipose Tissue: Involvement of Myocyte BKCa Channels and Adiponectin. Br. J. Pharmacol. 2013, 169, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Saxton, S.N.; Ryding, K.E.; Aldous, R.G.; Withers, S.B.; Ohanian, J.; Heagerty, A.M. Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 880–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Montagnani, M.; Funahashi, T.; Shimomura, I.; Quon, M.J. Adiponectin Stimulates Production of Nitric Oxide in Vascular Endothelial Cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Antonopoulos, A.S.; Digby, J.; Lee, R.; Reilly, S.; Coutinho, P.; Shirodaria, C.; Sayeed, R.; Petrou, M.; De Silva, R.; et al. Interactions between Vascular Wall and Perivascular Adipose Tissue Reveal Novel Roles for Adiponectin in the Regulation of Endothelial Nitric Oxide Synthase Function in Human Vessels. Circulation 2013, 127, 2209–2221. [Google Scholar] [CrossRef] [Green Version]
- Föller, M.; Jaumann, M.; Dettling, J.; Saxena, A.; Pakladok, T.; Munoz, C.; Ruth, P.; Sopjani, M.; Seebohm, G.; Rüttiger, L.; et al. AMP-Activated Protein Kinase in BK-Channel Regulation and Protection against Hearing Loss Following Acoustic Overstimulation. FASEB J. 2012, 26, 4243–4253. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci 2019, 20, 1190. [Google Scholar] [CrossRef] [Green Version]
- Christou, G.A.; Kiortsis, D.N. Adiponectin and Lipoprotein Metabolism. Obes. Rev. 2013, 14, 939–949. [Google Scholar] [CrossRef]
- Rahmouni, K.; Correia, M.L.G.; Haynes, W.G.; Mark, A.L. Obesity-Associated Hypertension. Hypertension 2005, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [Green Version]
- Bełtowski, J. Leptin and the Regulation of Endothelial Function in Physiological and Pathological Conditions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Jamroz-Wiśniewska, A.; Gertler, A.; Solomon, G.; Wood, M.E.; Whiteman, M.; Bełtowski, J. Leptin-Induced Endothelium-Dependent Vasorelaxation of Peripheral Arteries in Lean and Obese Rats: Role of Nitric Oxide and Hydrogen Sulfide. PLoS ONE 2014, 9, e86744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quehenberger, P.; Exner, M.; Sunder-Plassmann, R.; Ruzicka, K.; Bieglmayer, C.; Endler, G.; Muellner, C.; Speiser, W.; Wagner, O. Leptin Induces Endothelin-1 in Endothelial Cells in Vitro. Circ. Res. 2002, 90, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators Linking Adipose Tissue, Inflammation and Immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Ardanaz, N.; Pagano, P.J. Hydrogen Peroxide as a Paracrine Vascular Mediator: Regulation and Signaling Leading to Dysfunction. Exp. Biol. Med. 2006, 231, 237–251. [Google Scholar] [CrossRef]
- Montfort, W.R.; Wales, J.A.; Weichsel, A. Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor. Antioxid. Redox Signal. 2017, 26, 107–121. [Google Scholar] [CrossRef]
- Wang, R. Shared Signaling Pathways among Gasotransmitters. Proc. Natl. Acad. Sci. USA 2012, 109, 8801–8802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Cheng, Y.; Moore, P.K.; Bian, J.-S. Hydrogen Sulphide Regulates Intracellular PH in Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun 2007, 358, 1142–1147. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, Y.; Wu, J.; Sun, A.; Yang, F.; Zheng, D.; Li, T.; Dong, S.; Zhao, Y.; Yang, G.; et al. Calcium Sensing Receptor Regulating Smooth Muscle Cells Proliferation through Initiating Cystathionine-Gamma-Lyase/Hydrogen Sulfide Pathway in Diabetic Rat. Cell Physiol. Biochem. 2015, 35, 1582–1598. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. The Role of Perivascular Adipose Tissue in Obesity-induced Vascular Dysfunction. Br. J. Pharm. 2017, 174, 3425–3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.M.K.W.; Lu, C.; Su, L.-Y.; Gao, Y.-J. Endothelium-Dependent Relaxation Factor Released by Perivascular Adipose Tissue. J. Hypertens 2009, 27, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kusters, P.J.H.; Lutgens, E.; Seijkens, T.T.P. Exploring Immune Checkpoints as Potential Therapeutic Targets in Atherosclerosis. Cardiovasc. Res. 2018, 114, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Isolation of Nitric Oxide Synthetase, a Calmodulin-Requiring Enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric Oxide Synthase Isozymes. Characterization, Purification, Molecular Cloning, and Functions. Hypertension 1994, 23 Pt 2, 1121–1131. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Liang, X.; Liu, W.; Lv, S.; He, S.; Kuang, H.; Yang, S. Roles of ENOS in Atherosclerosis Treatment. Inflamm. Res. 2019, 68, 429–441. [Google Scholar] [CrossRef]
- Xia, N.; Horke, S.; Habermeier, A.; Closs, E.I.; Reifenberg, G.; Gericke, A.; Mikhed, Y.; Münzel, T.; Daiber, A.; Förstermann, U.; et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Félétou, M.; Vanhoutte, P.M. Endothelial Dysfunction: A Multifaceted Disorder (The Wiggers Award Lecture). Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Fredette, N.C.; Barton, M.; Prossnitz, E.R. Regulation of Vascular Smooth Muscle Tone by Adipose-Derived Contracting Factor. PLoS ONE 2013, 8, e79245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.A.; Randall, M.D.; Roberts, R.E. Sex Differences in the Role of Phospholipase A2-dependent Arachidonic Acid Pathway in the Perivascular Adipose Tissue Function in Pigs. J. Physiol. 2017, 595, 6623–6634. [Google Scholar] [CrossRef]
- Egan, K.M.; Lawson, J.A.; Fries, S.; Koller, B.; Rader, D.J.; Smyth, E.M.; Fitzgerald, G.A. COX-2-Derived Prostacyclin Confers Atheroprotection on Female Mice. Science 2004, 306, 1954–1957. [Google Scholar] [CrossRef] [Green Version]
- Mendizábal, Y.; Llorens, S.; Nava, E. Vasoactive Effects of Prostaglandins from the Perivascular Fat of Mesenteric Resistance Arteries in WKY and SHROB Rats. Life Sci. 2013, 93, 1023–1032. [Google Scholar] [CrossRef]
- Ayala-Lopez, N.; Watts, S.W. New Actions of an Old Friend: Perivascular Adipose Tissue’s Adrenergic Mechanisms. Br. J. Pharm. 2017, 174, 3454–3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala-Lopez, N.; Martini, M.; Jackson, W.F.; Darios, E.; Burnett, R.; Seitz, B.; Fink, G.D.; Watts, S.W. Perivascular Adipose Tissue Contains Functional Catecholamines. Pharm. Res. Perspect. 2014, 2, e00041. [Google Scholar] [CrossRef]
- Vargovic, P.; Ukropec, J.; Laukova, M.; Cleary, S.; Manz, B.; Pacak, K.; Kvetnansky, R. Adipocytes as a New Source of Catecholamine Production. FEBS Lett. 2011, 585, 2279–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.M.; Filgueira, F.P.; Tostes, R.C.; Carvalho, M.H.C.; Akamine, E.H.; Lobato, N.S. H2O2 Generated from Mitochondrial Electron Transport Chain in Thoracic Perivascular Adipose Tissue Is Crucial for Modulation of Vascular Smooth Muscle Contraction. Vasc. Pharm. 2016, 84, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Poitou, C.; Torcivia, A.; Bouillot, J.-L.; Dietrich, A.; Klöting, N.; Grégoire, C.; Lolmede, K.; Blüher, M.; Clément, K. Adipocyte Size Threshold Matters: Link with Risk of Type 2 Diabetes and Improved Insulin Resistance After Gastric Bypass. J. Clin. Endocrinol. Metab. 2014, 99, E1466–E1470. [Google Scholar] [CrossRef]
- Chandalia, M.; Lin, P.; Seenivasan, T.; Livingston, E.H.; Snell, P.G.; Grundy, S.M.; Abate, N. Insulin Resistance and Body Fat Distribution in South Asian Men Compared to Caucasian Men. PLoS ONE 2007, 2, e812. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, M.; Silambanan, S.; Chandran, A.S.; Prabhakaran, A.A.; Ramakrishnan, R. C-Reactive Protein (CRP) and Leptin Receptor in Obesity: Binding of Monomeric CRP to Leptin Receptor. Front. Immunol. 2018, 9, 1167. [Google Scholar] [CrossRef] [Green Version]
- Gnacińska, M.; Małgorzewicz, S.; Guzek, M.; Lysiak-Szydłowska, W.; Sworczak, K. Adipose Tissue Activity in Relation to Overweight or Obesity. Endokrynol. Pol. 2010, 61, 160–168. [Google Scholar]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [Green Version]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Tortajada-Girbés, M.; Simó-Jordá, R.; Alonso-Iglesias, E. Elevated Advanced Oxidation Protein Products (AOPPs) Indicate Metabolic Risk in Severely Obese Children. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 237–243. [Google Scholar] [CrossRef]
- Jiang, F.; Lim, H.K.; Morris, M.J.; Prior, L.; Velkoska, E.; Wu, X.; Dusting, G.J. Systemic Upregulation of NADPH Oxidase in Diet-Induced Obesity in Rats. Redox Rep. 2011, 16, 223–229. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric Oxide, Superoxide, and Peroxynitrite: The Good, the Bad, and the Ugly. Am. J. Physiol.-Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Mulvany, M.J. Small Artery Remodelling in Hypertension. Basic Clin. Pharm. Toxicol. 2012, 110, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Bizzarri, A.; Venteclef, N.; Essers, Y.; Cleutjens, J.P.; Konings, E.; Jocken, J.W.E.; Cajlakovic, M.; Ribitsch, V.; Clément, K.; et al. Increased Adipose Tissue Oxygen Tension in Obese Compared with Lean Men Is Accompanied by Insulin Resistance, Impaired Adipose Tissue Capillarization, and Inflammation. Circulation 2011, 124, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.-L.; et al. Reduction of Macrophage Infiltration and Chemoattractant Gene Expression Changes in White Adipose Tissue of Morbidly Obese Subjects after Surgery-Induced Weight Loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaca, Ü.; Schram, M.T.; Houben, A.J.H.M.; Muris, D.M.J.; Stehouwer, C.D.A. Microvascular Dysfunction as a Link between Obesity, Insulin Resistance and Hypertension. Diabetes Res. Clin. Pract. 2014, 103, 382–387. [Google Scholar] [CrossRef]
- Bussey, C.E.; Withers, S.B.; Aldous, R.G.; Edwards, G.; Heagerty, A.M. Obesity-Related Perivascular Adipose Tissue Damage Is Reversed by Sustained Weight Loss in the Rat. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1377–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withers, S.B.; Forman, R.; Meza-Perez, S.; Sorobetea, D.; Sitnik, K.; Hopwood, T.; Lawrence, C.B.; Agace, W.W.; Else, K.J.; Heagerty, A.M.; et al. Eosinophils Are Key Regulators of Perivascular Adipose Tissue and Vascular Functionality. Sci. Rep. 2017, 7, 44571. [Google Scholar] [CrossRef] [Green Version]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and Cardiovascular Disease: Revisiting an Old Relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Stanek, A.; Fazeli, B.; Bartuś, S.; Sutkowska, E. The Role of Endothelium in Physiological and Pathological States: New Data. BioMed Res. Int. 2018, 2018, e1098039. [Google Scholar] [CrossRef] [Green Version]
- Harte, A.; McTernan, P.; Chetty, R.; Coppack, S.; Katz, J.; Smith, S.; Kumar, S. Insulin-Mediated Upregulation of the Renin Angiotensin System in Human Subcutaneous Adipocytes Is Reduced by Rosiglitazone. Circulation 2005, 111, 1954–1961. [Google Scholar] [CrossRef] [Green Version]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes Produce Aldosterone Through Calcineurin-Dependent Signaling Pathways. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Belfiore, A.; Bernini, G.; Fabris, B.; Caridi, G.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; Mannelli, M.; et al. Body Mass Index Predicts Plasma Aldosterone Concentrations in Overweight-Obese Primary Hypertensive Patients. J. Clin. Endocrinol. Metab. 2008, 93, 2566–2571. [Google Scholar] [CrossRef] [Green Version]
- Boustany, C.M.; Bharadwaj, K.; Daugherty, A.; Brown, D.R.; Randall, D.C.; Cassis, L.A. Activation of the Systemic and Adipose Renin-Angiotensin System in Rats with Diet-Induced Obesity and Hypertension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R943–R949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniyappa, R.; Yavuz, S. Metabolic Actions of Angiotensin II and Insulin: A Microvascular Endothelial Balancing Act. Mol. Cell Endocrinol. 2013, 378, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Nogami, T.; Taguchi, K.; Matsumoto, T.; Kamata, K. Diabetic State, High Plasma Insulin and Angiotensin II Combine to Augment Endothelin-1-Induced Vasoconstriction via ET A Receptors and ERK. Br. J. Pharmacol. 2008, 155, 974–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.F.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-Derived Contracting Factors Mediate the Ang II-Induced Endothelial Dysfunction in the Rat Aorta: Preventive Effect of Red Wine Polyphenols. Pflug. Arch.-Eur. J. Physiol. 2010, 459, 671–679. [Google Scholar] [CrossRef]
- Schütten, M.T.J.; Houben, A.J.H.M.; de Leeuw, P.W.; Stehouwer, C.D.A. The Link Between Adipose Tissue Renin-Angiotensin-Aldosterone System Signaling and Obesity-Associated Hypertension. Physiology 2017, 32, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushibiki, M.; Yamada, M.; Oikawa, K.; Tomita, H.; Osanai, T.; Okumura, K. Aldosterone Causes Vasoconstriction in Coronary Arterioles of Rats via Angiotensin II Type-1 Receptor: Influence of Hypertension. Eur. J. Pharmacol. 2007, 572, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Aghamohammadzadeh, R.; Unwin, R.D.; Greenstein, A.S.; Heagerty, A.M. Effects of Obesity on Perivascular Adipose Tissue Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J. Vasc. Res. 2016, 52, 299. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory Phenotype of Perivascular Adipocytes: Influence of High-Fat Feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeter, M.R.; Eschholz, N.; Herzberg, S.; Jerchel, I.; Leifheit-Nestler, M.; Czepluch, F.S.; Chalikias, G.; Konstantinides, S.; Schäfer, K. Leptin-Dependent and Leptin-Independent Paracrine Effects of Perivascular Adipose Tissue on Neointima Formation. Arter. Thromb. Vasc. Biol. 2013, 33, 980–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T. Leptin-Induced Endothelial Dysfunction in Obesity. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance: A Possible Interface of Inflammation and Metabolism in Obesity-Related Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Hou, N.; Luo, J.-D. Leptin and Cardiovascular Diseases. Clin. Exp. Pharmacol. Physiol. 2011, 38, 905–913. [Google Scholar] [CrossRef]
- Harlan, S.M.; Morgan, D.A.; Agassandian, K.; Guo, D.-F.; Cassell, M.D.; Sigmund, C.D.; Mark, A.L.; Rahmouni, K. Ablation of the Leptin Receptor in the Hypothalamic Arcuate Nucleus Abrogates Leptin-Induced Sympathetic Activation. Circ. Res. 2011, 108, 808–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.H.; Tang, C.; et al. Increase or Decrease Hydrogen Sulfide Exert Opposite Lipolysis, but Reduce Global Insulin Resistance in High Fatty Diet Induced Obese Mice. PLoS ONE 2013, 8, e73892. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.; Bailey, S.M. Redox Biology of Hydrogen Sulfide: Implications for Physiology, Pathophysiology, and Pharmacology. Redox Biol. 2013, 1, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteman, M.; Gooding, K.M.; Whatmore, J.L.; Ball, C.I.; Mawson, D.; Skinner, K.; Tooke, J.E.; Shore, A.C. Adiposity Is a Major Determinant of Plasma Levels of the Novel Vasodilator Hydrogen Sulphide. Diabetologia 2010, 53, 1722–1726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Song, P.; Xu, J.; Zou, M.-H. Activation of NAD(P)H Oxidases by Thromboxane A2 Receptor Uncouples Endothelial Nitric Oxide Synthase. Arter. Thromb. Vasc. Biol. 2011, 31, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Drouin, A.; Farhat, N.; Bolduc, V.; Thorin-Trescases, N.; Gillis, M.-A.; Villeneuve, L.; Nguyen, A.; Thorin, E. Up-Regulation of Thromboxane A2 Impairs Cerebrovascular ENOS Function in Aging Atherosclerotic Mice. Pflug. Arch. 2011, 462, 371–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovati, M.; Mularoni, E.M.; Burzacca, S.; Ponziani, M.C.; Massucco, P.; Mattiello, L.; Piretto, V.; Cavalot, F.; Anfossi, G. Impaired Insulin-Induced Platelet Antiaggregating Effect in Obesity and in Obese NIDDM Patients. Diabetes 1995, 44, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.P.; Campbell, M.J.; Herring, M.P. Sex-Related Differences in Mood Responses to Acute Aerobic Exercise. Med. Sci. Sports Exerc. 2016, 48, 1798–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Sigala, W.; Adams, G.R.; Vaziri, N.D. Effect of Exercise on Cardiac Tissue Oxidative and Inflammatory Mediators in Chronic Kidney Disease. Am. J. Nephrol. 2009, 29, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Boa, B.C.S.; Yudkin, J.S.; van Hinsbergh, V.W.M.; Bouskela, E.; Eringa, E.C. Exercise Effects on Perivascular Adipose Tissue: Endocrine and Paracrine Determinants of Vascular Function. Br. J. Pharm. 2017, 174, 3466–3481. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.-M.; Jordy, A.B.; Poulsen, S.K.; Stender, S.; Pilegaard, H.; Astrup, A.; Larsen, T.M.; Wojtaszewski, J.F.P.; Richter, E.A.; et al. New Nordic Diet-Induced Weight Loss Is Accompanied by Changes in Metabolism and AMPK Signaling in Adipose Tissue. J. Clin. Endocrinol. Metab. 2015, 100, 3509–3519. [Google Scholar] [CrossRef]
- Ruffino, J.S.; Davies, N.A.; Morris, K.; Ludgate, M.; Zhang, L.; Webb, R.; Thomas, A.W. Moderate-Intensity Exercise Alters Markers of Alternative Activation in Circulating Monocytes in Females: A Putative Role for PPARγ. Eur. J. Appl. Physiol. 2016, 116, 1671–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meziat, C.; Boulghobra, D.; Strock, E.; Battault, S.; Bornard, I.; Walther, G.; Reboul, C. Exercise Training Restores ENOS Activation in the Perivascular Adipose Tissue of Obese Rats: Impact on Vascular Function. Nitric. Oxide 2019, 86, 63–67. [Google Scholar] [CrossRef]
- Cao, S.; Li, B.; Yi, X.; Chang, B.; Zhu, B.; Lian, Z.; Zhang, Z.; Zhao, G.; Liu, H.; Zhang, H. Effects of Exercise on AMPK Signaling and Downstream Components to PI3K in Rat with Type 2 Diabetes. PLoS ONE 2012, 7, e51709. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.S.; Sponton, A.C.S.; Trifone, C.B.; Delbin, M.A. Aerobic Exercise Training Prevents Perivascular Adipose Tissue-Induced Endothelial Dysfunction in Thoracic Aorta of Obese Mice. Front. Physiol. 2019, 10, 1009. [Google Scholar] [CrossRef] [Green Version]
- Saxton, S.N.; Toms, L.K.; Aldous, R.G.; Withers, S.B.; Ohanian, J.; Heagerty, A.M. Restoring Perivascular Adipose Tissue Function in Obesity Using Exercise. Cardiovasc. Drugs 2021. [Google Scholar] [CrossRef]
- Farah, C.; Kleindienst, A.; Bolea, G.; Meyer, G.; Gayrard, S.; Geny, B.; Obert, P.; Cazorla, O.; Tanguy, S.; Reboul, C. Exercise-Induced Cardioprotection: A Role for ENOS Uncoupling and NO Metabolites. Basic Res. Cardiol. 2013, 108. [Google Scholar] [CrossRef]
- DeVallance, E.; Branyan, K.W.; Lemaster, K.C.; Anderson, R.; Marshall, K.L.; Olfert, I.M.; Smith, D.M.; Kelley, E.E.; Bryner, R.W.; Frisbee, J.C.; et al. Exercise Training Prevents the Perivascular Adipose Tissue-Induced Aortic Dysfunction with Metabolic Syndrome. Redox Biol. 2019, 26, 101285. [Google Scholar] [CrossRef]
- Wang, J.; Polaki, V.; Chen, S.; Bihl, J.C. Exercise Improves Endothelial Function Associated with Alleviated Inflammation and Oxidative Stress of Perivascular Adipose Tissue in Type 2 Diabetic Mice. Oxid. Med. Cell Longev. 2020, 2020, 8830537. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Yin, H.; Huang, J.; Hu, M. Dysfunction of Perivascular Adipose Tissue in Mesenteric Artery Is Restored by Aerobic Exercise in High-Fat Diet Induced Obesity. Clin. Exp. Pharmacol. Physiol. 2021, 48, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.; Péneau, S.; Andreeva, V.A.; Méjean, C.; Fezeu, L.; Galan, P.; Hercberg, S. Weight-Loss Strategies Used by the General Population: How Are They Perceived? PLoS ONE 2014, 9, e97834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Reis Costa, D.E.F.; Silveira, A.L.M.; Campos, G.P.; Nóbrega, N.R.C.; de Araújo, N.F.; de Figueiredo Borges, L.; dos Santos Aggum Capettini, L.; Ferreira, A.V.M.; Bonaventura, D. High-Carbohydrate Diet Enhanced the Anticontractile Effect of Perivascular Adipose Tissue Through Activation of Renin-Angiotensin System. Front. Physiol. 2021, 11, 628101. [Google Scholar] [CrossRef]
- Sasoh, T.; Kugo, H.; Kondo, Y.; Miyamoto, K.; Minami, M.; Higashihara, M.; Kawamoto, H.; Takeshita, F.; Moriyama, T.; Zaima, N. Different effects of high-fat and high-sucrose diets on the physiology of perivascular adipose tissues of the thoracic and abdominal aorta. Adipocyte 2021, 10, 412–423. [Google Scholar] [CrossRef]
- Victorio, J.A.; Guizoni, D.M.; Freitas, I.N.; Araujo, T.R.; Davel, A.P. Effects of High-Fat and High-Fat/High-Sucrose Diet-Induced Obesity on PVAT Modulation of Vascular Function in Male and Female Mice. Front. Pharm. 2021, 12, 720224. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean Diet and Health Status: An Updated Meta-Analysis and a Proposal for a Literature-Based Adherence Score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [Green Version]
- Luisi, M.L.E.; Biffi, B.; Gheri, C.F.; Sarli, E.; Rafanelli, E.; Graziano, E.; Vidali, S.; Fattirolli, F.; Gensini, G.F.; Macchi, C. Efficacy of a Nutritional Education Program to Improve Diet in Patients Attending a Cardiac Rehabilitation Program: Outcomes of a One-Year Follow-Up. Intern. Emerg. Med. 2015, 10, 671–676. [Google Scholar] [CrossRef]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharm. 2019, 10, 1366. [Google Scholar] [CrossRef] [Green Version]
- Mantzoros, C.S.; Williams, C.J.; Manson, J.E.; Meigs, J.B.; Hu, F.B. Adherence to the Mediterranean Dietary Pattern Is Positively Associated with Plasma Adiponectin Concentrations in Diabetic Women. Am. J. Clin. Nutr. 2006, 84, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The Management of Very Low-Calorie Ketogenic Diet in Obesity Outpatient Clinic: A Practical Guide. J. Transl. Med. 2019, 17. [Google Scholar] [CrossRef]
- Monda, V.; Polito, R.; Lovino, A.; Finaldi, A.; Valenzano, A.; Nigro, E.; Corso, G.; Sessa, F.; Asmundo, A.; Di Nunno, N.; et al. Short-Term Physiological Effects of a Very Low-Calorie Ketogenic Diet: Effects on Adiponectin Levels and Inflammatory States. Int. J. Mol. Sci. 2020, 21, 3228. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.-E.; Maki, K.C. Review of Current Evidence and Clinical Recommendations on the Effects of Low-Carbohydrate and Very-Low-Carbohydrate (Including Ketogenic) Diets for the Management of Body Weight and Other Cardiometabolic Risk Factors: A Scientific Statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef] [Green Version]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular Disease Risk Factor Responses to a Type 2 Diabetes Care Model Including Nutritional Ketosis Induced by Sustained Carbohydrate Restriction at 1 Year: An Open Label, Non-Randomized, Controlled Study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef] [Green Version]
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of Cellular Biochemistry, Epigenetics and Metabolomics by Ketone Bodies. Implications of the Ketogenic Diet in the Physiology of the Organism and Pathological States. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef] [Green Version]
- Mattagajasingh, I.; Kim, C.-S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.-B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 Promotes Endothelium-Dependent Vascular Relaxation by Activating Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Parrado-Fernandez, C.; Csiszar, A.; de Cabo, R. Mechanisms Underlying Caloric Restriction and Lifespan Regulation: Implications for Vascular Aging. Circ. Res. 2008, 102, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Vink, R.G.; Roumans, N.J.; Mariman, E.C.; van Baak, M.A. Dietary Weight Loss-induced Changes in RBP4, FFA, and ACE Predict Weight Regain in People with Overweight and Obesity. Physiol. Rep. 2017, 5, e13450. [Google Scholar] [CrossRef] [Green Version]
- Lambert, E.A.; Sari, C.I.; Eikelis, N.; Phillips, S.E.; Grima, M.; Straznicky, N.E.; Dixon, J.B.; Esler, M.; Schlaich, M.P.; Head, G.A.; et al. Effects of Moxonidine and Low-Calorie Diet: Cardiometabolic Benefits from Combination of Both Therapies. Obesity 2017, 25, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Lugo, M.J.; Jiménez-Ortega, V.; Cano-Barquilla, P.; Mateos, P.F.; Spinedi, E.J.; Cardinali, D.P.; Esquifino, A.I. Melatonin Counteracts Changes in Hypothalamic Gene Expression of Signals Regulating Feeding Behavior in High-Fat Fed Rats. Horm. Mol. Biol. Clin. Investig. 2015, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunet-Marcassus, B.; Desbazeille, M.; Bros, A.; Louche, K.; Delagrange, P.; Renard, P.; Casteilla, L.; Pénicaud, L. Melatonin Reduces Body Weight Gain in Sprague Dawley Rats with Diet-Induced Obesity. Endocrinology 2003, 144, 5347–5352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szewczyk-Golec, K.; Rajewski, P.; Gackowski, M.; Mila-Kierzenkowska, C.; Wesołowski, R.; Sutkowy, P.; Pawłowska, M.; Woźniak, A. Melatonin Supplementation Lowers Oxidative Stress and Regulates Adipokines in Obese Patients on a Calorie-Restricted Diet. Oxid. Med. Cell Longev. 2017, 2017, 8494107. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Ssperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef]
- Liu, B.; Page, A.J.; Hatzinikolas, G.; Chen, M.; Wittert, G.A.; Heilbronn, L.K. Intermittent Fasting Improves Glucose Tolerance and Promotes Adipose Tissue Remodeling in Male Mice Fed a High-Fat Diet. Endocrinology 2019, 160, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, Q.; Liao, Q.; Li, M.; Zhang, P.; Santos, H.O.; Kord-Varkaneh, H.; Abshirini, M. Effects of Intermittent Fasting Diets on Plasma Concentrations of Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrition 2020, 79–80, 110974. [Google Scholar] [CrossRef]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of Adipose Tissue Inflammation Are Transiently Elevated during Intermittent Fasting in Women Who Are Overweight or Obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kim, Y.H.; Son, J.E.; Lee, J.H.; Kim, S.; Choe, M.S.; Moon, J.H.; Zhong, J.; Fu, K.; Lenglin, F.; et al. Intermittent Fasting Promotes Adipose Thermogenesis and Metabolic Homeostasis via VEGF-Mediated Alternative Activation of Macrophage. Cell Res. 2017, 27, 1309–1326. [Google Scholar] [CrossRef]
- Fuentes, L.; Roszer, T.; Ricote, M. Inflammatory Mediators and Insulin Resistance in Obesity: Role of Nuclear Receptor Signaling in Macrophages. Mediat. Inflamm. 2010, 2010, 219583. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Kajita, K.; Mune, T.; Ikeda, T.; Matsumoto, M.; Uno, Y.; Sugiyama, C.; Matsubara, K.; Morita, H.; Takemura, M.; Seishima, M.; et al. Effect of Fasting on PPARγ and AMPK Activity in Adipocytes. Diabetes Res. Clin. Pract. 2008, 81, 144–149. [Google Scholar] [CrossRef]
- Weir, H.J.; Yao, P.; Huynh, F.K.; Escoubas, C.C.; Goncalves, R.L.; Burkewitz, K.; Laboy, R.; Hirschey, M.D.; Mair, W.B. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab. 2017, 26, 884–896.e5. [Google Scholar] [CrossRef] [Green Version]
- Clemente-Postigo, M.; Tinahones, A.; El Bekay, R.; Malagón, M.M.; Tinahones, F.J. The Role of Autophagy in White Adipose Tissue Function: Implications for Metabolic Health. Metabolites 2020, 10, 179. [Google Scholar] [CrossRef]
- Rocchi, A.; He, C. Emerging Roles of Autophagy in Metabolism and Metabolic Disorders. Front. Biol. 2015, 10, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. https://doi.org/10.3390/nu13113843
Stanek A, Brożyna-Tkaczyk K, Myśliński W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients. 2021; 13(11):3843. https://doi.org/10.3390/nu13113843
Chicago/Turabian StyleStanek, Agata, Klaudia Brożyna-Tkaczyk, and Wojciech Myśliński. 2021. "The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis" Nutrients 13, no. 11: 3843. https://doi.org/10.3390/nu13113843
APA StyleStanek, A., Brożyna-Tkaczyk, K., & Myśliński, W. (2021). The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients, 13(11), 3843. https://doi.org/10.3390/nu13113843







