Health Promoting Properties of Bee Royal Jelly: Food of the Queens
Abstract
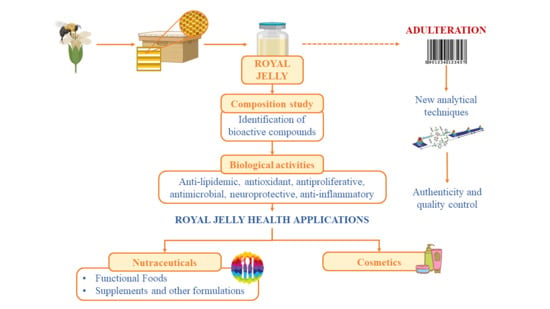
:1. Introduction
2. Composition of Royal Jelly
2.1. Carbohydrates
2.2. Proteins
2.3. Lipids
2.4. Vitamins
2.5. Minerals
2.6. Phenolic and Volatile Compounds
3. Royal Jelly Authenticity and Adulteration
4. Biological and Health Promoting Properties
4.1. Anti-Lipidemic Activity: Lipid Metabolism
4.2. Antioxidant Activity: Oxidative Stress
4.3. Antiproliferative Activity
4.4. Antimicrobial Activity
4.5. Neuroprotective Effect
4.6. Anti-Inflammatory Activity
4.7. Additional Effects
5. Royal Jelly Health Applications
5.1. Nutraceutical Industry
5.1.1. Functional Food
5.1.2. Supplements and Other Formulations
5.2. Cosmetic Industry
6. Final Remarks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RJ | Royal Jelly |
| 10-HDA | 10-hydroxy-2-decenoic acid |
| MRJP | Major Royal Jelly Proteins |
| FAA | Free Amino Acids |
| HDAA | 10-hydroxydecanoic acid |
| VC | Volatile Compounds |
| HDL-C | High-Density Lipoprotein Cholesterol |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| ROS | Reactive Oxygen Species |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| RNS | Reactive Nitrogen Species |
| AMPs | Antimicrobial Peptides Appear |
| AD | Alzheimer’s Disease |
| HS-SPME/GC–MS | Headspace Solid-Phase Microextraction/Gas Chromatography-Mass Spectrometry |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| RA | Rheumatoid Arthritis |
| VEGF | Vascular Endothelial Growth Factor |
| pRJ | protease-treated RJ |
References
- Fontana, R.; Mendes, M.A.; De Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Buttstedt, A.; Mureşan, C.I.; Lilie, H.; Hause, G.; Ihling, C.H.; Schulze, S.H.; Pietzsch, M.; Moritz, R.F.A. How Honeybees Defy Gravity with Royal Jelly to Raise Queens. Curr. Biol. 2018, 28, 1095–1100.e3. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.L.; Bíliková, K.; Casabianca, H.; Daniele, G.; Salmen Espindola, F.; Feng, M.; Guan, C.; Han, B.; Krištof Kraková, T.; Li, J.K.; et al. Standard methods for Apis mellifera royal jelly research. J. Apic. Res. 2019, 58, 1–68. [Google Scholar] [CrossRef] [Green Version]
- Scarselli, R.; Donadio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Towards royal jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Argüelles, S. Bee Products: Royal Jelly and Propolis; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128124918. [Google Scholar]
- Garcia-Amoedo, L.H.; De Almeida-Muradian, L.B. Physicochemical composition of pure and adulterated royal jelly. Quim. Nova 2007, 30, 257–259. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; de Almeida-Muradian, L.B. Quality and standardisation of Royal Jelly. J. ApiProd. ApiMed. Sci. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wytrychowski, M.; Chenavas, S.; Daniele, G.; Casabianca, H.; Batteau, M.; Guibert, S.; Brion, B. Physicochemical characterisation of French royal jelly: Comparison with commercial royal jellies and royal jellies produced through artificial bee-feeding. J. Food Compos. Anal. 2013, 29, 126–133. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Czyzewska, U.; Isidorova, A.G.; Bakier, S. Gas chromatographic and mass spectrometric characterization of the organic acids extracted from some preparations containing lyophilized royal jelly. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3776–3780. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic-mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 885–886, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Altaye, S.Z.; Meng, L.; Li, J. Molecular insights into the enhanced performance of royal jelly secretion by a stock of honeybee (Apis mellifera ligustica) selected for increasing royal jelly production. Apidologie 2019, 50, 436–453. [Google Scholar] [CrossRef] [Green Version]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Dimou, M.; Goras, G.; Rodopoulou, M.A.; Karazafiris, E.; Thrasyvoulou, A. A suggestion for royal jelly specifications. Arhiv za Higijenu Rada i Toksikologiju 2015, 66, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamyab, S.; Gharachorloo, M.; Honarvar, M.; Ghavami, M. Quantitative analysis of bioactive compounds present in Iranian royal jelly. J. Apic. Res. 2020, 59, 42–52. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Njeru, L.K.; Lattorff, H.M.G. African honeybee royal jelly: Phytochemical contents, free radical scavenging activity, and physicochemical properties. Food Biosci. 2020, 37, 100733. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Premratanachai, P.; Chanchao, C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.M.S.; Khalaf, A.A.; Moselhy, W.A.; Safwat, G.M. Royal jelly attenuates azathioprine induced toxicity in rats. Environ. Toxicol. Pharmacol. 2014, 37, 431–437. [Google Scholar] [CrossRef]
- Ibrahim, A.A.E.-M. Immunomodulatory effects of royal jelly on aorta CD3, CD68 and eNOS expression in hypercholesterolaemic rats. J. Basic Appl. Zool. 2014, 67, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Cihan, Y.B.; Ozturk, A.; Gokalp, S.S. Protective role of royal jelly against radiation-induced oxidative stress in rats. UHOD—Uluslararasi Hematoloji-Onkoloji Dergisi 2013, 23, 79–87. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Ferioli, F.; Armaforte, E.; Caboni, M.F. Comparison of the lipid content, fatty acid profile and sterol composition in local Italian and commercial royal jelly samples. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 875–884. [Google Scholar] [CrossRef]
- Wongchai, V.; Ratanavalachai, T. Seasonal variation of chemical composition of royal jelly produced in Thailand. Thammasat Int. J. Sci. Technol. 2002, 7, 1–8. [Google Scholar]
- Kimura, M.; Kimura, Y.; Tsumura, K.; Okihara, K.; Sugimoto, H.; Yamada, H.; Yonekura, M. 350-kDa royal jelly glycoprotein (Apisin), which stimulates proliferation of human monocytes, bears the β1-3galactosylated N-glycan: Analysis of the N-glycosylation site. Biosci. Biotechnol. Biochem. 2003, 67, 2055–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y.; et al. Comprehensive royal jelly (RJ) proteomics using one- and two-dimensional proteomics platforms reveals novel RJ proteins and potential phospho/glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.C.; Morgan, S.L.; Spencley, A.L.; Mariano, N.; Chang, E.Y.; Shankar, G.; Luo, Y.; Li, T.H.; Huh, D.; Huynh, S.K.; et al. Honey bee Royalactin unlocks conserved pluripotency pathway in mammals. Nat. Commun. 2018, 9, 5078. [Google Scholar] [CrossRef]
- Šimúth, J.; Bíliková, K.; Kováčová, E.; Kuzmová, Z.; Schroder, W. Immunochemical Approach to Detection of Adulteration in Honey: Physiologically Active Royal Jelly Protein Stimulating TNF-α Release Is a Regular Component of Honey. J. Agric. Food Chem. 2004, 52, 2154–2158. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Han, B.; Feng, M.; Fang, Y.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and Proteomic Investigations Reveal Major Royal Jelly Protein 1 Associated with Anti-hypertension Activity in Mouse Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kamakura, M.; Suenobu, N.; Fukushima, M. Fifty-seven-kDa protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum. Biochem. Biophys. Res. Commun. 2001, 282, 865–874. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Park, H.G.; Deng, Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia. Pac. Entomol. 2019, 22, 684–689. [Google Scholar] [CrossRef]
- Bíliková, K.; Mirgorodskaya, E.; Bukovská, G.; Gobom, J.; Lehrach, H.; Simúth, J. Towards functional proteomics of minority component of honeybee royal jelly: The effect of post-translational modifications on the antimicrobial activity of apalbumin2. Proteomics 2009, 9, 2131–2138. [Google Scholar] [CrossRef]
- Mostafa, R.E.; El-Marasy, S.A.; Abdel Jaleel, G.A.; Bakeer, R.M. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon 2020, 6, e03330. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; Habashy, N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019, 128, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Tamura, S.; Kono, T.; Harada, C.; Yamaguchi, K.; Moriyama, T. Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chem. 2009, 114, 1491–1497. [Google Scholar] [CrossRef]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Jung, B.; Choi, Y.S.; Kim, H.K.; Yoon, H.J.; Gui, Z.Z.; Lee, J.; Jin, B.R. Honeybee (Apis cerana) major royal jelly protein 4 exhibits antimicrobial activity. J. Asia-Pac. Entomol. 2019, 22, 175–182. [Google Scholar] [CrossRef]
- Santos, K.S.; Delazari Dos Santos, L.; Anita Mendes, M.; Monson De Souza, B.; Malaspina, O.; Palma, M.S. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.). Insect Biochem. Mol. Biol. 2005, 35, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Li, R.; Feng, M.; Han, B.; Zhou, T.; Li, J. Towards posttranslational modification proteome of royal jelly. J. Proteom. 2012, 75, 5327–5341. [Google Scholar] [CrossRef]
- Bachanová, K.; Klauduny, J.; Kopernický, J.; Simúth, J. Identification of honeybee peptide active against Paenibacillus larvae larvae through bacterial growth-inhibition assay on polyacrylamide gel. Apidologie 2002, 33, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Bílikova, K.; Huang, S.C.; Lin, I.P.; Šimuth, J.; Peng, C.C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef]
- Bărnuţiu, L.I.; Mărghitaş, L.A.; Dezmirean, D.S.; Mihai, C.M.; Bobiş, O. Chemical Composition and Antimicrobial Activity of Royal Jelly—Review. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 67–72. [Google Scholar]
- Bíliková, K.; Hanes, J.; Nordhoff, E.; Saenger, W.; Klaudiny, J.; Šimúth, J. Apisimin, a new serine-valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 2002, 528, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Fang, Y.; Feng, M.; Lu, X.; Huo, X.; Meng, L.; Wu, B.; Li, J. In-depth phosphoproteomic analysis of royal jelly derived from western and eastern honeybee species. J. Proteom. Res. 2014, 13, 5928–5943. [Google Scholar] [CrossRef]
- Alreshoodi, F.M.; Sultanbawa, Y. Antimicrobial activity of royal jelly. Antiinfect. Agents 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Garcia, M.C.; Finola, M.S.; Marioli, J.M. Bioassay directed identification of Royal Jelly’s active compounds against the growth of bacteria capable of infecting cutaneous wounds. Adv. Microbiol 2013, 3, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, D.; Wei, Z.; Wang, J.; Li, C.; Hui, Y.; Lei, K.; Chen, X.; Shen, N.; Jin, L.; et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethno-Pharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, D.; Rajkovic, I.; Chinou, I.; Colic, M. Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J. Funct. Foods 2013, 5, 838–846. [Google Scholar] [CrossRef]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10- Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid. Based Complement. Altern. Med 2009, 6, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, C.; Xue, Y. Contribution of Lipids in Honeybee (Apis mellifera) royal jelly to health. J. Med. Food 2013, 16, 96–102. [Google Scholar] [CrossRef]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid. Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [Green Version]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific hydroxy fatty acids in Royal Jelly activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-Decenoic Acid, the Major Lipid Component of Royal Jelly, Extends the Lifespan of Caenorhabditis Elegans Through Dietary Restriction and Target of Rapamycin Signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Hwang, E.; Lee, K.; Han, S.-M.; Cho, Y.; Kim, S. Royal Jelly Protects Against Ultraviolet B–Induced Photoaging in Human Skin Fibroblasts via Enhancing Collagen Production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Nazzi, F.; Bortolomeazzi, R.; Della Vedova, G.; Del Piccolo, F.; Annoscia, D.; Milani, N. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften 2009, 96, 309–314. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Ström, K.; Schnürer, J. Metabolite Profiles of Lactic Acid Bacteria in Grass Silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzopalic, T.; Vucevic, D.; Tomic, S.; Djokic, J.; Chinou, I.; Colic, M. 3,10-Dihydroxy-decanoic acid, isolated from royal jelly, stimulates Th1 polarising capability of human monocyte-derived dendritic cells. Food Chem. 2011, 126, 1211–1217. [Google Scholar] [CrossRef]
- De Paula, R.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.S.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y. Isolation and characterization of proteins and lipids from honeybee (Apis mellifera L.) queen larvae and royal jelly. Food Res. Int. 2013, 54, 330–337. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Chung-Eun, H. Chapter 36—Vitamin Metabolism. In Essentials of Medical Biochemistry; Bhagavan, N.V., Chung-Eun, H., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 683–699. ISBN 9780124166875. [Google Scholar]
- Awasthi, S.; Awasthi, A. Role of vitamin a in child health and nutrition. Clin. Epidemiol. Glob. Health 2020, 8, 1039–1042. [Google Scholar] [CrossRef]
- Bates, C.J. Pantothenic Acid. In Encyclopedia of Human Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 1–5. [Google Scholar]
- Bhagavan, N.V.; Chung-Eun, H. Chapter 25—Nucleotide Metabolism. In Essentials of Medical Biochemistry; Bhagavan, N.V., Chung-Eun, H., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 465–487. ISBN 9780124166875. [Google Scholar]
- Popescu, O.; Mărghitas, L.; Bobis, O.; Stanciu, O.; Bonta, V.; Moise, A.; Dezmirean, D. Sugar profile and total proteins content of fresh royal jelly. Bull. UASVM Anim. Sci. Biotechnol. 2009, 66, 265–269. [Google Scholar]
- Daniele, G.; Casabianca, H. Sugar composition of French royal jelly for comparison with commercial and artificial sugar samples. Food Chem. 2012, 134, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Sesta, G. Determination of sugars in royal jelly by HPLC. Apidologie 2006, 37, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Schmitzová, J.; Klaudiny, J.; Albert, Š.; Schröder, W.; Schreckengost, W.; Hanes, J.; Júdová, J.; Šimúth, J. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. C. 1998, 54, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Albert, Š.; Klaudiny, J. The MRJP/YELLOW protein family of Apis mellifera: Identification of new members in the EST library. J. Insect Physiol. 2004, 50, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Schönleben, S.; Sickmann, A.; Mueller, M.J.; Reinders, J. Proteome analysis of Apis mellifera royal jelly. Anal. Bioanal. Chem. 2007, 389, 1087–1093. [Google Scholar] [CrossRef]
- Tamura, S.; Amano, S.; Kono, T.; Kondoh, J.; Yamaguchi, K.; Kobayashi, S.; Ayabe, T.; Moriyama, T. Molecular characteristics and physiological functions of major royal jelly protein 1 oligomer. Proteomics 2009, 9, 5534–5543. [Google Scholar] [CrossRef]
- Cruz, G.C.N.; Garcia, L.; Silva, A.J.; Barbosa, J.A.R.G.; Ricart, C.A.O.; Freitas, S.M.; Sousa, M.V. Calcium effect and pH-dependence on self-association and structural stability of the Apis mellifera major royal jelly protein 1. Apidologie 2011, 42, 252–269. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Washino, N.; Yonekura, M. N-Linked Sugar Chains of 350-kDa Royal Jelly Glycoprotein. Biosci. Biotechnol. Biochem. 1995, 59, 507–509. [Google Scholar] [CrossRef]
- Šimúth, J. Some properties of the main protein of honeybee (Apis mellifera) royal jelly. Apidologie 2001, 32, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Li, M.; Guo, H.; Peng, W.; Xue, X.; Hu, Y.; Liu, Y.; Zhao, Y.; Fang, X.; Wang, K.; et al. Architecture of the native major royal jelly protein 1 oligomer. Nat. Commun. 2018, 9, 3373. [Google Scholar] [CrossRef]
- Kolayli, S.; Keskin, M. Natural Bee Products and Their Apitherapeutic Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 66, ISBN 9780128179079. [Google Scholar]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Liming, W.; Jinhui, Z.; Xiaofeng, X.; Yi, L.; Jing, Z. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compos. Anal. 2009, 22, 242–249. [Google Scholar] [CrossRef]
- Boselli, E.; Caboni, M.; Sabatini, A.; Marcazzan, G.; Giovanni, L. Determination and changes of free amino acids in royal jelly during storage. Apidologie 2003, 34, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Kanbur, M.; Eraslan, G.; Beyaz, L.; Silici, S.; Liman, B.C.; Altinordulu, Ş.; Atasever, A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Ekmekcioglu, O.; Eraslan, G.; Demirtas, A. Antioxidative Effect of Royal Jelly in Cisplatin-induced Testes Damage. Urology 2009, 74, 545–551. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Stocker, A.; Schramel, P.; Kettrup, A.; Bengsch, E. Trace and mineral elements in royal jelly and homeostatic effects. J. Trace Elem. Med. Biol. 2005, 19, 183–189. [Google Scholar] [CrossRef]
- Balkanska, R.; Mladenova, E.; Karadjova, I. Quantification of selected trace and mineral elements in royal jelly from Bulgaria by ICP-OES and etaas. J. Apic. Sci. 2017, 61, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Otero, J.L.; Paseiro, P.; Simal, J.; Cepeda, A. Mineral content of the honeys produced in Galicia (North-west Spain). Food Chem. 1994, 49, 169–171. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Li, Z.G.; Tian, W.L.; Fang, X.M.; Su, S.K.; Peng, W.J. Differential volatile organic compounds in royal jelly associated with different nectar plants. J. Integr. Agric. 2016, 15, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Ma, C.; Wang, W.; Zhang, L.; Hao, J.; Li, J. Gas chromatography-mass spectrometry analysis reveals the differences in volatile components of royal jelly from different honeybee stocks. LWT 2020, 124, 109143. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Machado, A.M.; Aazza, S.; Lyoussi, B.; Miguel, M.G.; Mateus, M.C.; Figueiredo, A.C. Chemical Characterization and Biological Properties of Royal Jelly Samples From the Mediterranean Area. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.; El-Guendouz, S. Volatile Compounds of Royal Jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 191–197. [Google Scholar]
- López-Gutiérrez, N.; del Aguilera-Luiz, M.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Virgiliou, C.; Kanelis, D.; Pina, A.; Gika, H.; Tananaki, C.; Zotou, A.; Theodoridis, G. A targeted approach for studying the effect of sugar bee feeding on the metabolic profile of Royal Jelly. J. Chromatogr. A 2020, 1616, 460783. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Wytrychowski, M.; Batteau, M.; Guibert, S.; Casabianca, H. Stable isotope ratio measurements of royal jelly samples for controlling production procedures: Impact of sugar feeding. Rapid Commun. Mass Spectrom. 2011, 25, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Antinelli, J.F.; Zeggane, S.; Davico, R.; Rognone, C.; Faucon, J.P.; Lizzani, L. Evaluation of (E)-10-hydroxydec-2-enoic acid as a freshness parameter for royal jelly. Food Chem. 2003, 80, 85–89. [Google Scholar] [CrossRef]
- Morgado Schmidt, E.; da Silva Cunha, I.B.; Nogueira Eberlin, M.; C H F Sawaya, A. Characterization of Royal Jelly by Electrospray Ionization Mass Spectrometry Fingerprinting. Mass Spectrom. Purif. Tech. 2015, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Abdelnur, P.V.; Abe, S.; Cunha, I.B.S.; Lima-Pallone, J.A.; Godoy, H.T.; Eberlin, M.N.; Catharino, R.R. Metabolic fingerprinting of royal jelly: Characterization and proof of authenticity. Qual. Assur. Saf. Crop. Foods 2011, 3, 185–190. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Xue, X.; Zhang, J.; Chen, F.; Li, Y.; Wu, L.; Li, C.; Mi, J. Hydrophilic interaction chromatography/tandem mass spectrometry for the determination of melamine in royal jelly and royal jelly lyophilized powder. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 4164–4170. [Google Scholar] [CrossRef] [PubMed]
- Reybroeck, W. Residues of antibiotics and chemotherapeutics in honey. J. Apic. Res. 2018, 57, 97–112. [Google Scholar] [CrossRef]
- Calvarese, S.; Forti, A.F.; Scortichini, G.; Diletti, G. Chloramphenicol in royal jelly: Analytical aspects and occurrence in Italian imports. Apidologie 2006, 37, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chen, H.; Lin, Z.-G.; Niu, Q.-S.; Wang, Z.; Gao, F.; Ji, T. Carbendazim exposure during the larval stage suppresses major royal jelly protein expression in nurse bees (Apis mellifera). Chemosphere 2020, 266, 129011. [Google Scholar] [CrossRef] [PubMed]
- Milone, J.P.; Chakrabarti, P.; Sagili, R.R.; Tarpy, D.R. Colony-level pesticide exposure affects honey bee (Apis mellifera L.) royal jelly production and nutritional composition. Chemosphere 2021, 263, 128183. [Google Scholar] [CrossRef] [PubMed]
- Giroud, B.; Bruckner, S.; Straub, L.; Neumann, P.; Williams, G.R.; Vulliet, E. Trace-level determination of two neonicotinoid insecticide residues in honey bee royal jelly using ultra-sound assisted salting-out liquid liquid extraction followed by ultra-high-performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 151, 104249. [Google Scholar] [CrossRef]
- Hou, J.; Xie, W.; Hong, D.; Zhang, W.; Li, F.; Qian, Y.; Han, C. Simultaneous determination of ten neonicotinoid insecticides and two metabolites in honey and Royal-jelly by solid−phase extraction and liquid chromatography−tandem mass spectrometry. Food Chem. 2019, 270, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Arribas, M.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Development and validation of UHPLC–MS/MS methods for determination of neonicotinoid insecticides in royal jelly-based products. J. Food Compos. Anal. 2018, 70, 105–113. [Google Scholar] [CrossRef]
- Yan, A.T.; Yan, R.T.; Tan, M.; Hackam, D.G.; Leblanc, K.L.; Kertland, H.; Tsang, J.L.; Jaffer, S.; Kates, M.L.; Leiter, L.A.; et al. Contemporary Management of Dyslipidemia in High-Risk Patients: Targets Still Not Met. Am. J. Med. 2006, 119, 676–683. [Google Scholar] [CrossRef]
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Foods 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef]
- Chiu, H.F.; Chen, B.K.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes. Res. Clin. Pract. 2018, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, D.; Fujiwara, N.; Suzuki, K. Antioxidants: Benefits and risks for long-term health. Maturitas 2010, 67, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Roden, M. Non-alcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2020, 101122. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Vinciguerra, M.; Oben, J.; Tarquini, R.; De Cosmo, S. Non-alcoholic fatty liver disease: The role of nuclear receptors and circadian rhythmicity. Liver Int. 2014, 34, 1133–1152. [Google Scholar] [CrossRef]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipič, B.; Gradišnik, L.; Rihar, K.; Šooš, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arhiv Higijenu Rada Toksikologiju 2015, 66, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y. Antitumor and antimetastatic actions of various natural products. Stud. Nat. Prod. Chem. 2008, 34, 35–76. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Townsend, G.F.; Morgan, J.F.; Tolnai, S.; Hazlett, B.; Morton, H.J.; Shuel, R.W. Studies on the in Vitro Antitumor Activity of Fatty Acids 10-Hydroxy-2-decenoic Acid from Royal Jelly. Cancer Res. 1960, 20, 503–510. [Google Scholar] [PubMed]
- Wang, J.; Zhang, W.; Zou, H.; Lin, Y.; Lin, K.; Zhou, Z.; Qiang, J.; Lin, J.; Chuka, C.M.; Ge, R.; et al. 10-Hydroxy-2-decenoic acid inhibiting the proliferation of fibroblast-like synoviocytes by PI3K-AKT pathway. Int. Immunopharmacol. 2015, 28, 97–104. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms: My perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.K.; Srivastava, S.; Singh, M.; Ghosh, J.K. Inducing toxicity by introducing a leucine-zipper-like motif in frog antimicrobial peptide, magainin 2. Biochem. J. 2011, 436, 609–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Šedivá, M.; Laho, M.; Kohútová, L.; Mojžišová, A.; Majtán, J.; Klaudiny, J. 10-HDA, A Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCleskey, C.S.; Melampy, R.M. Bactericidal Properties of Royal Jelly of the Honeybee*. J. Econ. Entomol. 1939, 32, 581–587. [Google Scholar] [CrossRef]
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [Green Version]
- Food, E.; Authority, S. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef] [Green Version]
- Altuntas, S.; Cinar, A.; Altuntas, V. Modelling of listeria monocytogenes growth and survival in presence of royal jelly, a promising anti-biofilm agent. J. Food Nutr. Res. 2020, 59, 7–15. [Google Scholar]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Romanelli, A.; Moggio, L.; Montella, R.C.; Campiglia, P.; Iannaccone, M.; Capuano, F.; Pedone, C.; Capparelli, R. Peptides from Royal Jelly: Studies on the antimicrobial activity of jelleins, jelleins analogs and synergy with temporins. J. Pept. Sci. 2011, 17, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, D.; Li, M.; Jin, F.; Din, M.; Parnell, L.D.; Lai, C.Q. Mechanism of Action of Recombinant Acc-Royalisin from Royal Jelly of Asian Honeybee against Gram-Positive Bacteria. PLoS ONE 2012, 7, e47194. [Google Scholar] [CrossRef]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K.; Milk, M.; Company, I. A Potent Antibacterial in Royal Jelly. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Gunalp, R.; Ulusoy, M.; Celebier, I.; Keskin, N. Antifungal effect of royal jelly on Candida albicans. J. Biotechnol. 2018, 280, S70–S71. [Google Scholar] [CrossRef]
- Alzheimer’s Association 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Mohamed, A.A.R.; Galal, A.A.A.; Elewa, Y.H.A. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem. 2015, 117, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Minami, A.; Matsushita, H.; Ieno, D.; Matsuda, Y.; Horii, Y.; Ishii, A.; Takahashi, T.; Kanazawa, H.; Wakatsuki, A.; Suzuki, T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric 2016, 19, 568–573. [Google Scholar] [CrossRef]
- Aslan, A.; Cemek, M.; Buyukokuroglu, M.E.; Altunbas, K.; Bas, O.; Yurumez, Y. Royal jelly can diminish secondary neuronal damage after experimental spinal cord injury in rabbits. Food Chem. Toxicol. 2012, 50, 2554–2559. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, J.; Jin, P.; Yang, Q.; Zhu, K.; You, M.; Hu, F.; Chen, M. Royal jelly ameliorates behavioral deficits, cholinergic system deficiency, and autonomic nervous dysfunction in ovariectomized cholesterol-fed rabbits. Molecules 2019, 24, 1149. [Google Scholar] [CrossRef] [Green Version]
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci. 2015, 143, 64–80. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Almeer, R.S.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly mitigates cadmium-induced neuronal damage in mouse cortex. Mol. Biol. Rep. 2019, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Poirier, P.; Carnethon, M.R.; Blair, S.N.; Church, T.S. Autonomic function and change in insulin for exercising postmenopausal women. Maturitas 2010, 65, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, J.; Wang, F.; Wei, C.; Zhou, A.; Jia, X.; Li, F.; Tang, M.; Chu, L.; Zhou, Y.; Zhou, C.; et al. The prevalence of dementia in urban and rural areas of China. Alzheimer’s Dement. 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Chin, J.; Kim, J.W.; Shin, M.H.; Ahn, S.; Lee, D.Y.; Seo, S.W.; Na, D.L. Menopausal hormone therapy and mild cognitive impairment: A randomized, placebo-controlled trial. Menopause 2018, 25, 870–876. [Google Scholar] [CrossRef]
- Jaya Prasanthi, R.P.; Schommer, E.; Thomasson, S.; Thompson, A.; Feist, G.; Ghribi, O. Regulation of β-amyloid levels in the brain of cholesterol-fed rabbit, a model system for sporadic Alzheimer’s disease. Mech. Ageing Dev. 2008, 129, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2018, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Aslan, Z.; Aksoy, L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petelin, A.; Kenig, S.; Kopinč, R.; Deželak, M.; Černelič Bizjak, M.; Jenko Pražnikar, Z. Effects of royal jelly administration on lipid profile, satiety, inflammation, and antioxidant capacity in asymptomatic overweight adults. Evid. Based Complement. Altern. Med. 2019, 2019, 4969720. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017, 79, 299–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaku, M.; Rocabado, J.M.R.; Kitami, M.; Ida, T.; Uoshima, K. Royal jelly affects collagen crosslinking in bone of ovariectomized rats. J. Funct. Foods 2014, 7, 398–406. [Google Scholar] [CrossRef]
- Møller, A.M.J.; Delaissé, J.M.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Søe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Hayashi, M.; Nagamatsu, K.; Ono, T.; Kamakura, M.; Iwata, T.; Nakashima, T. The key royal jelly component 10-hydroxy-2-decenoic acid protects against bone loss by inhibiting NF-κB signaling downstream of FFAR4. J. Biol. Chem. 2020, 295, 12224–12232. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Guo, H.; Guo, Y.; Ebihara, S.; Asada, M.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Kudo, Y.; et al. Royal jelly prevents the progression of sarcopenia in aged mice in vivo and in vitro. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1482–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Wang, H.; Pei, Y.; Li, Y.; Wu, H.; Song, Y.; Guo, Q.; Guo, H.; Fukushima, S.; Tatefuji, T.; et al. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: A randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017, 7, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal jelly delays motor functional impairment during aging in genetically heterogeneous male mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Hijikata, K.; Seike, K.; Nakano, S.; Banjo, M.; Sato, Y.; Takahashi, K.; Hatta, H. Effects of royal jelly administration on endurance training-induced mitochondrial adaptations in skeletal muscle. Nutrients 2018, 10, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protects, J.; Skeletal, A.D.; Atrophy, M. Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients 2020, 12, 3089. [Google Scholar]
- Alu’datt, M.H.; Rababah, T.; Obaidat, M.M.; Ereifej, K.; Alhamad, M.N.; Mhaidat, N.; Andrade, J.E.; Johargy, A.; Ayadi, W. Probiotics in Milk as Functional Food: Characterization and Nutraceutical Properties of Extracted Phenolics and Peptides from Fermented Skimmed Milk Inoculated with Royal Jelly. J. Food Saf. 2015, 35, 509–522. [Google Scholar] [CrossRef]
- Mendoza, M.R.; Olano, N.O.; Villamiel, M. Chemical Indicators of Heat Treatment in Fortified and Special Milks. J. Agric. Food Chem. 2005, 53, 2995–2999. [Google Scholar] [CrossRef]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef]
- Yakoot, M.; Salem, A.; Helmy, S. Effect of Memo®, a natural formula combination, on Mini-Mental State Examination scores in patients with mild cognitive impairment. Clin. Interv. Aging 2013, 8, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Taavoni, S.; Barkhordari, F.; Goushegir, A.; Haghani, H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement. Ther. Med. 2014, 22, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Osama, H.; Abdullah, A.; Gamal, B.; Emad, D.; Sayed, D.; Hussein, E.; Mahfouz, E.; Tharwat, J.; Sayed, S.; Medhat, S.; et al. Effect of Honey and Royal Jelly against Cisplatin- Induced Nephrotoxicity in Patients with Cancer Effect of Honey and Royal Jelly against Cisplatin-Induced Nephrotoxicity in Patients. J. Am. Coll. Nutr. 2017, 36, 342–346. [Google Scholar] [CrossRef]
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical Evaluation of a Royal Jelly Supplementation for the Restoration of Dry Eye: A Prospective Randomized Double Blind Placebo Controlled Study and an Experimental Mouse Model. PLoS ONE 2017, 12, e169069. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012, 11, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadnia, H.; Sharifi, N.; Alizadeh, S.; Kamalati, A.; Safari, M.S. Wonderful Effects of Royal Jelly on Treatment of Male- Factor Related Infertility. Austin J. Reprod. Med. Infertil. 2015, 2, 1031. [Google Scholar]
- Salari, R.; Salari, R.; Medicine, C. Electronic Physician (ISSN: 2008-5842). Electron. Physician 2017, 9, 3592–3597. [Google Scholar] [CrossRef] [Green Version]
- Questel, E.; Hernandez-pigeon, H.; Galliano, M.F.; Caruana, A.; Ceruti, I.; Ambonati, M.; Mejean, C.; Damour, O.; Castex-rizzi, N.; Bessou-touya, S.; et al. Effects of Hydroxydecine ® (10-hydroxy-2-decenoic acid) on skin barrier structure and function. Eur. J. Dermatol. 2011, 2, 906–915. [Google Scholar] [CrossRef]
- Seyyedi, F.; Rafiean, M.; Miraj, S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagnostic Res. 2016, 10, QC01–QC05. [Google Scholar] [CrossRef]
- Fern, B.; Mart, J.M.; Chas-barbeito, C.; Bautista-casta, I. Food & Function Determinants of specific food consumption in the Canary Islands (Spain) †. Food Funct. 2011, 2, 627–632. [Google Scholar] [CrossRef] [Green Version]



| Compound | Molecular Group | % in Fresh RJ | Biological Activity | Reference |
|---|---|---|---|---|
| Proteins | ||||
| Proteins | - | 9–18% | Stimulates proliferation of human monocytes and promotes proliferation of Jurkat lymphoid cell | [10,20,25,29,30] |
| MRJP1 | Protein | 5.89% | Nematicidal activity | [31] |
| Antitumor effect | [7,32] | |||
| Hypocholesterolemic effect | [15,33] | |||
| Anti-hypertensive activity | [34] | |||
| Allergen | [11,26] | |||
| Royalactin | Protein | 0.25% | Increase in lifespan in invertebrates | [11,20] |
| Stimulation of proliferation of rat hepatocytes | [35] | |||
| Queen differentiation in honeybees | [35] | |||
| Activation of a pathway that allows self-renewal of stem cells | [31] | |||
| MRJP2 and isoforms | Protein | 1.41% | Antitumor effect | [7,30,32] |
| Antimicrobial activity and Protection against oxidative stress | [36] | |||
| Antibacterial activity | [37] | |||
| Allergen | [11,26] | |||
| Hepato-renal protective effect | [38] | |||
| Antitumor effects | [39] | |||
| Hepatocyte protection | [39] | |||
| Wound-healing activity | [40] | |||
| MRJP3 | Protein | 1.66% | Modulation of immune responses of T cells | [30,41,42] |
| Suppression of proinflammatory cytokine secretion. | [41] | |||
| Immunomodulatory effect | [43] | |||
| Wound-healing bioactivity | [40] | |||
| MRJP4 | Protein | 0.89% | Antimicrobial activity | [30,44] |
| MRJP5 | Protein | 0.64% | - | [30] |
| MRJP6 | Protein | - | - | [30] |
| MRJP7 | Protein | 0.51% | Wound-healing bioactivity | [30,40] |
| MRJP8 | Protein | - | - | [45] |
| MRJP9 | Protein | - | - | [30] |
| Glucose oxidase | Enzyme | 0.08% | Carbohydrate metabolism | [20,30] |
| Antibacterial | [11,20,25,30] | |||
| Gluco-cerebrosidase | Enzyme | - | Hydrolysis | [46] |
| Alpha-glucosidase | Enzyme | - | Hydrolysis | [30,46] |
| Royalisin | Protein | 0.83% | Antibacterial activity | [11,20,25,47,48,49] |
| Antifungal activity | [11,20,47] | |||
| Apisimin | Peptide | 0.13% | Stimulation of proliferation of human monocytes | [20,29,50] |
| Jelleines I-III | Peptide | 0.37% | Antimicrobial activity | [1,20,25,49] |
| Jelleine IV | Peptide | - | - | [1,25,49] |
| Venom protein 2 | Enzyme | - | Protection of larvae from diseases infection | [46] |
| Apolipophorin-III-like protein | Protein | 0.08% | Antimicrobial | [20,25,51] |
| Lipids | ||||
| Lipids | - | 3–8% | - | [10] |
| 10-HDA | Fatty acid | 0.75–3.39% | Antimicrobial activity | [17,20,52,53] |
| Immunomodulatory activity | [54,55,56] | |||
| Inhibitor of cancer growth | [57,58,59] | |||
| Estrogenic activity | [58,60] | |||
| Anti-inflammatory effect | [61] | |||
| Activation of TRPA1 and TRPV1 receptors | [62] | |||
| Increase longevity in C. elegans | [63] | |||
| Neurogenesis inductor | [63] | |||
| Protective effect against ultraviolet B in Human Skin | [64] | |||
| 10-hydroxydecanoic acid (10-HDAA) | Fatty acid | 0.78–1.05% | Estrogenic activity | [58,59,60] |
| Activation of TRPA1 and TRPV1 receptors | [62] | |||
| 8-hydroxy octanoic acid | Fatty acid | 0.18–0.39% | Varroa-repellent activity | [59,65] |
| 3-hydroxydecanoic acid | Fatty acid | 0.05–0.09% | Antifungal activity | [59,66] |
| 3,10-dihydroxydecanoic acid | Fatty acid | 0.26–0.46% | Immunomodulatory activity: Stimulation of dendritic cell differentiation | [55,59,67] |
| 9-hydroxy-2-decenoic acid | Fatty acid | 0.07–0.15% | Signal components (pheromone) of honeybee queen | [14,59] |
| 1,10-decanedioic acid (sebacic) | Fatty acid | 0.15–0.24% | Estrogenic activity | [58,59,60] |
| - | Anti-inflammatory effect | [61] | ||
| 2-Decenedioic | Fatty acid | 0.18–0.33% | - | [59] |
| Phenols | Lipid | 0.24–0.6% | Antioxidant activity | [59,62,68] |
| Ferulic acid | Phenol | 12.95–18.93 µg/kg | Antioxidant activity | [59,69] |
| Waxes | Lipid | 0.3–0.36% | - | [62] |
| Steroids | Lipid | 0.18–0.24% | Effects on collagen synthesis | [59,62] |
| 24-methylene cholesterol | Steroid | 6.06 mg/lipid | Estrogenic activity | [27,60,70] |
| Phospholipids | Lipid | 0.02–0.04 | - | [62] |
| Vitamins | ||||
| Vitamin A | Vitamin | 1.10 mg/100 g | Immunity, maintenance of the visual system, and maintenance of epithelial cellular integrity | [71,72] |
| Vitamin B1 | Vitamin | 2.06 mg/100 g | Transketolation, metabolism of fats, proteins and nucleic acids | [71] |
| Vitamin B2 | Vitamin | 2.77 mg/100 g | Precursor of FMN and FAD | [71] |
| Niacin (B3) | Vitamin | 42.42 mg/100 g | Increase HDL cholesterol levels | [71] |
| Vitamin B5 (Pantothenic acid) | Vitamin | 52.80 mg/100 g | Constituent of coenzyme A, fatty acid metabolism | [71,73] |
| Vitamin B6 | Vitamin | 11.90 mg/100 g | Transamination and decarboxylation of amino acids | [71] |
| Vitamin B9 (Folic acid) | Vitamin | 0.40 mg/100 g | DNA biosynthesis and methylation | [74] |
| Vitamin B12 | Vitamin | 0.15 mg/100 g | Formation of red blood cells and maintenance of the central nervous system. | [71] |
| Vitamin C (Ascorbic acid) | Vitamin | 2.00 mg/100 g | Antioxidant | [71] |
| Vitamin D | Vitamin | 0.2 mg/100 g | Calcium absorption | [74] |
| Vitamin E | Vitamin | 5.00 mg/100 g | Antioxidant activity | [71] |
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida albicans | 1450 | 1250 | 700 | 1320 | 2.5 | 2.5–10 | 1100 | 0.125–1 | 1340 | 1250 | 200 | 1100 | |||
| Candida glabatra | 1170 | 1170 | 350 | 850 | 800 | 980 | 150 | 1250 | |||||||
| Candida tropicalis | 1120 | 900 | 490 | 900 | 970 | 950 | 180 | 950 | |||||||
| Paenibacillus larvae | 0.6–2.8 | 40 | 6 | ||||||||||||
| Vibrio parahaemolyticus | 4 | ||||||||||||||
| Enterobacter cloacae | 1630 | 770 | 570 | 1500 | 10 | 15 | 1500 | 1330 | 1200 | 1100 | 900 | ||||
| Escherichia coli | 1150 | 400 | 1100 | 1180 | 2.5 | 10–15 | 15 | 1100 | 10 | 980 | 950 | 1450 | 1500 | ||
| Lactobacillus acidophilus and Lactobacillus helveticus | 10 | ||||||||||||||
| Pseudomonas aeruginosa | 1120 | 650 | 640 | 1180 | 10 | 15–80 | 30 | 750 | 980 | 940 | 10 | 900 | 670 | ||
| Staphylococcus aureus | 670 | 550 | 350 | 350 | 10 | 15–200 | 30 | 450 | 700 | 670 | 7–250 | 950 | 990 | ||
| Staphylococcus epidermidis | 990 | 400 | 250 | 400 | 200 | 780 | 720 | 740 | 880 | 950 | |||||
| Staphylococcus intermedius and Staphylococcus xylosus | 4–12 | ||||||||||||||
| Staphylococcus saprophyticus | 15 | 30 | |||||||||||||
| Streptococcus mutans | 1370 | 780 | 350 | 670 | 980 | 800 | 720 | 1140 | 780 | ||||||
| Streptococcus viridans | 900 | 840 | 180 | 780 | 800 | 700 | 580 | 950 | 740 | ||||||
| Streptococcus alactolyticus | 9 | ||||||||||||||
| Bacillus subtilis | 10 | 30–40 | 60 | ||||||||||||
| Klebsiella pneumoniae | 1250 | 1450 | 470 | 10 | 15 | 900 | 1260 | 980 | 1350 | 1640 | |||||
| Listeria monocytogenes | 200 | ||||||||||||||
| Salmonella enterica Paratyphi | 200 | ||||||||||||||
| Salmonella cholearasuis | 9 | ||||||||||||||
| Salmonella (infantis, typhi-murium) | 10 | ||||||||||||||
| Clostridium tetani | 250 | ||||||||||||||
| Micrococcus luteus (Sarcina lutea) | 125 | ||||||||||||||
| Bifidobacterium (adolescentis, bifidum, breve, infantis, lon-gum) | 10 |
| Applications | Administration of RJ/Dose in Humans | Health Benefits | Ref. |
|---|---|---|---|
| NUTRACEUTICAL INDUSTRY | |||
| Functional foods | |||
| Skimmed milk fortified with RJ (Probiotics) | Lactobacillus acidophilus fermented milk with RJ | Increase in antioxidant activities. Accumulation of bioactive peptides with antihypertensive effect (inhibitory activity of angiotensin 1-converting enzyme). | [174] |
| Milk supplemented with RJ | Inhibits some Gram-positive and Gram-negative bacteria growth and mesophilic and thermophilic dairy starters. Prevention of the conversion of milk into fermented products | [175] | |
| Supplements/industry formulations | |||
| Capsules (1 g RJ/capsule) | Daily intake of oral 1 capsule for 8 weeks | Effectiveness in alleviating the menopausal symptoms. | [176] |
| Capsules (0.35 g RJ/capsule) | Consumption of 9 capsules per day for 3 months | Significantly reduction in the serum total cholesterol and low-density lipoprotein cholesterol levels. | [119] |
| Memo® Capsules (0.75 g of lyophilized RJ/capsule) | One Memo® capsule before breakfast daily for 4 weeks. | Cognitive impairment treatment representative of the early phases of Alzheimer’s disease (more studies needed). | [177] |
| Capsules (1 g RJ/capsule) | Two months intake of one RJ capsule daily | Effectiveness in reducing the premenstrual syndrome | [178] |
| Capsules (quantity not found). | Two capsules of RJ | Nephroprotective effect against cisplatin toxicity in cancer assisted for cisplatin chemotherapy. | [179] |
| Tablets (0.12 g RJ/tablet) | Ingestion of 6 tables daily for 8 weeks | Increased tear volume in patients with dry eye | [180] |
| Liquid formulation | Six-month intake of RJ (3 g of RJ in 100 mL liquid/day) | Improvement of erythropoiesis, glucose tolerance and mental health. | [181] |
| Positive results on sperms and its mobility in infertility treatments | [182] | ||
| COSMETIC INDUSTRY | |||
| Cream | RJ vaginal cream 15% | Estrogen-like effects | [183] |
| Cream | Cream with 10-Hydroxy-2-decenoic fatty acid exclusive of RJ. | Possible treatment in the dysfunction of the skin barrier | [184] |
| Cream | Vaginal cream of RJ 15% for 3 months | Estrogenic properties to treat sexual and urinary problems in women | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. https://doi.org/10.3390/nu13020543
Collazo N, Carpena M, Nuñez-Estevez B, Otero P, Simal-Gandara J, Prieto MA. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients. 2021; 13(2):543. https://doi.org/10.3390/nu13020543
Chicago/Turabian StyleCollazo, Nicolas, Maria Carpena, Bernabe Nuñez-Estevez, Paz Otero, Jesus Simal-Gandara, and Miguel A. Prieto. 2021. "Health Promoting Properties of Bee Royal Jelly: Food of the Queens" Nutrients 13, no. 2: 543. https://doi.org/10.3390/nu13020543
APA StyleCollazo, N., Carpena, M., Nuñez-Estevez, B., Otero, P., Simal-Gandara, J., & Prieto, M. A. (2021). Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients, 13(2), 543. https://doi.org/10.3390/nu13020543