‘An Apple a Day’?: Psychiatrists, Psychologists and Psychotherapists Report Poor Literacy for Nutritional Medicine: International Survey Spanning 52 Countries
Abstract
1. Introduction
2. Materials and Methods
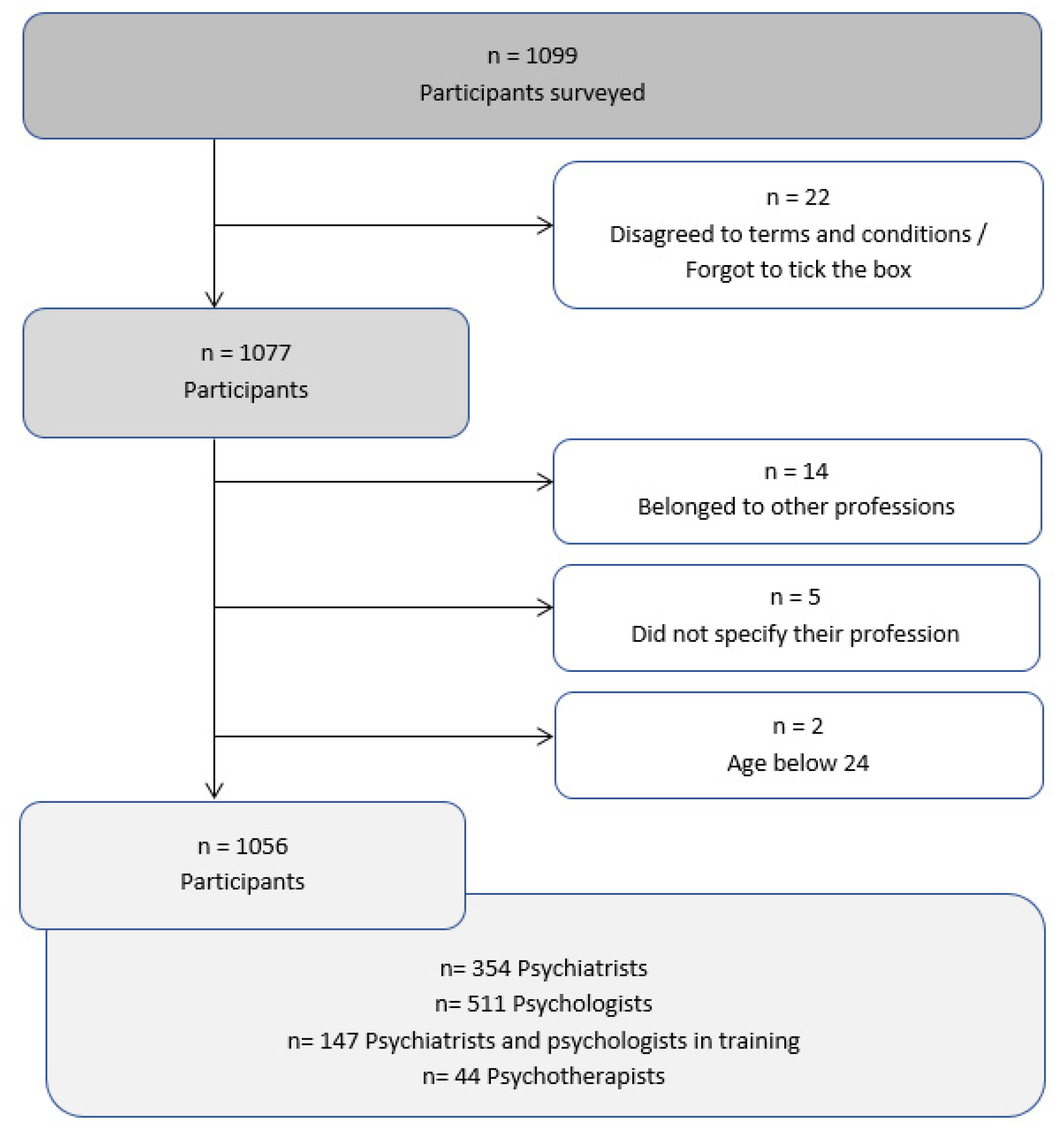
2.1. Recruitment of Participants and Group Characteristics
2.2. The Online Survey
2.3. Statistical Evaluation and Data Management
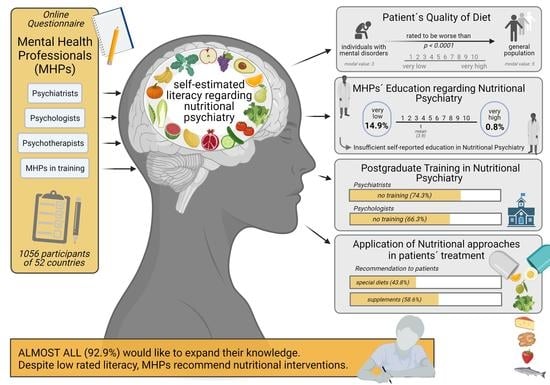
3. Results
3.1. Demographical Data of Study Participants
3.2. Nutritional Education
3.3. Treatment Practices
3.4. Recommendation of Diets and Dietary Supplements by MHPs
4. Discussion
4.1. Education
4.2. Treatment Practices
4.3. Recommended Diets and Supplements by MHPs
4.4. Strengths and Limitations
4.5. Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 10. [Google Scholar] [CrossRef]
- Chang, C.K.; Hayes, R.D.; Perera, G.; Broadbent, M.T.; Fernandes, A.C.; Lee, W.E.; Hotopf, M.; Stewart, R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE 2011, 6, e19590. [Google Scholar] [CrossRef]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Hancock, K.J.; Kisely, S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: Retrospective analysis of population based registers. BMJ 2013, 346, f2539. [Google Scholar] [CrossRef]
- Vancampfort, D.; Stubbs, B.; Mitchell, A.J.; De Hert, M.; Wampers, M.; Ward, P.B.; Rosenbaum, S.; Correll, C.U. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psychiatry 2015, 14, 339–347. [Google Scholar] [CrossRef]
- Bhering Martins, L.; Braga Tibães, J.R.; Sanches, M.; Jacka, F.; Berk, M.; Teixeira, A.L. Nutrition-based interventions for mood disorders. Expert Rev. Neurother. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Keller, C.; Li, L. Neuropeptides in gut-brain axis and their influence on host immunity and stress. Comput. Struct. Biotechnol. J. 2020, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Glabska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.T.; Albert, S.M.; Dew, M.A.; Lockovich, M.H.; Reynolds, C.F., 3rd. Coaching in healthy dietary practices in at-risk older adults: A case of indicated depression prevention. Am. J. Psychiatry 2014, 171, 499–505. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Hausteiner, C.; Bornschein, S.; Zilker, T.; Forstl, H.; Grassmann, J. The influence of diet on mental health. Nervenarzt 2007, 78, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Ward, P.B.; Samaras, K.; Firth, J.; Stubbs, B.; Tripodi, E.; Burrows, T.L. Dietary intake of people with severe mental illness: Systematic review and meta-analysis. Br. J. Psychiatry 2019, 214, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Happell, B. The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues Ment Health Nurs. 2011, 32, 589–597. [Google Scholar] [CrossRef]
- Berk, M.; Sarris, J.; Coulson, C.E.; Jacka, F.N. Lifestyle management of unipolar depression. Acta Psychiatry Scand. 2013, 127, 38–54. [Google Scholar] [CrossRef]
- Chung, M.; van Buul, V.J.; Wilms, E.; Nellessen, N.; Brouns, F.J. Nutrition education in European medical schools: Results of an international survey. Eur. J. Clin. Nutr. 2014, 68, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.M.; Lindell, K.C.; Kohlmeier, M.; Zeisel, S.H. Status of nutrition education in medical schools. Am. J. Clin. Nutr. 2006, 83, 941S–944S. [Google Scholar] [CrossRef]
- Crowley, J.; Ball, L.; Hiddink, G.J. Nutrition in medical education: A systematic review. Lancet Planet. Health 2019, 3, e379–e389. [Google Scholar] [CrossRef]
- Terry, N.; Reeves, A. How do counsellors and psychotherapists understand diet and nutrition as part of the therapy process? A heuristic study. Couns. Psychother. Res. 2015, 15, 309–319. [Google Scholar] [CrossRef]
- Burks, R.J.; Keeley, S.M. Exercise and diet therapy: Psychotherapists’ beliefs and practices. Prof. Psychol. Res. Pract. 1989, 20, 62–64. [Google Scholar] [CrossRef]
- Wittink, D.R.; Bayer, L.R. The measurement imperative. Mark. Res. 2003, 15, 19. [Google Scholar]
- Hewlett, E.; Moran, V. Making Mental Health Count: The Social and Economic Costs of Neglecting Mental Health Care; OECD Health Policy Studies, OECD Publishing: Paris, France, 2014. [Google Scholar] [CrossRef]
- Laster, J.; Frame, L.A. Beyond the Calories-Is the Problem in the Processing? Curr. Treat. Opt. Gastroenterol. 2019, 17, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Chronobiology and nutrition. Neuroscience 2013, 253, 78–88. [Google Scholar] [CrossRef]
- Kushi, L.H.; Cunningham, J.E.; Hebert, J.R.; Lerman, R.H.; Bandera, E.V.; Teas, J. The macrobiotic diet in cancer. J. Nutr. 2001, 131, 3056S–3064S. [Google Scholar] [CrossRef]
- Witasek, A. Diagnosis and therapy after Dr. F. X. Mayr. Forsch. Komplement. 1999, 6, 45–46. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Meffert, C.; Gerdes, N. Program adherence and effectiveness of a commercial nutrition program: The metabolic balance study. J. Nutr. Metab. 2010, 2010, 197656. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Russell, J.L. Tcm Foods, Cooking with the Five Elements: A Reference Guide; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2015. [Google Scholar]
- Cavelius, A.; Pape, D.; Ilies, A.; Gillessen, H.; Schwarz, R.; Trunz-Carlisi, E. Schlank im Schlaf: Das Basisbuch. Die Revolutionäre Formel: So Nutzen Sie Ihre Bio-Uhr zum Abnehmen; Gräfe und Unzer Verlag GmbH: Munich, Germany, 2014. [Google Scholar]
- Zhao, X.; Tan, X.; Shi, H.; Xia, D. Nutrition and traditional Chinese medicine (TCM): A system’s theoretical perspective. Eur. J. Clin. Nutr. 2021, 75, 267–273. [Google Scholar] [CrossRef]
- Javelle, F.; Lampit, A.; Bloch, W.; Haussermann, P.; Johnson, S.L.; Zimmer, P. Effects of 5-hydroxytryptophan on distinct types of depression: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 77–88. [Google Scholar] [CrossRef]
- Chen, T.S.; Liou, S.Y.; Chang, Y.L. Antioxidant evaluation of three adaptogen extracts. Am. J. Chin. Med. 2008, 36, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Fuladi, S.; Emami, S.A.; Mohammadpour, A.H.; Karimani, A.; Manteghi, A.A.; Sahebkar, A. Assessment of Withania somnifera root extract efficacy in patients with generalized anxiety disorder: A randomized double-blind placebo-controlled trial. Curr. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010. [Google Scholar] [CrossRef]
- Chang, S.M.; Chen, C.H. Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: A randomized controlled trial. J. Adv. Nurs. 2016, 72, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef]
- Ulbricht, C.; Chao, W.; Nummy, K.; Rusie, E.; Tanguay-Colucci, S.; Iannuzzi, C.M.; Plammoottil, J.B.; Varghese, M.; Weissner, W. Chia (Salvia hispanica): A systematic review by the natural standard research collaboration. Rev. Recent Clin. Trials 2009, 4, 168–174. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Dietary reference values for choline. EFSA J. 2016, 14, e04484. [Google Scholar] [CrossRef]
- Davidson, J.R.; Abraham, K.; Connor, K.M.; McLeod, M.N. Effectiveness of chromium in atypical depression: A placebo-controlled trial. Biol. Psychiatry 2003, 53, 261–264. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Yasrebifar, F.; Haghighi, M.; Mohammadi, Y.; Jahangard, L. Evaluating the Effect of Coenzyme Q10 Augmentation on Treatment of Bipolar Depression: A Double-Blind Controlled Clinical Trial. J. Clin. Psychopharmacol. 2018, 38, 460–466. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Hu, Z.; Oh, S.; Ha, T.W.; Hong, J.T.; Oh, K.W. Sleep-Aids Derived from Natural Products. Biomol. Ther. 2018, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Pfaffenrath, V.; Schnitker, J.; Friede, M.; Henneicke-von Zepelin, H.H. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention—A randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia Int. J. Headache 2005, 25, 1031–1041. [Google Scholar] [CrossRef]
- Zhao, M.X.; Dong, Z.H.; Yu, Z.H.; Xiao, S.Y.; Li, Y.M. Effects of Ginkgo biloba extract in improving episodic memory of patients with mild cognitive impairment: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2012, 10, 628–634. [Google Scholar] [CrossRef]
- Parsons, C.G.; Danysz, W.; Hesselink, M.; Hartmann, S.; Lorenz, B.; Wollenburg, C.; Quack, G. Modulation of NMDA receptors by glycine--introduction to some basic aspects and recent developments. Amino Acids 1998, 14, 207–216. [Google Scholar] [CrossRef]
- Shimbo, M.; Nakamura, K.; Jing Shi, H.; Kizuki, M.; Seino, K.; Inose, T.; Takano, T. Green tea consumption in everyday life and mental health. Public Health Nutr. 2005, 8, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, D.O.; Zhang, L. Mechanisms Underlying the Anti-Depressive Effects of Regular Tea Consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Lojewski, M.; Rojowski, J.; Opoka, W.; Sulkowska-Ziaja, K. Natural products of relevance in the prevention and supportive treatment of depression. Psychiatria Polska 2015, 49, 435–453. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Tian, J.; Liu, J.P. Huperzine A for Alzheimer’s disease: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2013, 8, e74916. [Google Scholar] [CrossRef]
- Wang, R.; Yan, H.; Tang, X.C. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharm. Sin. 2006, 27, 1–26. [Google Scholar] [CrossRef]
- Benjamin, J.; Levine, J.; Fux, M.; Aviv, A.; Levy, D.; Belmaker, R.H. Double-blind, placebo-controlled, crossover trial of inositol treatment for panic disorder. Am. J. Psychiatry 1995, 152, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Fux, M.; Levine, J.; Aviv, A.; Belmaker, R.H. Inositol treatment of obsessive-compulsive disorder. Am. J. Psychiatry 1996, 153, 1219–1221. [Google Scholar] [CrossRef]
- Penetar, D.M.; Toto, L.H.; Lee, D.Y.; Lukas, S.E. A single dose of kudzu extract reduces alcohol consumption in a binge drinking paradigm. Drug Alcohol Depend. 2015, 153, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, A.H.; Overstreet, D.H.; Perfumi, M.; Massi, M. Plant derivatives in the treatment of alcohol dependency. Pharm. Biochem. Behav. 2003, 75, 593–606. [Google Scholar] [CrossRef]
- Hess, S.; Baker, G.; Gyenes, G.; Tsuyuki, R.; Newman, S.; Le Melledo, J.M. Decreased serum L-arginine and L-citrulline levels in major depression. Psychopharmacology 2017, 234, 3241–3247. [Google Scholar] [CrossRef]
- Yi, J.; Horky, L.L.; Friedlich, A.L.; Shi, Y.; Rogers, J.T.; Huang, X. L-arginine and Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2009, 2, 211–238. [Google Scholar] [PubMed]
- Lillehei, A.S.; Halcon, L.L.; Savik, K.; Reis, R. Effect of Inhaled Lavender and Sleep Hygiene on Self-Reported Sleep Issues: A Randomized Controlled Trial. J. Altern. Complement. Med. 2015, 21, 430–438. [Google Scholar] [CrossRef]
- Kasper, S.; Gastpar, M.; Muller, W.E.; Volz, H.P.; Moller, H.J.; Dienel, A.; Schlafke, S. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: A randomized, double-blind, placebo controlled trial. Int. Clin. Psychopharmacol. 2010, 25, 277–287. [Google Scholar] [CrossRef]
- Higgins, J.P.; Flicker, L. Lecithin for dementia and cognitive impairment. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Wass, C.; Klamer, D.; Katsarogiannis, E.; Palsson, E.; Svensson, L.; Fejgin, K.; Bogren, I.B.; Engel, J.A.; Rembeck, B. L-lysine as adjunctive treatment in patients with schizophrenia: A single-blinded, randomized, cross-over pilot study. BMC Med. 2011, 9, 40. [Google Scholar] [CrossRef]
- Smriga, M.; Ghosh, S.; Mouneimne, Y.; Pellett, P.L.; Scrimshaw, N.S. Lysine fortification reduces anxiety and lessens stress in family members in economically weak communities in Northwest Syria. Proc. Natl. Acad. Sci. USA 2004, 101, 8285–8288. [Google Scholar] [CrossRef]
- Zheng, W.; Li, W.; Qi, H.; Xiao, L.; Sim, K.; Ungvari, G.S.; Lu, X.B.; Huang, X.; Ning, Y.P.; Xiang, Y.T. Adjunctive folate for major mental disorders: A systematic review. J. Affect. Disord. 2020, 267, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Matsunaga, S.; Nomura, I.; Okuya, M.; Kishi, T.; Iwata, N. Folic acid/methylfolate for the treatment of psychopathology in schizophrenia: A systematic review and meta-analysis. Psychopharmacology 2018, 235, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Sugino, T.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr. Res. 2008, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.; Turner, J.; Del Mar, C. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst. Rev. 2002. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Tovar, M.; Estrada-Reyes, R.; Solis-Chagoyan, H.; Argueta, J.; Dorantes-Barron, A.M.; Quero-Chavez, D.; Cruz-Garduno, R.; Cercos, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian modulation of neuroplasticity by melatonin: A target in the treatment of depression. Br. J. Pharmacol. 2018, 175, 3200–3208. [Google Scholar] [CrossRef]
- Hansen, M.V.; Halladin, N.L.; Rosenberg, J.; Gogenur, I.; Moller, A.M. Melatonin for pre- and postoperative anxiety in adults. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Lin, A.; Nguy, C.H.; Shic, F.; Ross, B.D. Accumulation of methylsulfonylmethane in the human brain: Identification by multinuclear magnetic resonance spectroscopy. Toxicol. Lett. 2001, 123, 169–177. [Google Scholar] [CrossRef]
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev. A J. Clin. Ther. 2002, 7, 22–44. [Google Scholar]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. PTR 2006, 20, 619–633. [Google Scholar] [CrossRef]
- da Fonseca, L.R.; Rodrigues, R.A.; Ramos, A.S.; da Cruz, J.D.; Ferreira, J.L.P.; Silva, J.R.A.; Amaral, A.C.F. Herbal Medicinal Products from Passiflora for Anxiety: An Unexploited Potential. Sci. World J. 2020, 2020, 6598434. [Google Scholar] [CrossRef] [PubMed]
- Modica-Napolitano, J.S.; Renshaw, P.F. Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: Implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol. Psychiatry 2004, 55, 273–277. [Google Scholar] [CrossRef]
- Silvan, J.M.; Michalska-Ciechanowska, A.; Martinez-Rodriguez, A.J. Modulation of Antibacterial, Antioxidant, and Anti-Inflammatory Properties by Drying of Prunus domestica L. Plum Juice Extracts. Microorganisms 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Travica, N.; D’Cunha, N.M.; Naumovski, N.; Kent, K.; Mellor, D.D.; Firth, J.; Georgousopoulou, E.N.; Dean, O.M.; Loughman, A.; Jacka, F.; et al. The effect of blueberry interventions on cognitive performance and mood: A systematic review of randomized controlled trials. Brain Behav. Immun. 2020, 85, 96–105. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. (Bethesda Md.) 2020, 11, 602–615. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Barbosa, R.S.D.; Vieira-Coelho, M.A. Probiotics and prebiotics: Focus on psychiatric disorders—A systematic review. Nutr. Rev. 2020, 78, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Rubin, L.H.; Fornelli, D.; Drogos, L.; Banuvar, S.; Shulman, L.P.; Geller, S.E. Effects of botanicals and combined hormone therapy on cognition in postmenopausal women. Menopause 2009, 16, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.K.; Perry, R.; Ernst, E. The effectiveness and efficacy of Rhodiola rosea L.: A systematic review of randomized clinical trials. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 235–244. [Google Scholar] [CrossRef]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst. Rev. 2016, 10, CD011286. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Rocks, T.; Ruusunen, A.; Loughman, A.; Lopresti, A.; Marshall, S.; Berk, M.; Jacka, F.; Dean, O.M. Effect of saffron supplementation on symptoms of depression and anxiety: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Hood, S.D.; Drummond, P.D. Efficacy of a standardised saffron extract (affron(R)) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: A randomised, double-blind, placebo-controlled study. J. Psychopharmacol. (Oxf. Engl.) 2019, 33, 1415–1427. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.B.; Lutjohann, D.; Mulder, M.; Vanmierlo, T. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef]
- Ayati, Z.; Sarris, J.; Chang, D.; Emami, S.A.; Rahimi, R. Herbal medicines and phytochemicals for obsessive-compulsive disorder. Phytother. Res. 2020, 34, 1889–1901. [Google Scholar] [CrossRef]
- Cui, C.; Birru, R.L.; Snitz, B.E.; Ihara, M.; Kakuta, C.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; Mathis, C.A.; Miyamoto, Y.; et al. Effects of soy isoflavones on cognitive function: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 134–144. [Google Scholar] [CrossRef]
- Haller, H.; Anheyer, D.; Cramer, H.; Dobos, G. Complementary therapies for clinical depression: An overview of systematic reviews. BMJ Open 2019, 9, e028527. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Savage, K.; Firth, J.; Stough, C.; Sarris, J. GABA-modulating phytomedicines for anxiety: A systematic review of preclinical and clinical evidence. Phytother. Res. 2018, 32, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kataria, A.; Kolla, B.P.; Thusius, N.; Loukianova, L.L. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin. Proc. 2019, 94, 1065–1072. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef]
- Plevin, D.; Galletly, C. The neuropsychiatric effects of vitamin C deficiency: A systematic review. BMC Psychiatry 2020, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.H. Bone health in patients with anorexia nervosa. Clin. Calcium 2013, 23, 263–269. [Google Scholar]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed]
- Delavarian, M.; Hassanvand, A.; Gharibzadeh, S. Increasing performance in children with ADHD by trapping lead with a nano-zeolite. J. Neuropsychiatry Clin. Neurosci. 2013, 25, E23. [Google Scholar] [CrossRef]
- Royak-Schaler, R.; Feldman, R.H. Health behaviors of psychotherapists. J. Clin. Psychol. 1984, 40, 705–710. [Google Scholar] [CrossRef]
- Ryan, V.C.; Rao, L.O.; Rekers, G. Nutritional practices, knowledge, and attitudes of psychiatric healthcare professionals: Unexpected results. Psychiatr. Hosp. 1990, 21, 125–127. [Google Scholar] [PubMed]
- Hark, L.A.; Deen, D. Position of the Academy of Nutrition and Dietetics: Interprofessional Education in Nutrition as an Essential Component of Medical Education. J. Acad. Nutr. Diet. 2017, 117, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.; Wynaden, D.; Heslop, K. Who is responsible for metabolic screening for mental health clients taking antipsychotic medications? Int. J. Ment. Health Nurs. 2018, 27, 196–203. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Liew, A.Y.; Yusof, M.S.D.; Duffy, R.; Breen, E.G.; Kinsley, B.; Kelly, B.D. Screening for metabolic syndrome in long-term psychiatric illness: Audit of patients receiving depot antipsychotic medication at a psychiatry clinic. Eur. J. Psychiatry 2011, 25, 213–222. [Google Scholar] [CrossRef]
- World Health Organization. Management of Physical Health Conditions in Adults with Severe Mental Disorders: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Sanchez-Villegas, A.; Delgado-Rodriguez, M.; Alonso, A.; Schlatter, J.; Lahortiga, F.; Serra Majem, L.; Martinez-Gonzalez, M.A. Association of the Mediterranean dietary pattern with the incidence of depression: The Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch. Gen. Psychiatry 2009, 66, 1090–1098. [Google Scholar] [CrossRef]
- Vicinanza, R.; Bersani, F.S.; D’Ottavio, E.; Murphy, M.; Bernardini, S.; Crisciotti, F.; Frizza, A.; Mazza, V.; Biondi, M.; Troisi, G.; et al. Adherence to Mediterranean diet moderates the association between multimorbidity and depressive symptoms in older adults. Arch. Gerontol. Geriatr. 2020, 88, 104022. [Google Scholar] [CrossRef]
- Mantzorou, M.; Vadikolias, K.; Pavlidou, E.; Tryfonos, C.; Vasios, G.; Serdari, A.; Giaginis, C. Mediterranean diet adherence is associated with better cognitive status and less depressive symptoms in a Greek elderly population. Aging Clin. Exp. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Bandinelli, S.; Penninx, B.W.; Vogelzangs, N.; Corsi, A.M.; Lauretani, F.; Kisialiou, A.; Vazzana, R.; Terracciano, A.; Guralnik, J.M.; et al. Depressive symptoms and inflammation increase in a prospective study of older adults: A protective effect of a healthy (Mediterranean-style) diet. Mol. Psychiatry 2011, 16, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Sanchez-Villegas, A.; Martinez-Gonzalez, M.A.; Estruch, R.; Salas-Salvado, J.; Corella, D.; Covas, M.I.; Aros, F.; Romaguera, D.; Gomez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Stough, C.; Bousman, C.; Mischoulon, D.; Murphy, J.; Macdonald, P.; Adams, L.; Nazareth, S.; Oliver, G. Nutraceuticals for major depressive disorder-more is not merrier: An 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 2019, 245, 1007–1015. [Google Scholar] [CrossRef]
- Firth, J.; Teasdale, S.B.; Allott, K.; Siskind, D.; Marx, W.; Cotter, J.; Veronese, N.; Schuch, F.; Smith, L.; Solmi, M.; et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019, 18, 308–324. [Google Scholar] [CrossRef]
- Mörkl, S.; Wagner-Skacel, J.; Lahousen, T.; Lackner, S.; Holasek, S.J.; Bengesser, S.A.; Painold, A.; Holl, A.K.; Reininghaus, E. The role of nutrition and the gut-brain axis in psychiatry: A review of the literature. Neuropsychobiology 2020, 79, 80–88. [Google Scholar] [CrossRef]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schefft, C.; Kilarski, L.L.; Bschor, T.; Kohler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R.K.; Hamazaki, K.; Freeman, M.P.; Maes, M.; Matsuoka, Y.J.; Belmaker, R.H.; et al. A multi-national, multi-disciplinary Delphi consensus study on using omega-3 polyunsaturated fatty acids (n-3 PUFAs) for the treatment of major depressive disorder. J. Affect. Disord. 2020, 265, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Glenn, T.; Conell, J.; Rasgon, N.; Marsh, W.; Sagduyu, K.; Munoz, R.; Lewitzka, U.; Bauer, R.; Pilhatsch, M.; et al. Common use of dietary supplements for bipolar disorder: A naturalistic, self-reported study. Int. J. Bipolar Disord. 2015, 3, 29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donohue, J.M.; Pincus, H.A. Reducing the societal burden of depression: A review of economic costs, quality of care and effects of treatment. Pharm. Econ. 2007, 25, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Niv, N.; Shatkin, J.P.; Hamilton, A.B.; Unutzer, J.; Klap, R.; Young, A.S. The use of herbal medications and dietary supplements by people with mental illness. Comm. Ment. Health J. 2010, 46, 563–569. [Google Scholar] [CrossRef]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Antidepressant adherence: Are patients taking their medications? Innov. Clin. Neurosci. 2012, 9, 41. [Google Scholar]
- Klesse, C.; Berger, M.; Bermejo, I.; Bschor, T.; Gensichen, J.; Harfst, T.; Hautzinger, M.; Kolada, C.; Kühner, C.; Matzat, J.; et al. Evidenzbasierte Psychotherapie der Depression. Psychotherapeut 2010, 55, 247–263. [Google Scholar] [CrossRef]
- Kessler, R.C.; Soukup, J.; Davis, R.B.; Foster, D.F.; Wilkey, S.A.; Van Rompay, M.I.; Eisenberg, D.M. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am. J. Psychiatry 2001, 158, 289–294. [Google Scholar] [CrossRef]
- Quirk, S.E.; Williams, L.J.; O’Neil, A.; Pasco, J.A.; Jacka, F.N.; Housden, S.; Berk, M.; Brennan, S.L. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry 2013, 13, 175. [Google Scholar] [CrossRef]
- Murakami, K.; Mizoue, T.; Sasaki, S.; Ohta, M.; Sato, M.; Matsushita, Y.; Mishima, N. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition 2008, 24, 140–147. [Google Scholar] [CrossRef]
- Fava, M.; Mischoulon, D. Folate in depression: Efficacy, safety, differences in formulations, and clinical issues. J. Clin. Psychiatry 2009, 70 (Suppl. S5), 12–17. [Google Scholar] [CrossRef]
- Byerley, W.F.; Judd, L.L.; Reimherr, F.W.; Grosser, B.I. 5-Hydroxytryptophan: A review of its antidepressant efficacy and adverse effects. J. Clin. Psychopharmacol. 1987, 7, 127–137. [Google Scholar] [CrossRef]
- Chavez, M.L.; Jordan, M.A.; Chavez, P.I. Evidence-based drug-herbal interactions. Life Sci. 2006, 78, 2146–2157. [Google Scholar] [CrossRef]
- Tang, S.W.; Tang, W.; Leonard, B.E. Patients on psychotropic medications and herbal supplement combinations: Clinical considerations. Int. Clin. Psychopharmacol. 2017, 32, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Griffith, K.A.; DeCastro, R.A.; Ubel, P. Sex, role models, and specialty choices among graduates of US medical schools in 2006-2008. J. Am. Coll. Surg. 2014, 218, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Cope, C.; Michalski, D.S.; Fowler, G.A. Summary Report: Student Demographics. Graduate Study in Psychology 2017; American Psychological Association: Washington, DC, USA, 2016. [Google Scholar]
- Fagerli, R.A.; Wandel, M. Gender differences in opinions and practices with regard to a “healthy diet”. Appetite 1999, 32, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Minogue, V.; Lucock, M. Nutrition and mental health recovery. Ment. Health Learn. Disabil. Res. Pract. 2010, 7, 43. [Google Scholar] [CrossRef]
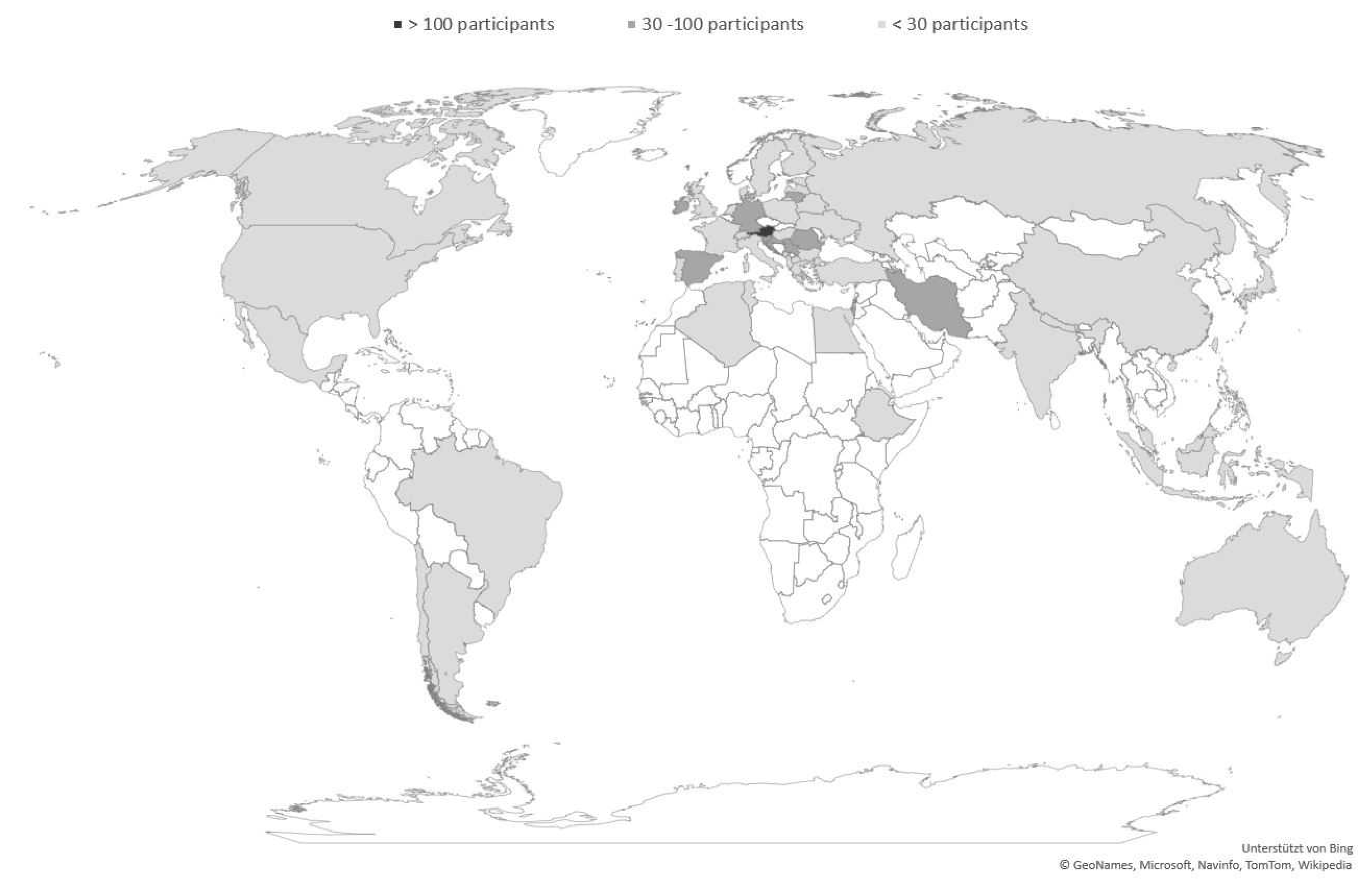
| Income Group (June 2020) | Country | Continent | Participants (per Country) | Psychiatrists Working in Mental Health Sector (per 100,000 Population) | Psychologists Working in Mental Health Sector (per 100,000 Population) |
|---|---|---|---|---|---|
| High income | Australia | Oceania | 19 | 13.5 | 103.0 |
| Austria | Europe | 481 | 20.7 * | 0.2 ** | |
| Canada | North America | 4 | 14.7 | 48.7 | |
| Chile | South America | 1 | 7.00 | NA | |
| Croatia | Europe | 32 | 11.1 | 4.4 | |
| Denmark | Europe | 2 | 17 † | NA | |
| Estonia | Europe | 11 | 16.2 | 6.5 | |
| Finland | Europe | 2 | 23.6 | 109.5 | |
| France | Europe | 4 | 20.9 | 48.7 | |
| Germany | Europe | 40 | 13.2 | 49.6 | |
| Greece | Europe | 2 | 5.8 | 8.8 | |
| Hungary | Europe | 1 | 11.1 | 2.5 | |
| Ireland | Europe | 33 | 19.0 *** | 6.0 † | |
| Israel | Asia | 52 | 9.9 | 88.1 | |
| Italy | Europe | 8 | 6.0 | 3.8 | |
| Japan | Asia | 4 | 11.9 | 3.0 | |
| Latvia | Europe | 1 | 1.0 | NA | |
| Lithuania | Europe | 30 | 18.5 | 15.9 | |
| Malta | Europe | 1 | NA | NA | |
| Netherlands | Europe | 5 | 20.9 | 123.5 | |
| Poland | Europe | 3 | 24.2 | 16.4 | |
| Portugal | Europe | 2 | 11† | NA | |
| Romania | Europe | 54 | 5.7 | 1.5 | |
| Slovenia | Europe | 31 | 12.0 | 9.3 | |
| Spain | Europe | 32 | 9.7 | NA | |
| Sweden | Europe | 3 | 22† | 66 † | |
| Switzerland | Europe | 19 | 44.0 | 84.1 | |
| Taiwan | Asia | 1 | NA | NA | |
| UK | Europe | 15 | 20† | 16 † | |
| USA | North America | 12 | 10.54 | 29.9 | |
| Upper-middle income | Albania | Europe | 1 | 1.5 | 1.2 |
| Argentina | South America | 2 | 21.7 | 222.6 | |
| Belarus | Europe | 2 | 13.5 | 5.5 | |
| Brazil | South America | 9 | 3.2 | 12.4 | |
| Bulgaria | Europe | 1 | 7.2 | 1.9 | |
| China | Asia | 2 | 2.2 | NA | |
| Indonesia | Asia | 2 | 0.3 | 0.2 | |
| Iran | Asia | 34 | 2.0 | 5.2 | |
| Macedonia | Europe | 2 | 14.4 | 2.4 | |
| Malaysia | Asia | 2 | 1.1 | 1.0 | |
| Mexico | North America | 18 | 0.2 | 3.5 | |
| Montenegro | Europe | 1 | 8.3 | NA | |
| Russia | Asia (Europe) | 7 | 8.5 | 4.6 | |
| Serbia | Europe | 35 | 8.6 | 4.6 | |
| Turkey | Europe | 3 | 1.6 | 2.5 | |
| Lower-middle income | Algeria | Africa | 1 | NA | NA |
| Egypt | Africa | 3 | 1.6 | 0.3 | |
| India | Asia | 3 | 0.3 | 0.1 | |
| Nepal | Asia | 1 | 0.4 | 0.5 | |
| Tunisia | Africa | 3 | NA | 0.01 | |
| Ukraine | Europe | 9 | 6.9 | NA | |
| Low income | Ethiopia | Africa | 1 | NA | NA |
| Psychiatrists | Psychologists | Psychotherapists | Psychiatrists and Psychologists in Training | p-Value | ||
|---|---|---|---|---|---|---|
| (n = 354) | (n = 511) | (n = 44) | (n = 147) | |||
| Sex | n | 193 | 428 | 34 | 102 | <0.001 |
| (female) | % | 54.5 | 84.3 | 77.3 | 69.4 | |
| Age | mean | 40.9 | 42.1 | 40.2 | 30.5 | <0.001 |
| (years) | standard deviation | 11.1 | 10.5 | 11.5 | 4.6 | |
| Working experience | mean | 12.1 | 10.9 | 10.2 | 13.9 | <0.001 |
| (years) | standard deviation | 9.9 | 9.4 | 8.8 | 9.5 |
| Free-Text Answers from Mental Health Professionals Regarding Recommended Diets for Individuals with Psychiatric Disorders | Description |
|---|---|
| “Alkaline diet/ Base-fasting” | Only foods that have a supposed alkaline metabolism are allowed for a preset period of time |
| “Blue Zone diet” | Low meat intake, high fiber, and minimally processed foods, originating from so-called ‘Blue zones’ described as being healthiest communities worldwide [26] |
| “Chrononutrition” | Nutrition considering the circadian system [27] |
| “Clean 9” | Diet using a supplement regime |
| “Dietary advices based on macrobiotic diet” | A basic nutritional scheme which is individualized depending on sex, age, level of activity, indivudal needs, and environment [28] |
| “Elimination reintroduction trials” | Individuals forgo certain foods or ingredients to find out if they have a negative effect on them |
| “F. X. Mayr Kur” | Detoxifying diet originally with bread rolls and milk [29] |
| “fasting” | Willful refrainment from eating |
| “FDH (=“Friss die Hälfte”) diet” | German phrase saying “eat half” (of what you normally would eat) |
| “FODMAP” | Restriction of rapidly fermentable, short-chain carbohydrates for patients with functional gut symptoms [30] |
| “Intermittent fasting” | Alternating time periods of regular food intake and fasting |
| “Metabolic balance diet” | Nutrition program aiming to change lifestyle permanently through individualized nutrition plans taking releant blood paramters into account for laboratory support [31] |
| “MIND” | Mediterranean DASH (Dietary Approaches to Stop Hypertension) Intervention for Neurodegenerative Delay [32] |
| “Nutrition according to the five elements” | Nutrional approach arised from Traditional Chinese Medicine (TCM). Local foods are categorized from an energetic pont of view [33] |
| “Sleep diet by Dr. Pape” | Diet concentrating on eating the right thing at the right time and take longer breaks between meals, in addition to an active everyday life and a lot of sleep [34] |
| “TCM (Traditional Chinese Medicine)” | TCM aims to achieve harmony and balance in ones body through food. It has its own internal logic and concepts [35] |
| “Traffic light system diet” | Foods are labeled in different groups according to their amount of health-relevant nutrients |
| in general | “balanced, diversified” |
| “healthy” | |
| “unprocessed” | |
| “seasonal”, “regional” | |
| “reasonable with regard to carbs” | |
| effects | “deacidifying” |
| “warming dishes” | |
| “sleep promoting” | |
| “acid -base balance” | |
| high/rich in | “whole foods” |
| “(plant based) fresh fruit and vegetables” | |
| “nuts” | |
| “fiber” | |
| “omegas” | |
| “proteins” | |
| “antioxidants” | |
| inclusion of | “fatty fish” |
| “probiotic food such as fermented cabbage” | |
| “serotonin rich food” | |
| free of or reduction of | “gluten” |
| “casein” | |
| “caffeine/soft drings with caffeine” | |
| “wheat” | |
| “dairy” | |
| “allergenic foods” | |
| “meat” | |
| “sugar/sweets” | |
| “calories” | |
| “portion quantity” | |
| “instant meals” | |
| “conservats” | |
| “cholesterol” | |
| “salt” | |
| “fat” | |
| “sodium” | |
| “sodium-glutamate” | |
| “alcoholic foods” |
| Depending on Time | |
|---|---|
| “regular nutritional intake” | |
| “intermittent fasting” | |
| “no stimulating foods in the evening” | |
| “two meals a day” | |
| depending on the process of the intake | |
| “mindful eating” | |
| “awareness of nutrition” | |
| “no eating for stress relief” | |
| adapted to individual requirements | |
| diabetes | “diabetes diet” |
| eating disorders malnourished obesity | “diet plans, main and in-between meals” “fortification” “hypocaloric diet” |
| cachexia | “high calorie intake” |
| hyponatremia | “salty nutrition” |
| cancer | “depending on specific cancer” |
| serotonin side effects | “avoiding foods with tryptophan if suspected serotonin side effects are present” |
| Morbus Wilson | “low copper in nutrition in case of Morbus Wilson” |
| Geriatric patients | “I recommend old people having difficulties with eating or are anxious about it especially eating what gives them pleasure” |
| Monoamidoxidase (MAO-) inhibitors | “diet low in tyramine when thereis intake of MAO-inhibitors” |
| Other answers | |
| Referral to colleagues | “I refer to a Nutritionist” “I refer to Dietologists” “I feel not qualified to give advice” |
| Other lifestyle interventions | “I recommend to stay slim without diet” |
| “I recommend evidence-based interventions” | |
| “not necessarily through diet” “sports” “ban of illegal drugs” | |
| Free-Text Answers from Mental Health Professionals Regarding Recommended Supplements and Foods for Psychiatric Disorders | [Linnean Name], Specifications, and Supposed (Psychiatric) Effects of Supplements * Named by Mental Health Professionals |
|---|---|
| “5-Hydroxytryptophane (5-HTP)” | Naturally occurring amino acid, chemical precursor of serotonin, used as a nonpharmacological treatment for depression [36] |
| “Adaptogenic herbs” | Substances used in traditional and herbal medicine with the aim of stabilization and promoting adaptation to environmental factors [37] |
| “Albumin” | Family of globular proteins, found in blood plasma |
| “Alkalising supplements” | For example, sodium bicarbonate |
| “Aloe vera juice” | Juice of [Aloe barbadensis] |
| “Amino acids (several)/Protein powder” | Precursor to neurotransmitters (tryptophane, tyrosine) |
| “Ashwagandha” | [Withania somnifera], herb with gamma-aminobutyric acid (GABA-)ergic properties used in traditional medicine to reduce stress and enhance wellbeing [38,39] |
| “Astaxanthine” | Carotenoid with antioxidant and anti-inflammatory properties, produced by several freshwater and marine microorganisms, including bacteria, yeast, fungi, and microalgae [40] |
| “Beta-glucan” | Sugars found in cell walls of bacteria, fungi, yeasts, algae, lichens, and plants (barley, oats) [41] |
| “Bitter substances” | Substances found in vegetables and spices (e.g., radicchio, chicory, endive, cardamom, ginger) used to treat digestive issues in traditional medicine systems |
| “Calcium” | Essential element needed in large quantities, acting as an electrolyte with important functions in nerve conduction and building of bone mass |
| “Cannabidiol (CBD)” | Phytocannabinoid, traditionally used for treatment of anxiety, cognition, movement disorders, and pain [42] |
| “Chamomile tea” | Tea made from dried flowers of [Chamaemelum nobile]; traditionally used as a supplementary approach to treat sleep problems and depression [43] |
| “Cherries” | [Prunus cerasus], source of polyphenols and vitamin C with anti-oxidant and anti-inflammatory properties [44,45] |
| “Chia seeds” | Seeds of [Salvia hispanica], novel food, under preliminary research for potential effects on health [46] |
| “Chlorella” | [Chlorella species]; single-celled green algae, consumed as a health supplement primarily in the United States and in Japan |
| “Choline” | Essential nutrient for humans needed for the production of the neurotransmitter acetylcholine; dietary sources of choline and choline phospholipids include egg yolk, wheat germ, and meats, especially organ meats, such as beef liver [47] |
| “Chromium” | Chemical element, used as a dietary supplement, showing decreases of the sensitivity of 5-HT2A receptors [48] |
| “Coenzyme Q10” | Ubichinone, a coenzyme present in all cells, mainly in mitochondria, as an element of the electron transport chain [49] |
| “Copper” | Chemical element, with high serum levels in Wilson’s disease [50] |
| “Epigallocatechin gallate (EGCG)” | Abundant catechin in green tea; polyphenol, found to enhance sleep [51] |
| “Evening primrose oil” | [Oenothera], contains gamma-linolenic acid |
| “Feverfew” | [Tanacetum parthenium], medicinal herb, traditionally used for the prevention of migraine [52] |
| “Ginkgo” | [Ginkgo biloba], traditionally used alone or as an add-on therapy, in the treatment of mild cognitive impairment and dementia [53] |
| “Glycine” | Amino acid, inhibitory neurotransmitter in the central nervous system, required co-agonist along with glutamate for N-methyl-D-aspartate (NMDA) receptors [54] |
| “Green tea” | Infusion prepared from [Camellia sinensis], containing L-theanine, polyphenols, and polyphenol metabolites, used historically in medicine with conflicting results for psychiatric disorders [55,56] |
| “Griffonia” | [Griffonia simplicifolia]; a tropical plant native to West Africa, rich in 5-hydroxy-l-tryptophan (5-HTP), a precursor in the synthesis of serotonin (5-HT), traditionally used for the treatment of depression [57] |
| “Herbal medicines” | Traditional plant-derived medicines, often given in combinations |
| “Herbal mixture (seven herbs from Heidelberg: anise, cumin, fennel, wormwood, yarrow, burnet, and juniper)” | Traditional herbal mixture used in Germany (Heidelberg) for the treatment of digestive issues |
| “Huperzine” | Alkaloid compound found in [Huperzia serrata], a traditional Chinese medicine supplement. Huperzine has strong acetylcholine inhibiting properties and is used as an over the counter supplement for neurological disorders such as Alzheimer’s disease [58,59] |
| “Inositol” | Carbocyclic sugar, abundant in the brain and important for cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors, as well as osmoregulation; some studies investigated inositol for panic disorders and obsessive compulsive disorders [60,61] |
| “Iodine” | Chemical element; used to treat iodine-deficiency or thyreotoxicosis |
| “Kudzu/Japanese arrowroot” | [Pueraria montana] trailing perennial vines native to East Asia; a food supplement traditionally recommended for the treatment of alcohol abuse and dependence [62,63] |
| “L-Arginine” | Amino acid that is used in the biosynthesis of proteins with possible roles in atherosclerosis, redox stress and the inflammatory process, regulation of synaptic plasticity and neurogenesis, and modulation of glucose metabolism and insulin activity [64,65] |
| “L-Aspartate” | Amino acid that is used in the biosynthesis of proteins |
| “Lavender oil” | Oil derived from [Lavandula], herbal oil traditionally used for anxiety and sleep disturbances [66,67] |
| “Lecithin” | Group of yellow-brownish fatty substances occurring in animal and plant tissues, used as a dietary supplement for dementia [68] |
| “Lemon balm” | [Melissa officinalis]; used as a sleep-aid in traditional medicine; anxiolytic effects on mood, cognition, and memory have been shown in clinical trials. AChE inhibitory activity, stimulation of the acetylcholine and GABA-A receptors and matrix metallo proteinase-2 are potential mechanisms of action [69,70] |
| “L-Lysine” | Essential amino-acid in humans; found to reduce positive symptoms in schizophrenia in small pilot trials; reduced anxiety and improved stress response [71,72] |
| “L-Methylfolate” | Primary biologically active form of folate; used as an adjunctive antidepressant in major depressive disorder [73] and as a therapy for negative symptoms in schizophrenia [74] |
| “L-Ornithine” | Non-proteinogenic amino acid, which plays a role in the urea cycle [75] |
| “L-Theanine” | Amino acid analogue of the proteinogenic amino acids L-glutamate and L-glutamine, constituent of green tea; |
| “L-Tryptophan” | Amino acid that is used in the biosynthesis of proteins, which is converted into 5-hydroxytryptophan (5-HTP), which is then converted into serotonin and melatonin; dietary supplement used as an antidepressant, anxiolytic, and sleep aid with limited evidence for depression [76] |
| “Melatonin” | Hormone of the pineal gland, which regulates the sleep–wake cycle with antioxidant properties; improves sleep and has anti-depressant and anti-anxiety effects [77,78] |
| “Methylsulfonylmethane (MSM)” | Organosulfur compound; used in alternative medicine which crosses the blood–brain barrier with no known medical benefits for psychiatric disorders [79,80] |
| “Mineral tablets” | Common constituent of dietary supplements |
| “Mint tea/Peppermint” | Herbal infusion of [Mentha piperita, Mentha spicata]; used in traditional medicine for irritable bowel syndrome (IBS) symptoms; limited human studies, no clinical trials for psychiatric indications [81] |
| “Multivitamins” | A supplement containing a range of vitamins and/or dietary minerals. |
| “Passion flower” | [Passiflora L.], traditionally used as a sedative and anxiolytic [82] |
| “Phosphorylethanolamine” | Ethanolamine derivative; found to have specific effects on mitochondrial function [83] |
| “Plum juice” | [Prunus spec.] traditionally used as a dietary laxative; has anti-oxidant and anti-inflammatory properties [84] |
| “Polyphenols” | Naturally occurring organic compounds characterized by multiples of phenol units, used for improving cognitive performance and symptoms of depression [85,86] |
| “Potassium” | Given as potassium chloride used in the treatment of hypokalemia |
| “Prebiotics (several)” | Non-digestible fiber, promoting growth of microorganisms; did not differ from placebo in trials for depression and anxiety in a recent meta-analysis [87], use of prebiotics still lacks sufficiently robust evidence for psychiatric disorders [88] |
| “Propolis” | Mixture of bees wax and saliva produced by honey bees; used in traditional medicine |
| “Pycnogenol” | Chemical compound found in the bark of European pine trees/[Pinus pinaster]; nutritional supplement used in alternative medicine for the treatment of attention deficit hyperactivity disorder (ADHD); |
| “Red clover” | [Trifolium pratense], a herb containing phytoestrogens, shown to increase cognitive function in postmenopausal women [89] |
| “Rose root” | [Rhodiola rosea], adaptogen traditionally used for the reduction of stress-related syndromes, such as fatigue and burnout [90] |
| “S-Adenosyl-L-Methionine (SAMe)” | Co-substrate involved in methyl group transfers, transsulfuration, and aminopropylation, used as an add-on therapy for depression [91] |
| “Saffron” | [Crocus sativus], used for symptoms of depression and anxiety [92,93] |
| “Salt (Sodiumchloride, NaCl)” | Used as saline solution for a number of indications in clinical medicine |
| “Seaweed oil” | Oil from macroalgae rich in phytosterols, carotenoids, and polysaccharides; extracts used in diet pills to lose weight; compounds cross the blood–brain barrier and exert neuro-protective functions [94,95] |
| “Silibinum” | [Silybum marianum] active compound from the milk thistle; traditionally used for hepatic disorders; plant-based intervention used for obsessive compulsive disorder and anxiety disorder [96] |
| “Sip foods” | Used as additional calorie sources in the treatment of anorexia nervosa |
| “Soy products” | Products made from soybeans [Glycine max]; used for improvement of cognitive function in adults [97] |
| “St. Johns Wort” | [Hypericum perforatum], used for mild to moderate major depression [98] |
| “Tumeric, Curcumin” | [Curcuma longa], spice frequently used in Asian countries with anti-inflammatory and anti-oxidant properties with effects on depressive and anxiety symptoms [99] |
| “Tyrosine” | Amino acid, precursor of dopamine and noradrenaline, thyroid hormones, and melanin |
| “Valerian” | [Valeriana officinalis], GABA-modulating phytochemical traditionally used as an anxiolytic [100] |
| “Vitamin B1” | Thiamine, used for the prevention of Wernicke-Korsakoff-syndrome in alcohol dependency disorders [101] |
| “Vitamin B3” | Vitamin family that includes three forms or vitamers: nicotinamide (niacinamide), niacin (nicotinic acid), and nicotinamide riboside; deficiencies cause pellagra (fatigue, loss of appetite, abdominal pain); co-factor in serotonin-synthesis [102] |
| “Vitamin B-Complex” | Complex of water-soluble B vitamins in food supplements |
| “Vitamin C” | Co-factor in serotonin-synthesis, with deficiencies linked to depression and cognitive impairment [103] |
| “Vitamin K2” | Menachinone, one of three types of vitamin K with protective effects on bone mineral density. May be beneficial for prevention of bone loss in patients with anorexia nervosa [104] |
| “Wild yams” | [Dioscorea villosa]; traditionally used root containing phytoestrogens |
| “Zeolite” | Aluminosilicate minerals used as adsorbents [105,106] |
| Free Text Answers from Psychiatrists |
| “I refer to a dietitian” |
| “I recommend monitoring the blood sugar level” |
| “If a patient had a problem of malabsorption, bad digestion, cancer, operated bypass sleeve, etc., I recommend protein with specific check-ups and specific products and laboratory results (before I worked as a doctor in internal medicine)” |
| “I warn my patients to be cautious for interactions when they are taking supplements together with psychopharmacological medication” |
| Free Text Answers from Psychologists and Psychotherapists |
| “I recommended to see a medical doctor when a patient asked me to recommend a supplement” |
| “I send my clients to see a doctor if they ask me about nutrition” |
| “I ask my patients to go to the doctor and check up and take some proper supplements. Or telling them to have some special fruits and vegetables to get vitamin B, iron, omega 3 etc.” |
| “Any discussion I have is by asking them to go to a general practitioner (GP) to discuss above” |
| “I recommended to see a naturopath” |
| “I recommended the patient to inform them about supplements” |
| “Do not see it as my role as a psychologist” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mörkl, S.; Stell, L.; Buhai, D.V.; Schweinzer, M.; Wagner-Skacel, J.; Vajda, C.; Lackner, S.; Bengesser, S.A.; Lahousen, T.; Painold, A.; et al. ‘An Apple a Day’?: Psychiatrists, Psychologists and Psychotherapists Report Poor Literacy for Nutritional Medicine: International Survey Spanning 52 Countries. Nutrients 2021, 13, 822. https://doi.org/10.3390/nu13030822
Mörkl S, Stell L, Buhai DV, Schweinzer M, Wagner-Skacel J, Vajda C, Lackner S, Bengesser SA, Lahousen T, Painold A, et al. ‘An Apple a Day’?: Psychiatrists, Psychologists and Psychotherapists Report Poor Literacy for Nutritional Medicine: International Survey Spanning 52 Countries. Nutrients. 2021; 13(3):822. https://doi.org/10.3390/nu13030822
Chicago/Turabian StyleMörkl, Sabrina, Linda Stell, Diana V. Buhai, Melanie Schweinzer, Jolana Wagner-Skacel, Christian Vajda, Sonja Lackner, Susanne A. Bengesser, Theresa Lahousen, Annamaria Painold, and et al. 2021. "‘An Apple a Day’?: Psychiatrists, Psychologists and Psychotherapists Report Poor Literacy for Nutritional Medicine: International Survey Spanning 52 Countries" Nutrients 13, no. 3: 822. https://doi.org/10.3390/nu13030822
APA StyleMörkl, S., Stell, L., Buhai, D. V., Schweinzer, M., Wagner-Skacel, J., Vajda, C., Lackner, S., Bengesser, S. A., Lahousen, T., Painold, A., Oberascher, A., Tatschl, J. M., Fellinger, M., Müller-Stierlin, A., Serban, A. C., Ben-Sheetrit, J., Vejnovic, A.-M., Butler, M. I., Balanzá-Martínez, V., ... Holasek, S. J. (2021). ‘An Apple a Day’?: Psychiatrists, Psychologists and Psychotherapists Report Poor Literacy for Nutritional Medicine: International Survey Spanning 52 Countries. Nutrients, 13(3), 822. https://doi.org/10.3390/nu13030822