Executive Functions and Body Weight at Different Ages: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.3. Apparatus
- -
- The Stroop task [38] is one of the most adopted instruments for assessing executive functions, specifically cognitive inhibition and interference control.
- -
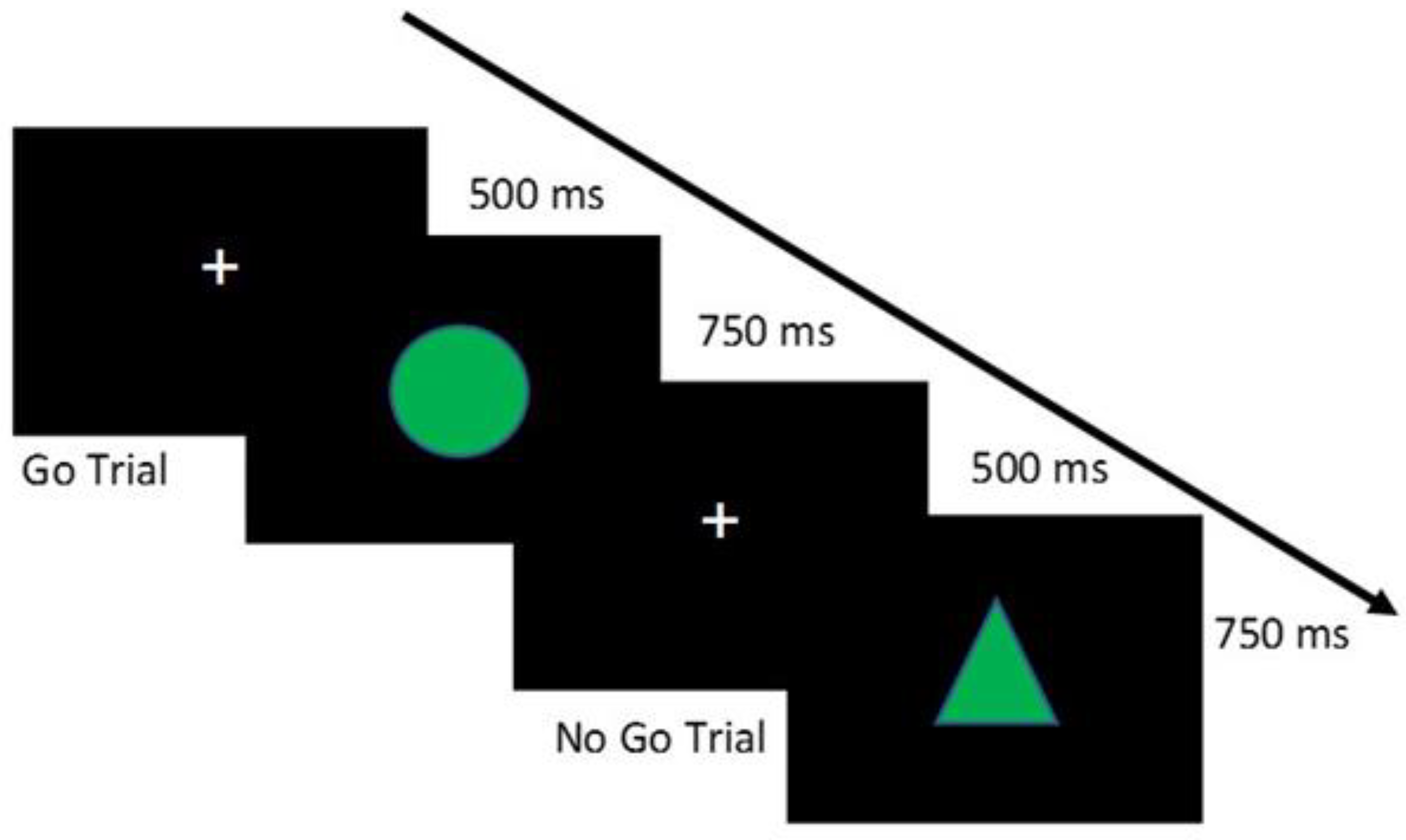
- The Go/No-Go Task [39] allows assessing motor inhibition, i.e., the ability to control an inadequate motor response.
- -
- The n-back task [40] is largely adopted to assess working memory.
- -
- The Wisconsin Card Sorting Test (WCST, [42]), a computerized version of the WCST was adopted to assess cognitive flexibility. The test requires the participant to match some cards according to specific characteristics of four Stimulus Cards.
2.4. General Procedure
2.5. Data Analysis
3. Results
3.1. General Data of the Sample
3.2. Regression Analysis
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Body Mass Index—BMI. Available online: http://www.euro.who.int/en/healthtopics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 28 May 2019).
- Gettens, K.M.; Gorin, A.A. Executive function in weight loss and weight loss maintenance: A conceptual review and novel neuropsychological model of weight control. J. Behav. Med. 2017, 40, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Cserjési, R.; Luminet, O.; Poncelet, A.S.; Lénárd, L. Altered executive function in obesity. Exploration of the role of affective states on cognitive abilities. Appetite 2009, 52, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Prickett, C.; Brennan, L.; Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 2015, 9, 93–113. [Google Scholar] [CrossRef]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Gordon, E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr. Psychiatry 2007, 48, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Hay, P.; Campbell, L.; Trollor, J.N. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes. Rev. 2011, 12, 740–755. [Google Scholar] [CrossRef] [PubMed]
- European Health Information Gateway. Available online: https://gateway.euro.who.int/en/ (accessed on 1 December 2020).
- Hernández-Quevedo, C.; Rechel, B. Obesity. In The Role of Public Health Organizations in Addressing Public Health Problems in Europe: The Case of Obesity, Alcohol and Antimicrobial Resistance; Health Policy Series, No. 51.; Rechel, B., Maresso, A., Sagan, A., Hernandez-Quevado, C., Richardson, E., Jakubowski, E., McKee, M., Nolte, E., Eds.; European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536186/ (accessed on 16 January 2021.).
- Calitri, R.; Pothos, E.M.; Tapper, K.; Brunstrom, J.M.; Rogers, P.J. Cognitive biases to healthy and unhealthy food words predict change in BMI. Obesity 2010, 18, 2282–2287. [Google Scholar] [CrossRef]
- Kadouh, H.C.; Acosta, A. Current paradigms in the etiology of obesity. Tech. Gastrointest. Endosc. 2017, 19, 2–11. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef]
- Mehrzad, R. Etiology of obesity. In the Obesity; Elsevier: Amsterdam, The Nederlands, 2020; pp. 43–54. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef]
- Favieri, F.; Forte, G.; Marotta, A.; Casagrande, M. Food-Related Attentional Bias in Individuals with Normal Weight and Overweight: A Study with a Flicker Task. Nutrients 2020, 12, 492. [Google Scholar] [CrossRef]
- Reinert, K.R.; Po’e, E.K.; Barkin, S.L. The relationship between executive function and obesity in children and adolescents: A systematic literature review. J. Obes. 2013, 2013, 820956. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Nederkoorn, C.; Houben, K.; Hofmann, W.; Roefs, A.; Jansen, A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010, 29, 389. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M. Interplay between the gut-brain axis, obesity and cognitive function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Sellbom, K.S.; Gunstad, J. Cognitive function and decline in obesity. J. Alzheimer's Dis. 2012, 30, S89–S95. [Google Scholar] [CrossRef]
- Vainik, U.; Baker, T.E.; Dadar, M.; Zeighami, Y.; Michaud, A.; Zhang, Y.; Alanis, J.C.; Misic, B.; Collins, D.L.; Dagher, A. Neurobehavioral correlates of obesity are largely heritable. Proc. Natl. Acad. Sci. USA 2018, 115, 9312–9317. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Davidson, T.L.; Jones, S.; Roy, M.; Stevenson, R.J. The cognitive control of eating and body weight: It’s more than what you “think”. Front. Psychol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A metaanalysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Wirt, T.; Schreiber, A.; Kesztyüs, D.; Steinacker, J.M. Early life cognitive abilities and body weight: Cross-sectional study of the association of inhibitory control, cognitive flexibility, and sustained attention with BMI percentiles in primary school children. J. Obes. 2015, 2015, 534651. [Google Scholar] [CrossRef]
- Mamrot, P.; Hanć, T. The Association of the Executive Functions with overweight and obesity indicators in children and adolescents: A literature review. Neurosci. Biobehav. Rev. 2019, 107, 59–68. [Google Scholar] [CrossRef]
- Groppe, K.; Elsner, B. The influence of hot and cool executive function on the development of eating styles related to overweight in children. Appetite 2015, 87, 127–136. [Google Scholar] [CrossRef]
- Best, J.R.; Miller, P.H.; Jones, L.L. Executive functions after age 5: Changes and correlates. Dev. Rev. 2009, 29, 180–200. [Google Scholar] [CrossRef]
- Gunstad, J.; Lhotsky, A.; Wendell, C.R.; Ferrucci, L.; Zonderman, A.B. Longitudinal examination of obesity and cognitive function: Results from the Baltimore longitudinal study of aging. Neuroepid 2010, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Coppin, G.; Nolan-Poupart, S.; Jones-Gotman, M.; Small, D.M. Working memory and reward association learning impairments in obesity. Neuropsychologia 2014, 65, 146–155. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Body Mass Index—BMI. Available online: https://www.who.int/toolkits/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 1 December 2020).
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- De Onis, M.; Garza, C.; Onyango, A.W.; Borghi, E. Comparison of the WHO child growth standards and the CDC 2000 growth charts. J. Nutr. 2007, 137, 144–14834. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Müller, U. Executive function in typical and atypical development. In The Wiley-Blackwell Handbook of Childhood Cognitive Development; Goswami, U., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 574–603. [Google Scholar]
- Treit, S.; Chen, Z.; Rasmussen, C.; Beaulieu, C. White matter correlates of cognitive inhibition during development: A diffusion tensor imaging study. Neuroscience 2014, 276, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Banzato, C.; Talamini, G. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Stroop, R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Simson, R.; Vaughan, H.G., Jr.; Ritter, W. The scalp topography of potentials in auditory and visual Go/NoGo tasks. Clin. Neurophysiol. 1977, 43, 864–875. [Google Scholar] [CrossRef]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef]
- Pelegrina, S.; Lechuga, M.T.; García-Madruga, J.A.; Elosúa, M.R.; Macizo, P.; Carreiras, M.; Fuentes, L.J.; Bajo, M.T. Normative data on the n-back task for children and young adolescents. Front. Psychol. 2015, 6, 1544. [Google Scholar] [CrossRef] [PubMed]
- Milner, B. Effects of different brain lesions on card sorting: The role of the frontal lobes. Arch. Neurol. 1963, 9, 100–110. [Google Scholar] [CrossRef]
- Wessel, J.R. Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology 2018, 55, e12871. [Google Scholar] [CrossRef]
- Reyes, S.; Peirano, P.; Peigneux, P.; Lozoff, B.; Algarin, C. Inhibitory control in otherwise healthy overweight 10-year-old children. Int. J. Obes. 2015, 39, 1230. [Google Scholar] [CrossRef]
- Alarcón, G.; Ray, S.; Nagel, B.J. Lower working memory performance in overweight and obese adolescents is mediated by white matter microstructure. J. Int. Neuropsychol. 2016, 22, 281–292. [Google Scholar] [CrossRef]
- Verdejo-García, A.; Pérez-Expósito, M.; Schmidt-Río-Valle, J.; Fernández-Serrano, M.J.; Cruz, F.; Pérez-García, M.; López-Belmonte, G.; Martín-Matillas, M.; Martín-Lagos, J.A.; Marcos, A.; et al. Selective alterations within executive functions in adolescents with excess weight. Obesity 2010, 18, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Altman, N. Neural networks of motor and cognitive inhibition are dissociated between brain hemispheres: An fMRI study. Int. J. Neurosci. 2009, 119, 1848–1880. [Google Scholar] [CrossRef]
- Chen, E.Y.; Zeffiro, T.A. Hunger and BMI modulate neural responses to sweet stimuli: fMRI meta-analysis. Int. J. Obes. 2020, 44, 1636–1652. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Lee, H.J.; Lumeng, J.C. Obesity-associated biomarkers and executive function in children. Pediatr. Res. 2015, 77, 143–147. [Google Scholar] [CrossRef]
- Perpiñá, C.; Segura, M.; Sánchez-Reales, S. Cognitive flexibility and decision-making in eating disorders and obesity. Eat. Weight Disord. 2017, 22, 435–444. [Google Scholar] [CrossRef]
- Darcy, A.M.; Fitzpatrick, K.K.; Colborn, D.; Datta, N.; Aspen, V.; Shields, C.S.; Le Grange, D.; Lock, J. Set-shifting among adolescents with bulimic spectrum eating disorders. Psychosom. Med. 2012, 74, 869. [Google Scholar] [CrossRef]
- Fitzpatrick, K.K.; Darcy, A.; Colborn, D.; Gudorf, C.; Lock, J. Set-shifting among adolescents with anorexia nervosa. Int. J. Eat. Disord. 2012, 45, 909–912. [Google Scholar] [CrossRef]
- Delgado-Rico, E.; Río-Valle, J.S.; González-Jiménez, E.; Campoy, C.; Verdejo-García, A. BMI predicts emotion-driven impulsivity and cognitive inflexibility in adolescents with excess weight. Obesity 2012, 20, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Galioto Wiedemann, R.; Calvo, D.; Meister, J.; Spitznagel, M.B. Self-reported physical activity is associated with cognitive function in lean, but not obese individuals. Clin. Obes. 2014, 4, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Galioto, R.; Gunstad, J.; Spitznagel, M.B. Uncontrolled eating is associated with reduced executive functioning. Clin. Obes. 2014, 4, 172–179. [Google Scholar] [CrossRef]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32, S56–S59. [Google Scholar] [CrossRef] [PubMed]


| Children | Adolescents | Young Adults | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Weight | Overweight | Normal Weight | Overweight | Normal Weight | Overweight | F2,160 | p | ηp2 * | |
| N (m/f) | 31 (17/14) | 15 (5/10) | 38 (13/25) | 12 (2/10) | 38 (15/23) | 32 (13/19) | |||
| Age | 8.00 (0.93) | 7.93 (0.80) | 16.71 (0.77) | 16.42 (0.67) | 22.39 (2.28) | 24.44 (2.47) | 8.12 | 0.001 | 0.10 |
| Years of Education | 2.9 (0.75) | 2.93 (0.80) | 11.71 (0.77) | 11.42 (0.67) | 15.89 (1.33) | 16.84 (1.22) | 5.36 | 0.01 | 0.06 |
| Weight (kg) | 27.79 (5.35) | 38.24 (7.96) | 58.10 (7.38) | 75.33 (11.36) | 61.45 (8.78) | 80.03 (15.18) | 2.39 | 0.10 | 0.03 |
| Height (m) | 1.32 (0.08) | 1.35 (0.07) | 1.68 (0.07) | 1.68 (0.11) | 1.69 (0.09) | 1.70 (0.08) | <1 | 0.59 | 0.01 |
| BMI | 15.83 (1.70) | 20.74 (3.00) | 20.46 (1.97) | 26.74 (3.30) | 21.34 (1.74) | 27.35 (3.05) | <1 | 0.37 | 0.01 |
| BMI-to-age ratio | 1.99 (0.23) | 2.64 (0.43) | 1.23 (0.13) | 1.63 (0.22) | 0.97 (0.13) | 1.11 (0.13) | 20.78 | 0.001 | 0.21 |
| Waist-to-height ratio | 0.45 (0.04) | 0.50 (0.06) | 0.40 (0.08) | 0.47 (0.04) | 0.43 (0.04) | 0.50 (0.10) | <1 | 0.72 | 0.004 |
| Body Adiposity Index | 24.56 (4.52) | 29.61 (6.43) | 20.48 (8.59) | 26.18 (5.67) | 21.23 (3.13) | 26.82 (9.79) | <1 | 0.97 | 0.001 |
| Systolic Blood Pressure | 108.52 (17.71) | 113.65 (7.55) | 104.75 (12.36) | 111.41 (12.48) | 114.42 (10.68) | 119.39 (11.46) | <1 | 0.95 | 0.001 |
| Diastolic Blood Pressure | 67.74 (11.14) | 76.25 (10.73) | 68.16 (7.13) | 72.54 (7.74) | 69.30 (8.30) | 74.70 (6.84) | <1 | 0.61 | 0.01 |
| Hunger (0–100) | 50.80 (33.83) | 37.17 (27.05) | 40.28 (34.63) | 45.83 (31.48) | 61.06 (26.65) | 45.13 (33.63) | 1.40 | 0.25 | 0.01 |
| Cognitive Inhibition | |||||||||
| Stroop Effect (Stroop Task: reaction times) | 91.96 (81.22) | 108.23 (82.46) | 66.59 (44.94) | 103.61 (63.78) | 91.91 (60.18) | 98.24 (69.83) | <1 | 0.53 | 0.01 |
| False Alarms (Go-No Task) | 7.74 (4.15) | 8.83 (5.20) | 4.66 (3.53) | 5.67 (4.08) | 3.97 (3.14) | 4.55 (2.79) | <1 | 0.92 | 0.001 |
| Working memory | |||||||||
| 1-back task accuracy (%) | 0.70 (0.28) | 0.72 (0.25) | 0.93 (0.10) | 0.88 (0.13) | 0.94 (0.10) | 0.93 (0.11) | <1 | 0.60 | 0.01 |
| 2-back task accuracy (%) | 0.50 (0.27) | 0.51 (0.24) | 0.67 (0.22) | 0.71 (0.21) | 0.79 (0.17) | 0.82 (0.21) | <1 | 0.98 | 0.002 |
| Set-shifting | |||||||||
| Global Score (WCST) | 33.63 (20.98) | 37.47 (16.90) | 21.13 (16.62) | 25.17 (19.09) | 16.63 (16.01) | 13.16 (10.85) | <1 | 0.70 | 0.004 |
| Perseveration (WCST) | 10.50 (9.05) | 11.93 (10.44) | 6.03 (3.74) | 7.92 (4.91) | 4.87 (4.21) | 4.38 (3.59) | <1 | 0.61 | 0.01 |
| Models | B | Standard Error | Beta | t | Sign. (p=) | 95% CI Lower | 95% CI Upper | Zero-Order Correlation | % of Explenation | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Stroop Effect | 0.00 | 0.000 | 0.04 | 0.99 | 0.33 | 0.0 | 0.001 | 0.10 | 72 |
| False Alarms | 0.01 | 0.006 | 0.14 | 2.94 | 0.004 | 0.006 | 0.03 | 0.48 | ||
| 2 | Stroop Effect | 0.0 | 0.000 | 0.04 | 0.93 | 0.35 | 0.0 | 0.001 | 0.10 | 73 |
| False Alarms | 0.02 | 0.007 | 0.14 | 2.94 | 0.004 | 0.006 | 0.03 | 0.48 | ||
| 1-back task accuracy | −0.17 | 0.145 | −0.06 | −1.14 | 0.25 | −0.45 | 0.12 | −0.42 | ||
| 2-back task accuracy | 0.16 | 0.115 | 0.07 | 1.35 | 0.18 | −0.07 | 0.38 | −0.41 | ||
| 3 | Stroop Effect | 0.0 | 0.000 | 0.04 | 0.88 | 0.38 | 0.0 | 0.001 | 0.10 | 73 |
| False Alarms | 0.02 | 0.007 | 0.14 | 2.92 | 0.004 | 0.006 | 0.03 | 0.48 | ||
| 1-back task accuracy | −0.14 | 0.148 | −0.05 | −0.96 | 0.34 | −0.43 | 0.15 | −0.42 | ||
| 2-back task accuracy | 0.15 | 0.116 | 0.07 | 1.33 | 0.19 | −0.07 | 0.38 | −0.41 | ||
| Global Score (WCST) | 0.002 | 0.002 | 0.06 | 0.91 | 0.36 | −0.002 | 0.006 | 0.42 | ||
| Perseveration (WCST) | −0.001 | 0.01 | −0.01 | −0.14 | 0.89 | −0.012 | 0.01 | 0.36 | ||
| Age-Adjusted BMI | Stroop Effect | False Alarms | 1-Back Task | 2-Back Task | Global Score WCST | Perseveration WCST | |
|---|---|---|---|---|---|---|---|
| Stroop Effect | 0.08 p = 0.20 | 1.00 | |||||
| False Alarms | 0.48 p = 0.0001 | 0.17 p = 0.01 | 1.00 | ||||
| 1-back task | −0.42 p = 0.0001 | −0.07 p = 0.15 | −0.32 p = 0.0001 | 1.00 | |||
| 2-back task | −0.41 p = 0.0001 | −0.03 p = 0.20 | −0.32 p = 0.0001 | 0.54 p = 0.0001 | 1.00 | ||
| Global Score (WCST) | 0.42 p = 0.0001 | 0.06 p = 0.20 | 0.19 p = 0.01 | −0.32 p = 0.0001 | −0.27 p = 0.0001 | 1.00 | |
| Perseveration (WCST) | 0.36 p = 0.0001 | 0.12 p = 0.10 | 0.13 p = 0.10 | −0.25 p = 0.0001 | −0.29 p = 0.0001 | 0.74 p = 0.0001 | 1.00 |
| Stroop Effect (Stroop Task) | False Alarms (Go/No-Go Task) | 1-Back Accuracy | 2-Back Accuracy | Global Score (WCST) | Perseveration (WCST) | ||
|---|---|---|---|---|---|---|---|
| BMI | r | 0.07 | 0.15 | 0.15 | 0.21 | −0.01 | 0.02 |
| p | 0.60 | 0.37 | 0.37 | 0.20 | 0.98 | 0.88 | |
| waist-to-height | r | −0.04 | −0.05 | 0.28 | 0.24 | 0.04 | −0.03 |
| p | 0.82 | 0.75 | 0.08 | 0.14 | 0.83 | 0.84 | |
| BAI | r | 0.16 | 0.18 | 0.24 | 0.21 | −0.10 | −0.19 |
| p | 0.32 | 0.27 | 0.13 | 0.19 | 0.55 | 0.23 | |
| Self-reported hunger | r | −0.03 | −0.13 | −0.17 | −0.20 | 0.13 | −0.02 |
| p | 0.86 | 0.42 | 0.30 | 0.24 | 0.41 | 0.91 |
| Stroop Effect (Stroop Task) | False Alarms (Go-Nogo Task) | 1-Back Accuracy | 2-Back Accuracy | Global Score (WCST) | Perseveration (WCST) | ||
|---|---|---|---|---|---|---|---|
| BMI | r | −0.05 | −0.28 | −0.001 | 0.11 | 0.43 | 0.41 |
| p | 0.81 | 0.16 | 0.98 | 0.60 | 0.02 | 0.03 | |
| waist-to-height | r | 0.06 | 0.12 | −0.08 | 0.04 | 0.30 | 0.29 |
| p | 0.75 | 0.55 | 0.69 | 0.86 | 0.13 | 0.14 | |
| BAI | r | 0.02 | 0.12 | −0.03 | −0.01 | 0.24 | 0.22 |
| p | 0.90 | 0.54 | 0.88 | 0.94 | 0.24 | 0.27 | |
| Self-reported hunger | r | 0.18 | 0.55 | −0.39 | −0.06 | −0.11 | −0.13 |
| p | 0.38 | 0.003 | 0.05 | 0.76 | 0.57 | 0.52 |
| Stroop Effect (Stroop Task) | False Alarms (Go-Nogo Task) | 1-Back Accuracy | 2-Back Accuracy | Global Score (WCST) | Perseveration (WCST) | ||
|---|---|---|---|---|---|---|---|
| BMI | r | 0.05 | 0.28 | −0.23 | 0.14 | −0.16 | −0.18 |
| p | 0.75 | 0.05 | 0.10 | 0.33 | 0.25 | 0.20 | |
| waist-to-height | r | −0.07 | 0.13 | −0.10 | 0.05 | −0.04 | −0.05 |
| p | 0.64 | 0.36 | 0.48 | 0.74 | 0.79 | 0.73 | |
| BAI | r | 0.04 | 0.14 | −0.15 | 0.08 | 0.07 | 0.03 |
| p | 0.79 | 0.34 | 0.30 | 0.58 | 0.61 | 0.81 | |
| Self-reported hunger | r | 0.01 | 0.06 | −0.12 | −0.10 | 0.13 | 0.14 |
| p | 0.92 | 0.66 | 0.41 | 0.51 | 0.35 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favieri, F.; Chen, E.; Casagrande, M. Executive Functions and Body Weight at Different Ages: A Preliminary Study. Nutrients 2021, 13, 1174. https://doi.org/10.3390/nu13041174
Favieri F, Chen E, Casagrande M. Executive Functions and Body Weight at Different Ages: A Preliminary Study. Nutrients. 2021; 13(4):1174. https://doi.org/10.3390/nu13041174
Chicago/Turabian StyleFavieri, Francesca, Eunice Chen, and Maria Casagrande. 2021. "Executive Functions and Body Weight at Different Ages: A Preliminary Study" Nutrients 13, no. 4: 1174. https://doi.org/10.3390/nu13041174
APA StyleFavieri, F., Chen, E., & Casagrande, M. (2021). Executive Functions and Body Weight at Different Ages: A Preliminary Study. Nutrients, 13(4), 1174. https://doi.org/10.3390/nu13041174






