A Systematic Review, Meta-Analysis and Meta-Regression on the Effects of Carbohydrates on Sleep
Abstract
:1. Introduction
- Examine the effect of the quantity of CHO consumption on sleep quantity, continuity and architecture.
- Address potential effects of the quality of CHO (e.g., Glycemic Index or Glycemic Load) on sleep quantity, continuity and architecture.
- Investigate the effect of the timing of CHO consumption on sleep quantity, continuity and architecture.
2. Methodology
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Evidence Quality Appraisal
2.5. Definitions of Nutrition-Related and Sleep-Related Parameters
- Total Sleep Time (TST): The total time spent asleep during the recording night.
- Sleep Efficiency % (SE): The percentage of the Ratio of Total Sleep Time (TST) to Time in Bed.
- Sleep Onset Latency (SOL): Time from lights out until sleep onset (generally defined as first epoch of sleep Stage 2).
- Wake After Sleep Onset (WASO): The duration of wake during the night after initial sleep onset. This term was used in parallel with “Total Wake Time” because of their similarity and the interchangeable use of them in the shortlisted studies.
- REM Onset Latency (ROL): the interval between sleep onset and the onset of the first REM sleep period.
- Stage 1 (N1): Duration of sleep Stage 1, presented as minutes or percentage of TST.
- Stage 2 (N2): Duration of sleep Stage 2, presented as minutes or percentage of TST.
- Stage 3 (N3): Duration of Slow Wave Sleep (SWS), presented as minutes or percentage of TST.
- REM Sleep (REM): Duration of REM generally presented as minutes or percentage of TST.
2.6. Data Synthesis
- Sleep Depth (min): defined by shorter duration of N1 and N2 sleep stage, and longer duration of N3 sleep stage in minutes.
- Sleep Depth (%): defined by lower TST percentage of N1 and N2 sleep stage, and higher percentage of N3 sleep stage.
- REM attainment (min): defined by shorter ROL and increased REM duration in minutes.
- REM attainment (%): defined by shorter ROL and increased % REM.
2.7. Statistical Analyses and Meta-Analytic Calculations
3. Results
3.1. Studies’ Selection
3.2. Studies’ Characteristics
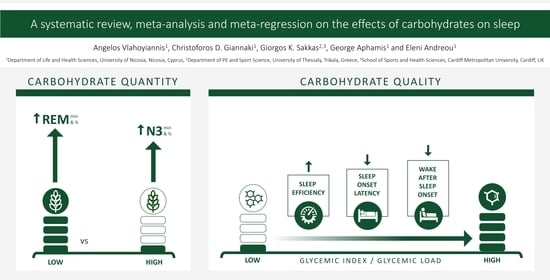
3.3. Effect of Carbohydrate Quantity on Sleep
3.4. Effect of Carbohydrate Quality on Sleep
3.5. Carbohydrate Timing and Sleep
3.6. Meta-Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, R.B.; Gamaldo, C.E.; Harding, S.M.; Brooks, R.; Lloyd, R.M.; Vaughn, B.V.; Marcus, C.L. AASM Scoring Manual Version 2.2 Updates: New Chapters for Scoring Infant Sleep Staging and Home Sleep Apnea Testing. J. Clin. Sleep Med. 2015, 11, 1253–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaghi, A.; O’Connor, H.; Chow, C.M. Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosci. 2008, 11, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Vlahoyiannis, A.; Aphamis, G.; Andreou, E.; Samoutis, G.; Sakkas, G.K.; Giannaki, C.D. Effects of High vs. Low Glycemic Index of Post-Exercise Meals on Sleep and Exercise Performance: A Randomized, Double-Blind, Counterbalanced Polysomnographic Study. Nutrients 2018, 10, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, D.L.; Wolkow, A.P.; Rajaratnam, S.M.W.; Drummond, S.P.A. Personal sleep debt and daytime sleepiness mediate the relationship between sleep and mental health outcomes in young adults. Depression Anxiety 2018, 35, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlahoyiannis, A.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Andreou, E.; Giannaki, C.D. Deconstructing athletes’ sleep: A systematic review of the influence of age, sex, athletic expertise, sport type, and season on sleep characteristics. J. Sport Heal. Sci. 2020, 25, S2095–S2546. [Google Scholar] [CrossRef] [PubMed]
- Ham, O.K.; Kim, J.; Lee, B.G.; Choi, E. Behavioral Characteristics and Cardiovascular Disease Risks Associated with Insomnia and Sleep Quality Among Middle-Aged Women in South Korea. Res. Nurs. Health 2017, 40, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Vlahoyiannis, A.; Sakkas, G.K.; Manconi, M.; Aphamis, G.; Giannaki, C.D. Athletes’ sleep assessment: From lifestyle to pharmacological interventions and vice versa. Sleep Med. 2021, 78, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.; Hudson, S.P.; Hecht, T.; MacKenzie, J. Protein source tryptophan versus pharmaceutical grade tryptophan as an efficacious treatment for chronic insomnia. Nutr. Neurosci. 2005, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Tsai, P.-S.; Fang, S.-C.; Liu, J.-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174. [Google Scholar]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Kapás, L.; Obál, F., Jr.; Opp, M.R.; Johannsen, L.; Krueger, J.M. Intraperitoneal injection of cholecystokinin elicits sleep in rabbits. Physiol. Behav. 1991, 50, 1241–1244. [Google Scholar] [CrossRef]
- Kapás, L.; Obál, F.; Alföldi, P.; Rubicsek, G.; Penke, B. Effects of nocturnal intraperitoneal administration of cholecystokinin in rats: Simultaneous increase in sleep, increase in EEG slow-wave activity, reduction of motor activity, suppression of eating, and decrease in brain temperature. Brain Res. 1988, 438, 155–164. [Google Scholar] [CrossRef]
- Akanmu, M.A.; Ukponmwan, O.E.; Katayama, Y.; Honda, K. Neuropeptide-Y Y2-receptor agonist, PYY3–36 promotes non-rapid eye movement sleep in rat. Neurosci. Res. 2006, 54, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010, 11, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Wurtman, R.J. Brain Serotonin Content: Physiological Dependence on Plasma Tryptophan Levels. Science 1971, 173, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, K.A.; Punjabi, N.M. Effects of Sleep Fragmentation on Glucose Metabolism in Normal Subjects. Chest 2010, 137, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.P.; Smith, K.; Atkinson, F.; Ruell, P.; Chow, C.M.; O’Connor, H.; Brand-Miller, J. High-glycaemic index and -glycaemic load meals increase the availability of tryptophan in healthy volunteers. Br. J. Nutr. 2011, 105, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005, 9, 41–50. [Google Scholar] [CrossRef]
- Noorwali, E.; Hardie, L.; Cade, J. Bridging the Reciprocal Gap between Sleep and Fruit and Vegetable Consumption: A Review of the Evidence, Potential Mechanisms, Implications, and Directions for Future Work. Nutrients 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2019, 85, 5–13. [Google Scholar] [CrossRef]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.; Chen, C.N.; Crisp, A.H.; Koval, J.; McGuinness, B.; Kalucy, R.S.; Kalucy, E.C.; Lacey, J.H. Isocaloric diet changes and electroencephalographic sleep. Lancet 1975, 2, 723–725. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Institute of Health Economics: Edmonton, AB, Canada, 2004. [Google Scholar]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suurmond, R.; Van Rhee, H.; Hak, T. Introduction, comparison, and validation ofMeta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindseth, G.; Lindseth, P.; Thompson, M. Nutritional effects on sleep. West. J. Nurs. Res. 2011, 35, 497–513. [Google Scholar] [CrossRef] [Green Version]
- Lindseth, G.; Murray, A. Dietary Macronutrients and Sleep. West. J. Nurs. Res. 2016, 38, 938–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, J.; Horne, J. Bed-time food supplements and sleep: Effects of different carbohydrate levels. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 426–433. [Google Scholar] [CrossRef]
- Jalilolghadr, S.; Afaghi, A.; O’Connor, H.; Chow, C.M. Effect of low and high glycaemic index drink on sleep pattern in children. J. Pak. Med. Assoc. 2011, 61, 533–536. [Google Scholar]
- Daniel, N.V.; Zimberg, I.Z.; Estadella, D.; Garcia, M.C.; Padovani, R.C.; Juzwiak, C.R. Effect of the intake of high or low glycemic index high carbohydrate-meals on athletes’ sleep quality in pre-game nights. An. Acad. Bras. Ciências 2019, 91, e20180107. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.M.F.; Thomas, S.; Mir, M.A. Effects of a Low Carbohydrate Isoenergetic Diet on Sleep Behavior and Pulmonary Functions in Healthy Female Adult Humans. J. Nutr. 1986, 116, 2393–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buman, M.P.; Phillips, B.A.; Youngstedt, S.D.; Kline, C.E.; Hirshkowitz, M. Does nighttime exercise really disturb sleep? Results from the 2013 National Sleep Foundation Sleep in America Poll. Sleep Med. 2014, 15, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Ollila, H.M.; Kettunen, J.; Pietiläinen, O.; Aho, V.; Silander, K.; Kronholm, E.; Perola, M.; Lahti, J.; Räikkönen, K.; Widen, E.; et al. Genome-wide association study of sleep duration in the Finnish population. J. Sleep Res. 2014, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.S.; Read, N.; Uvnas-Moberg, K.; Alster, P. Influences of Fat and Carbohydrate on Postprandial Sleepiness, Mood, and Hormones. Physiol. Behav. 1997, 61, 679–686. [Google Scholar] [CrossRef]
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1187–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dockray, G.J. Cholecystokinin and gut–brain signalling. Regul. Pept. 2009, 155, 6–10. [Google Scholar] [CrossRef]
- Steiger, A.; Dresler, M.; Schüssler, P.; Kluge, M. Ghrelin in mental health, sleep, memory. Mol. Cell. Endocrinol. 2011, 340, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuessler, P.; Uhr, M.; Ising, M.; Schmid, D.; Weikel, J.; Steiger, A. Nocturnal ghrelin levels-relationship to sleep EEG, the levels of growth hormone, ACTH and cortisol—and gender differences. J. Sleep Res. 2005, 14, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Plat, L. Physiology of growth hormone secretion during sleep. J. Pediatr. 1996, 128, S32–S37. [Google Scholar] [CrossRef]
- Friess, E.; Tagaya, H.; Grethe, C.; Trachsel, L.; Holsboer, F. Acute Cortisol Administration Promotes Sleep Intensity in Man. Neuropsychopharmacology 2003, 29, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Bohlhalter, S.; Murck, H.; Holsboer, F.; Steiger, A. Cortisol Enhances non-REM Sleep and Growth Hormone Secretion in Elderly Subjects. Neurobiol. Aging 1997, 18, 423–429. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J.; Regan, M.M.; McDermott, J.M.; Tsay, R.H.; Breu, J.J. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am. J. Clin. Nutr. 2003, 77, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Moller-Loswick, A.C.; Zachrisson, H.; Hyltander, A.; Körner, U.; Matthews, D.E.; Lundholm, K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. Am. J. Physiol. Metab. 1994, 266, E645–E652. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Kalucy, R.S.; Hartmann, M.K.; Lacey, J.H.; Crisp, A.H.; Bailey, J.E.; Eccleston, E.G.; Coppen, A. Plasma tryptophan and sheep. Br. Med. J. 1974, 4, 564–566. [Google Scholar] [CrossRef] [Green Version]
- Parker, D.C.; Rossman, L.G. Human Growth Hormone Release in Sleep: Nonsuppression by Acute Hyperglycemia. J. Clin. Endocrinol. Metab. 1971, 32, 65–69. [Google Scholar] [CrossRef]
- Howorka, K.; Heger, G.; Schabmann, A.; Anderer, P.; Tribl, G.; Zeitlhofer, J. Severe hypoglycaemia unawareness is associated with an early decrease in vigilance during hypoglycaemia. Psychoneuroendocrinology 1996, 21, 295–312. [Google Scholar] [CrossRef]
- Boyle, P.J.; Scott, J.C.; Krentz, A.J.; Nagy, R.J.; Comstock, E.; Hoffman, C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J. Clin. Investig. 1994, 93, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.F.; Fernández-Elías, V.E.; Hamouti, N.; Pallares, J.G.; Rodríguez, R.M. Higher Insulin-sensitizing Response after Sprint Interval Compared to Continuous Exercise. Int. J. Sports Med. 2015, 36, e4. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I. Sleep as restorative process: Human clues. Prog. Brain Res. 1980, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A. From slow waves to sleep homeostasis: New perspectives. Arch. Ital. Biol. 2001, 139, 53–61. [Google Scholar] [PubMed]
- Takahashi, Y.; Kipnis, D.M.; Daughaday, W.H. Growth hormone secretion during sleep. J. Clin. Investig. 1968, 47, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; De Gennaro, L.; Bertini, M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: Do we need a fixed SWS amount per night? Sleep Res. Online 1999, 2, 15–19. [Google Scholar]
- Vogel, G.W. A Review of REM Sleep Deprivation. Arch. Gen. Psychiatry 1975, 32, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, Y.; Tanuma, N.; Miyata, R.; Hayashi, M. Melatonin alterations and brain acetylcholine lesions in sleep disorders in Cockayne syndrome. Brain Dev. 2014, 36, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Vlahoyiannis, A.; Sakkas, G.K.; Manconi, M.; Aphamis, G.; Giannaki, C.D. A critical review on sleep assessment methodologies in athletic populations: Factors to be considered. Sleep Med. 2020, 74, 211–223. [Google Scholar] [CrossRef]



| Author, Year, Reference | Country | Study Design | N | Sex | Age (Year) | BMI (kg/m2) | Quality Score |
|---|---|---|---|---|---|---|---|
| Phillips et al., 1975 [24] | UK | Crossover | 8 | M | NR | 18.5–25 | Moderate |
| Porter and Horne, 1981 [33] | UK | Crossover | 6 | M | NR | NR * | Strong |
| Kwan et al., 1986 [36] | UK | Single Group pre-post design | 6 | F | 20–23 | 19–24 | Moderate |
| Afaghi et al., 2007 [23] | Australia | Crossover | 12 | M | 18–35 | 18.5–25 | Strong |
| Afaghi et al., 2008 [2] | Australia | Single Group pre-post design | 14 | M | 18–35 | 23.4 ± 1.9 | Moderate |
| Jalilolghadr et al., 2011 [34] | Australia | Crossover | 8 | M & F | 8–12 | 18.9 ± 2.2 | Strong |
| Lindseth et al., 2013 [31] | USA | Crossover | 44 | NR | 19–22 | 24.8 ± 3.5 | Strong |
| Lindseth and Murray, 2016 [32] | USA | Crossover | 36 | M & F | 20.9 ± 1.9 | 24.6 ± 4.1 | Strong |
| St-Onge et al., 2016 [37] | USA | Single-Group pre-post design | 26 | M & F | 30–45 | 22–26 | Moderate |
| Vlahoyiannis et al., 2018 [3] | Cyprus | Crossover | 10 | M | 18–26 | 24.9 ± 4 | Strong |
| Daniel et al., 2019 [35] | Brazil | Crossover | 9 | M | 18.0 ± 0.7 | 23.9 ± 1.5 | Moderate |
| Author, Year, Reference | Type of Intervention (A = CHO Quantity; B = CHO Quality; C = CHO Timing) | Duration of Intervention (A = Acute; B = Short -Term) | Timing of Intervention (A = 45 − 1 h; B = 4 h) | Trial | Nutrition Intervention Macronutrient Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kcal | CHO (g) | CHO (%) | Fat (g) | Fat (%) | Protein (g) | Protein (%) | |||||
| Phillips et al., 1975 [24] | A | B | NA | HCI | 2997 | 600.0 | 80.1 | 33.0 | 9.9 | 75.0 | 10.0 |
| B | NA | LCI | 2995 | 100.0 | 13.4 | 255.0 | 76.6 | 75.0 | 10.0 | ||
| Porter and Horne, 1981 [33] | A | A | A | HCI | 714 | 130.0 | 72.8 | 18.0 | 22.7 | 8.0 | 4.5 |
| A | A | ZCI | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| A | A | LCI | 401 | 47.0 | 46.9 | 21.0 | 47.1 | 6.0 | 6.0 | ||
| Kwan et al., 1986 [36] | A | B | NA | HCI | 1929 | 240.0 | 49.8 | 83.0 | 38.7 | 64.0 | 13.3 |
| B | NA | LCI | 2066 | 49.0 | 9.5 | 164.0 | 71.4 | 103.0 | 19.9 | ||
| Afaghi et al., 2007 [23] | B & C | A | A | HGI (1 h) | 764 | 173.0 | 90.6 | 1.3 | 1.5 | 15.0 | 7.9 |
| A | B | HGI (4 h) | 764 | 173.0 | 90.6 | 1.3 | 1.5 | 15.0 | 7.9 | ||
| A | B | LGI (4 h) | 764 | 173.0 | 90.6 | 1.3 | 1.5 | 15.0 | 7.9 | ||
| Afaghi et al., 2008 [2] | A | A & B | B | HCI | 1090 | 196.0 | 71.9 | 15.0 | 12.4 | 42.0 | 15.4 |
| A | B | LCI (acute) | 1090 | 2.0 | 0.7 | 74.0 | 61.1 | 103.0 | 37.8 | ||
| A & B | B | LCI (2 days) | 1090 | 2.0 | 0.7 | 74.0 | 61.1 | 103.0 | 37.8 | ||
| Lindseth et al., 2013 [31] | A | B | NA | HCI | NR | NR | 56.0 | NR | 22.0 | NR | 22.0 |
| B | NA | HCI | NR | NR | 50.0 | NR | 35.0 | NR | 15.0 | ||
| B | NA | LCI | NR | NR | 22.0 | NR | 56.0 | NR | 22.0 | ||
| Jalilolghadr et al., 2011 [34] | B | A | A | HGI | 238.4 | 45.1 | 75.6 | 0.6 | 2.3 | 13.2 | 22.1 |
| A | A | LGI | 277 | 25.9 | 37.3 | 13.6 | 44.2 | 12.8 | 18.5 | ||
| Lindseth and Murray, 2016 [32] | B | B | NA | HCI | NR | NR | 80.0 | NR | 10.0 | NR | 10.0 |
| B | NA | HCI | NR | NR | 50.0 | NR | 35.0 | NR | 15.0 | ||
| B | NA | LCI | NR | NR | 25.0 | NR | 65.0 | NR | 10.0 | ||
| St-Onge et al., 2016 [37] | A | B | NA | HCI | NR | NR | 53.5 | NR | 31.0 | NR | 17.0 |
| B | NA | LCI | NR | NR | 54.6 | NR | 32.7 | NR | 14.0 | ||
| Vlahoyiannis et al., 2018 [3] | B | A | B | HGI | 801.2 | 178.0 | 88.9 | 2.4 | 2.7 | 16.9 | 8.4 |
| A | B | LGI | 801.2 | 178.0 | 88.9 | 2.4 | 2.7 | 16.9 | 8.4 | ||
| Daniel et al., 2019 [35] | B | A | A | HGI | 1058 | 169.5 | 64.1 | 27.9 | 10.5 | 29.9 | 25.4 |
| A | A | LGI | 1083 | 160.3 | 59.2 | 33.1 | 12.2 | 34.4 | 28.6 | ||
| Author, Year, Reference | Sleep Monitoring Method | Familiarization | Nights Recorded (Per Trial) | TST (min) | SE (%) | SOL (min) | WASO (min) | ROL (min) | N1 (min) | N1 (%) | N2 (min) | N2 (%) | N3 (min) | N3 (%) | REM (min) | REM (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phillips et al., 1975 [24] | EEG | Y | 2 | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | ||
| Porter and Horne, 1981 [33] | PSG | Y | 3 | Χ | Χ | Χ | Χ | Χ | Χ | |||||||
| Kwan et al., 1986 [36] | EEG | Y | 2 | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | ||
| Afaghi et al., 2007 [23] | PSG | Y | 1 | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | ||||
| Afaghi et al., 2008 [2] | PSG | Y | 1 | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ |
| Jalilolghadr et al., 2011 [34] | PSG | Y | 1 | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | ||||
| Lindseth et al., 2013 [31] | PSG | Y | 1 | Χ | Χ | Χ | Χ | Χ | Χ | |||||||
| Lindseth and Murray, 2016 [32] | Actigraphy | N | 4 | Χ | Χ | |||||||||||
| St-Onge et al., 2016 [37] | Actigraphy | Y | 4 | Χ | Χ | Χ | Χ | |||||||||
| Vlahoyiannis et al., 2018 [3] | PSG | Y | 1 | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ | Χ |
| Daniel et al., 2019 [35] | Actigraphy | N | 1 | Χ | Χ | Χ | Χ |
| CHO Quantity | CHO Quality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hedges’ g | SE | z-Value | p | I2 Index | Results a | Hedges’ g | SE | z-Value | p | I2 Index | Results a | |
| TST | 0.01 | 0.09 | 0.08 | 0.936 | 12.33 | NS | −0.25 | 0.16 | −1.57 | 0.059 | 24.19 | NS |
| SE | 0.12 | 0.07 | 1.69 | 0.092 | 0 | NS | −0.27 | 0.21 | −1.27 | 0.203 | 63.09 | NS |
| SOL | −0.13 | 0.11 | −1.24 | 0.213 | 58.38 | NS | 0.58 | 0.47 | 1.24 | 0.213 | 83.54 | NS |
| ROL | 0.21 | 0.14 | 1.55 | 0.121 | 20.29 | NS | 0.07 | 0.20 | 0.34 | 0.731 | 46.08 | NS |
| WASO | −0.22 | 0.12 | −1.78 | 0.075 | 31.25 | NS | 0.11 | 0.20 | 0.57 | 0.569 | 58.09 | NS |
| N1 min | 0.06 | 0.1 | 0.60 | 0.551 | 0 | NS | ||||||
| N2 min | −0.02 | 0.09 | −0.19 | 0.849 | 0 | NS | ||||||
| N3 min | 0.37 | 0.07 | 5.13 | <0.001 | 0 | + | ||||||
| REM min | −0.38 | 0.05 | −8.05 | <0.001 | 0 | − | ||||||
| % N1 | 0.02 | 0.12 | 0.16 | 0.872 | 0 | NS | −0.21 | 0.16 | −1.37 | 0.171 | 0.00 | NS |
| % N2 | 0.03 | 0.08 | 0.39 | 0.698 | 0 | NS | 0.07 | 0.11 | 0.63 | 0.532 | 0.00 | NS |
| % N3 | 0.51 | 0.06 | 8.90 | <0.001 | 0 | + | 0.02 | 0.06 | 0.29 | 0.772 | 0.00 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlahoyiannis, A.; Giannaki, C.D.; Sakkas, G.K.; Aphamis, G.; Andreou, E. A Systematic Review, Meta-Analysis and Meta-Regression on the Effects of Carbohydrates on Sleep. Nutrients 2021, 13, 1283. https://doi.org/10.3390/nu13041283
Vlahoyiannis A, Giannaki CD, Sakkas GK, Aphamis G, Andreou E. A Systematic Review, Meta-Analysis and Meta-Regression on the Effects of Carbohydrates on Sleep. Nutrients. 2021; 13(4):1283. https://doi.org/10.3390/nu13041283
Chicago/Turabian StyleVlahoyiannis, Angelos, Christoforos D. Giannaki, Giorgos K. Sakkas, George Aphamis, and Eleni Andreou. 2021. "A Systematic Review, Meta-Analysis and Meta-Regression on the Effects of Carbohydrates on Sleep" Nutrients 13, no. 4: 1283. https://doi.org/10.3390/nu13041283