Magnesium for Pain Treatment in 2021? State of the Art
Abstract
1. Introduction
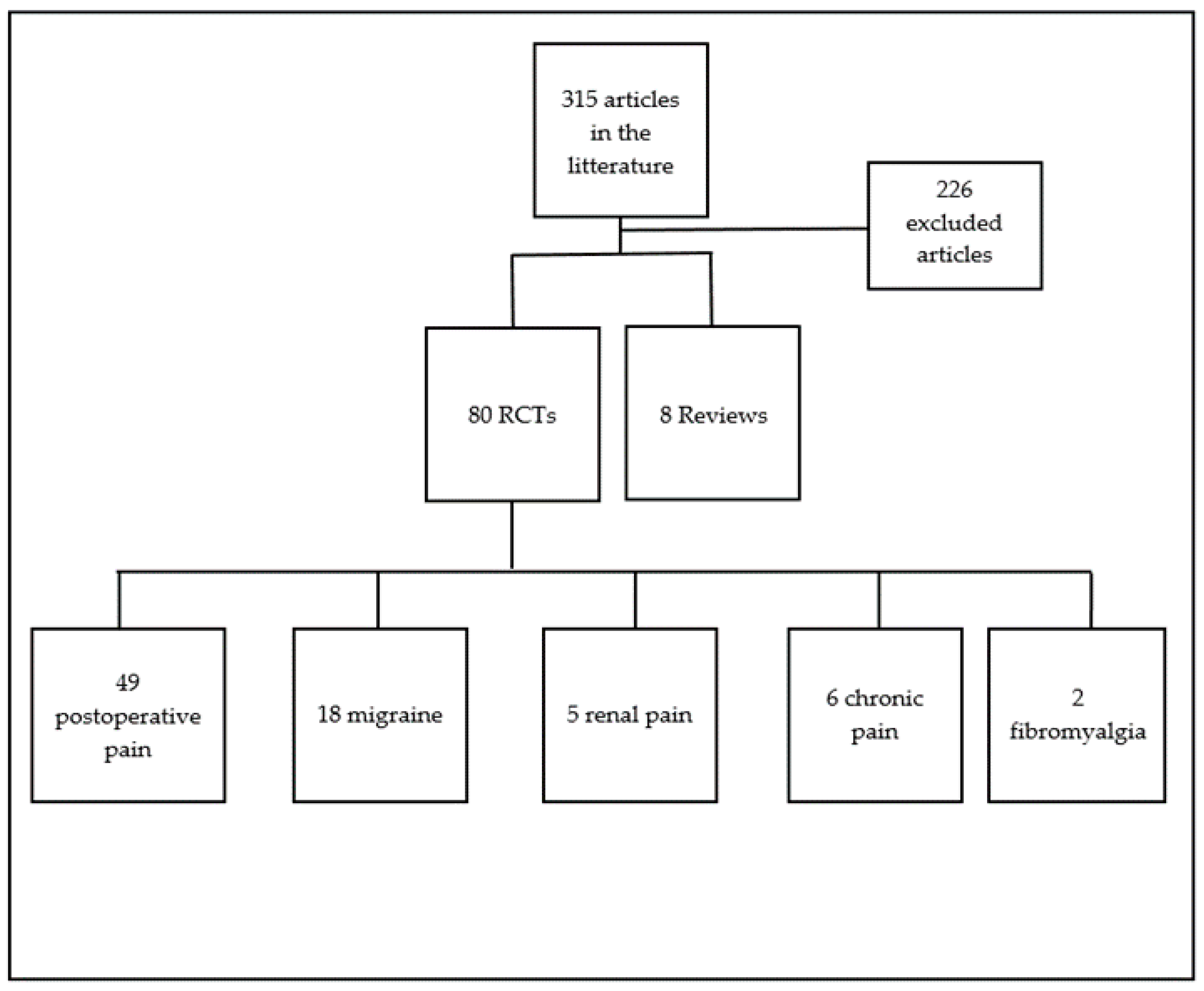
2. Materials and Methods
3. Results
3.1. Magnesium and Pain Diminution

3.2. Magnesium and Analgesics Consumption
3.3. Bioavailability of Magnesium Salts
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garland, E.L. Pain Processing in the Human Nervous System. Prim. Care Clin. Off. Pract. 2012, 39, 561–571. [Google Scholar] [CrossRef]
- Martucci, K.T.; Mackey, S.C. Neuroimaging of Pain. Anesthesioogy 2018, 128, 1241–1254. [Google Scholar] [CrossRef]
- Loeser, J.D.; Melzack, R. Pain: An overview. Lancet 1999, 353, 1607–1609. [Google Scholar] [CrossRef]
- Jank, R.; Gallee, A.; Boeckle, M.; Fiegl, S.; Pieh, C. Chronic Pain and Sleep Disorders in Primary Care. Pain Res. Treat. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef]
- Silberstein, S.; Loder, E.; Diamond, S.; Reed, M.L.; Bigal, E.M.; Lipton, R.B. Probable Migraine in the United States: Results of The American Migraine Prevalence and Prevention (AMPP) Study. Cephalalgia 2007, 27, 220–229. [Google Scholar] [CrossRef]
- Boyle, N.B.; Lawton, C.; Dye, L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review. Nutrients 2017, 9, 429. [Google Scholar] [CrossRef]
- Fuentes, J.C.; Salmon, A.A.; Silver, M.A. Acute and Chronic Oral Magnesium Supplementation: Effects on Endothelial Function, Exercise Capacity, and Quality of Life in Patients with Symptomatic Heart Failure. Congest. Hear. Fail. 2006, 12, 9–13. [Google Scholar] [CrossRef]
- Magnesium Saw Huge Cross-Channel Sales Growth Last Year. Here’s What’s Driving the Ingredient in 2020: 2020 Ingredient Trends to Watch for Foods, Drinks, and Dietary Supplements. Available online: https://www.nutritionaloutlook.com/view/magnesium-saw-huge-cross-channel-sales-growth-last-year-heres-whats-driving-ingredient (accessed on 14 December 2020).
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; DuBray, C. Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Ng, K.T.; Yap, J.L.; Izham, I.N.; Teoh, W.Y.; Kwok, P.E.; Koh, W.J. The effect of intravenous magnesium on postoperative morphine consumption in noncardiac surgery. Eur. J. Anaesthesiol. 2020, 37, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Nadeson, R.; Tucker, A.; Bajunaki, E.; Goodchild, C. Potentiation by ketamine of fentanyl antinociception. I. An experimental study in rats showing that ketamine administered by non-spinal routes targets spinal cord antinociceptive systems. Br. J. Anaesth. 2002, 88, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Pereira, B.; Morel, V.; Corriger, A.; Giron, F.; Marcaillou, F.; Bidar-Beauvallot, A.; Chandeze, E.; Lambert, C.; Bernard, L.; et al. Ketamine and Magnesium for Refractory Neuropathic Pain. Anesthesiology 2020, 133, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C.; Zarate, C.A. Ketamine, Sleep, and Depression: Current Status and New Questions. Curr. Psychiatry Rep. 2013, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Corriger, A.; Pickering, G. Ketamine and depression: A narrative review. Drug Des. Dev. Ther. 2019, 13, 3051–3067. [Google Scholar] [CrossRef]
- Felsby, S.; Nielsen, J.; Arendt-Nielsen, L.; Jensen, T.S. NMDA receptor blockade in chronic neuropathic pain: A comparison of ketamine and magnesium chloride. Pain 1996, 64, 283–291. [Google Scholar] [CrossRef]
- Nikolaev, M.V.; Magazanik, L.G.; Tikhonov, D.B. Influence of external magnesium ions on the NMDA receptor channel block by different types of organic cations. Neuropharmacology 2012, 62, 2078–2085. [Google Scholar] [CrossRef]
- Li, X.-H.; Miao, H.-H.; Zhuo, M. NMDA Receptor Dependent Long-term Potentiation in Chronic Pain. Neurochem. Res. 2019, 44, 531–538. [Google Scholar] [CrossRef]
- Blanke, M.L.; Van Dongen, A.M.J. Activation Mechanisms of the NMDA Receptor. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Gambrill, A.C.; Storey, G.P.; Barria, A. Dynamic Regulation of NMDA Receptor Transmission. J. Neurophysiol. 2011, 105, 162–171. [Google Scholar] [CrossRef]
- Fukunaga, K.; Muller, D.; Miyamoto, E. CaM Kinase II in Long-Term Potentiation. Neurochem. Int. 1996, 28, 343–358. [Google Scholar] [CrossRef]
- Ives. NMDA Receptors Play Key Role in Sleep Deficits that Accompany Psychiatric Disorders. News-Medicalnet 2020. Available online: https://www.news-medical.net/news/20200605/NMDA-receptors-play-key-role-in-sleep-deficits-that-accompany-psychiatric-disorders.aspx (accessed on 14 December 2020).
- Barkus, C.; McHugh, S.B.; Sprengel, R.; Seeburg, P.H.; Rawlins, J.N.P.; Bannerman, D.M. Hippocampal NMDA receptors and anxiety: At the interface between cognition and emotion. Eur. J. Pharmacol. 2010, 626, 49–56. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Chen, F.; Zhou, C.; Zhuang, C.; Shao, S.; Yu, J.; Huang, D.; Chen, B.; Yu, Z. Relationship between NMDA receptor and postoperative fatigue syndrome and its associated central mechanism. Chin. J. Gastrointest. Surg. 2015, 18, 376–381. [Google Scholar]
- Chen, L.-F.; Yang, C.-H.; Lin, T.-Y.; Pao, P.-J.; Chu, K.C.-W.; Hsu, C.-W.; Bai, C.-H.; Du, M.-H.; Hsu, Y.-P. Effect of magnesium sulfate on renal colic pain. Medicine 2020, 99, e23279. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Pickering, G.; Giacomoni, E.; Cazzaniga, A.; Pellegrino, P. Headaches and Magnesium: Mechanisms, Bioavailability, Therapeutic Efficacy and Potential Advantage of Magnesium Pidolate. Nutrients 2020, 12, 2660. [Google Scholar] [CrossRef]
- Park, R.; Ho, A.M.-H.; Pickering, G.; Arendt-Nielsen, L.; Mohiuddin, M.; Gilron, I. Efficacy and Safety of Magnesium for the Management of Chronic Pain in Adults: A Systematic Review. Anesthesia Analg. 2020, 131, 764–775. [Google Scholar] [CrossRef]
- Choi, H.; Parmar, N. The use of intravenous magnesium sulphate for acute migraine. Eur. J. Emerg. Med. 2013, 21, 2–9. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Yeh, T.-H.; Huang, Y.-C.; Chen, P.-Y. Effects of Intravenous and Oral Magnesium on Reducing Migraine: A Meta-analysis of Randomized Controlled Trials. Pain Physician 2016, 19, 97–112. [Google Scholar]
- Shi, L.; Zhu, H.; Ma, J.; Shi, L.L.; Gao, F.; Sun, W. Intra-articular magnesium to alleviate postoperative pain after arthroscopic knee surgery: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Gupta, R.; Verma, D. Evaluation of a single-dose of intravenous magnesium sulphate for prevention of postoperative pain after inguinal surgery. Indian J. Anaesth. 2011, 55, 31–35. [Google Scholar] [CrossRef]
- Haryalchi, K.; Abedinzade, M.; Khanaki, K.; Ghanaie, M.M.; Zadeh, F.M. Whether preventive low dose magnesium sulphate infusion has an influence on postoperative pain perception and the level of serum beta-endorphin throughout the total abdominal hysterectomy. Rev. Esp. Anestesiol. Reanim. Engl. Ed. 2017, 64, 384–390. [Google Scholar] [CrossRef]
- Dabbagh, A.; Elyasi, H.; Razavi, S.S.; Fathi, M.; Rajaei, S. Intravenous magnesium sulfate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol. Scand. 2009, 53, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Kocman, I.B.; Krobot, R.; Premuzić, J.; Kocman, I.; Stare, R.; Katalinić, L.; Basić-Jukić, N. The effect of preemptive intravenous low-dose magnesium sulfate on early postoperative pain after laparoscopic cholecystectomy. Acta Clin. Croat. 2013, 52, 289–294. [Google Scholar] [PubMed]
- Levaux, C.; Bonhomme, V.; Dewandre, P.Y.; Brichant, J.F.; Hans, P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia 2003, 58, 131–135. [Google Scholar] [CrossRef]
- Demiroglu, M.; Ün, C.; Ornek, D.H.; Kıcı, O.; Yıldırım, A.E.; Horasanlı, E.; Başkan, S.; Fikir, E.; Gamli, M.; Dikmen, B. The Effect of Systemic and Regional Use of Magnesium Sulfate on Postoperative Tramadol Consumption in Lumbar Disc Surgery. BioMed Res. Int. 2016, 2016, 1–5. [Google Scholar] [CrossRef][Green Version]
- Gucyetmez, B.; Atalan, H.; Aslan, S.; Yazar, S.; Polat, K. Effects of Intraoperative Magnesium Sulfate Administration on Postoperative Tramadol Requirement in Liver Transplantation: A Prospective, Double-Blind Study. Transplant. Proc. 2016, 48, 2742–2746. [Google Scholar] [CrossRef] [PubMed]
- Tauzin-Fin, P.; Sesay, M.; Delort-Laval, S.; Krol-Houdek, M.C.; Maurette, P. Intravenous magnesium sulphate decreases postoperative tramadol requirement after radical prostatectomy*. Eur. J. Anaesthesiol. 2006, 23, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Mireskandari, S.M.; Pestei, K.; Hajipour, A.; Jafarzadeh, A.; Samadi, S.; Nabavian, O. Effects of Preoperative Magnesium Sulphate on Post-Cesarean Pain, A Placebo Controlled Double Blind Study. J. Fam. Reprod. Health 2015, 9, 29–33. [Google Scholar]
- Arora, M.K.; Muthiah, T.; Trikha, A.; Sunder, A.R.; Prasad, G.; Singh, P.M. Efficacy of magnesium as an adjuvant to bupivacaine in 3-in-1 nerve block for arthroscopic anterior cruciate ligament repair. Indian J. Anaesth. 2016, 60, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, F.; Eroglu, A. The Effect of Intravenous Magnesium Sulfate Infusion on Sensory Spinal Block and Postoperative Pain Score in Abdominal Hysterectomy. BioMed Res. Int. 2014, 2014, 1–5. [Google Scholar] [CrossRef][Green Version]
- Schulz-Stubner, S.; Wettmann, G.; Reyle-Hahn, S.M.; Rossaint, R. Magnesium as part of balanced general anaesthesia with propofol, remifentanil and mivacurium: A double-blind, randomized prospective study in 50 patients. Eur. J. Anaesthesiol. 2001, 18, 723–729. [Google Scholar] [CrossRef]
- Taheri, A.; Haryalchi, K.; Ghanaie, M.M.; Arejan, N.H. Effect of Low-Dose (Single-Dose) Magnesium Sulfate on Postoperative Analgesia in Hysterectomy Patients Receiving Balanced General Anesthesia. Anesthesiol. Res. Pract. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Shin, C.S.; Lee, Y.C.; Lee, H.S.; Ban, M.; Kim, S.Y. Beneficial effect of intravenous magnesium during endoscopic submucosal dissection for gastric neoplasm. Surg. Endosc. 2015, 29, 3795–3802. [Google Scholar] [CrossRef] [PubMed]
- Mentes, O.; Harlak, A.; Yigit, T.; Balkan, A.; Balkan, M.; Cosar, A.; Savaser, A.; Kozak, O.; Tufan, T. Effect of intraoperative magnesium sulphate infusion on pain relief after laparoscopic cholecystectomy. Acta Anaesthesiol. Scand. 2008, 52, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Walia, C.; Gupta, R.; Kaur, M.; Mahajan, L.; Kaur, G.; Kaur, B. Propofol sparing effect of dexmedetomidine and magnesium sulfate during BIS targeted anesthesia: A prospective, randomized, placebo controlled trial. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 335–340. [Google Scholar]
- Saadawy, I.M.; Kaki, A.M.; El Latif, A.A.A.; Abd-Elmaksoud, A.M.; Tolba, O.M. Lidocaine vs. magnesium: Effect on analgesia after a laparoscopic cholecystectomy. Acta Anaesthesiol. Scand. 2010, 54, 549–556. [Google Scholar] [CrossRef]
- Ko, S.-H.; Lim, H.-R.; Kim, D.-C.; Han, Y.-J.; Choe, H.; Song, H.-S. Magnesium Sulfate Does Not Reduce Postoperative Analgesic Requirements. Anesthesiology 2001, 95, 640–646. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Na, H.-S.; Jeon, Y.-T.; Ro, Y.-J.; Kim, C.-S.; Do, S.-H.I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br. J. Anaesth. 2010, 104, 89–93. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kang, M.-H.; Park, K.-S.; Do, S.-H. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. Br. J. Anaesth. 2008, 100, 397–403. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, B.W.; Kim, B.G.; Oh, A.Y.; Kim, H.H.; Park, D.J.; Lee, C.M.; Kim, S.T.; Do, S.H. Prospective, randomized and controlled trial on magnesium sulfate administration during laparoscopic gastrectomy: Effects on surgical space conditions and recovery profiles. Surg. Endosc. 2016, 30, 4976–4984. [Google Scholar] [CrossRef]
- Bhatia, A.; Kashyap, L.; Pawar, D.K.; Trikha, A. Effect of intraoperative magnesium infusion on perioperative analgesia in open cholecystectomy. J. Clin. Anesth. 2004, 16, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Kim, E.-Y.; Na, H.-S.; Kim, T.K.; Kim, M.-H.; Do, S.-H. Magnesium sulphate attenuates acute postoperative pain and increased pain intensity after surgical injury in staged bilateral total knee arthroplasty: A randomized, double-blinded, placebo-controlled trial. Br. J. Anaesth. 2016, 117, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.-M.; Jheon, S.-H.; Nam, S.; Do, S.-H. Magnesium sulphate improves pulmonary function after video-assisted thoracoscopic surgery. Eur. J. Anaesthesiol. 2017, 34, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Jaoua, H.; Zghidi, S.M.; Wissem, L.; Laassili, S.; Ammar, N.; Ali, J.; Darmoul, S.; Askri, A.; Khelifi, S.; Ben Maamer, A.; et al. Effectiveness of intravenous magnesium on postoperative pain after abdominal surgery versus placebo: Double blind randomized controlled trial. Tunis. Med. 2010, 88, 317–323. [Google Scholar]
- Kumar, M.; Dayal, N.; Rautela, R.S.; Sethi, A.K. Effect of intravenous magnesium sulphate on postoperative pain following spinal anesthesia. A randomized double blind controlled study. Middle East J. Anaesthesiol. 2013, 22, 251–256. [Google Scholar]
- Asadollah, S.; Vahdat, M.; Yazdkhasti, P.; Nikravan, N. The effect of magnesium sulphate on postoperative analgesia requirements in gynecological surgeries. J. Turk. Soc. Obstet. Gynecol. 2015, 12, 34–37. [Google Scholar] [CrossRef]
- Khafagy, H.F.; Ebied, R.S.; Osman, E.S.; Ali, M.Z.; Samhan, Y.M. Perioperative effects of various anesthetic adjuvants with TIVA guided by bispectral index. Korean J. Anesthesiol. 2012, 63, 113–119. [Google Scholar] [CrossRef][Green Version]
- Ayoglu, H.; Karadeniz, U.; Kunduracilar, Z.; Ayoglu, F.N.; Erdemli, O. The analgesic effect of magnesium sulfate and ketamine in patients undergoing laparoscopic cholecystectomy. Pain Clin. 2005, 17, 45–53. [Google Scholar] [CrossRef]
- Koinig, H.; Wallner, T.; Marhofer, P.; Andel, H.; Hörauf, K.; Mayer, N. Magnesium Sulfate Reduces Intra- and Postoperative Analgesic Requirements. Anesth. Analg. 1998, 87, 206–210. [Google Scholar] [CrossRef]
- Çizmeci, P.; Ozkose, Z. Magnesium Sulphate as an Adjuvant to Total Intravenous Anesthesia in Septorhinoplasty: A Randomized Controlled Study. Aesthetic Plast. Surg. 2007, 31, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Benhaj, A.M.; Barakette, M.; Dhahri, S.; Ouezini, R.; Lamine, K.; Jebali, A.; Ferjani, M. Effect of intra and postoperative magnesium sulphate infusion on postoperative pain. Tunis Med. 2008, 86, 550–555. [Google Scholar]
- Seyhan, T.; Tuğrul, M.; Sungur, M.; Kayacan, S.; Telci, L.; Pembeci, K.; Akpir, K. Effects of three different dose regimens of magnesium on propofol requirements, haemodynamic variables and postoperative pain relief in gynaecological surgery. Br. J. Anaesth. 2005, 96, 247–252. [Google Scholar] [CrossRef]
- Frassanito, L.; Messina, A.; Vergari, A.; Colombo, D.; Chierichini, A.; Della Corte, F.; Navalesi, P.; Antonelli, M. Intravenous infusion of magnesium sulfate and postoperative analgesia in total knee arthroplasty. Minerva Anestesiol. 2015, 81, 1184–1191. [Google Scholar]
- Olgun, B.; Oğuz, G.; Kaya, M.; Şavlı, S.; Eskiçırak, H.E.; Güney, I.; Kadıoğulları, N. The effects of magnesium sulphate on desflurane requirement, early recovery and postoperative analgesia in laparascopic cholecystectomy. Magnes. Res. 2012, 25, 72–78. [Google Scholar] [CrossRef]
- Kizilcik, N.; Köner, Ö. Magnesium Sulfate Reduced Opioid Consumption in Obese Patients Undergoing Sleeve Gastrectomy: A Prospective, Randomized Clinical Trial. Obes. Surg. 2018, 28, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Oguzhan, N.; Gunday, I.; Turan, A. Effect of magnesium sulfate infusion on sevoflurane consumption, hemodynamics, and perioperative opioid consumption in lumbar disc surgery. J. Opioid Manag. 2008, 4, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zarauza, R.; Sáez-Fernández, A.N.; Iribarren, M.J.; Carrascosa, F.; Adame, M.; Fidalgo, I.; Monedero, P. A Comparative Study with Oral Nifedipine, Intravenous Nimodipine, and Magnesium Sulfate in Postoperative Analgesia. Anesth. Analg. 2000, 91, 938–943. [Google Scholar] [CrossRef] [PubMed]
- El Shal, S.M.; Lotfy, E. Evaluation of effect of intravenous Magnesium Sulfate infusion on tourniquet induced hypertension and pain in arthroscopic knee surgery patients under epidural anesthesia. Egypt. J. Anaesth. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Vicković, S.; Pjević, M.; Uvelin, A.; Pap, D.; Nikolić, D.; Lalić, I. Magnesium Sulfate as an Adjuvant to Anesthesia in Patients with Arterial Hypertension. Acta Clin. Croat. 2016, 55, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Lee, Y.-W.; Yoon, K.B.; Park, S.J.; Shim, Y.H. Magnesium Sulfate Prevents Remifentanil-Induced Postoperative Hyperalgesia in Patients Undergoing Thyroidectomy. Anesth. Analg. 2011, 113, 390–397. [Google Scholar] [CrossRef] [PubMed]
- ElSersy, H.E.; Metyas, M.C.; Elfeky, H.A.; Hassan, A.A. Intraoperative magnesium sulphate decreases agitation and pain in patients undergoing functional endoscopic surgery. Eur. J. Anaesthesiol. 2017, 34, 658–664. [Google Scholar] [CrossRef]
- Mavrommati, P.D.; Gabopoulou, Z.T.; Papadimos, C.N.; Petsikopoulos, M.G.; Vrettou, V.A.; Konstantinidou, M.G.; Velmachou, K.G. The perioperative infusion of low doses of magnesium sulfate reduces analgesic requirements in patients undergoing abdominal hernioplasty. Acute Pain 2004, 5, 81–87. [Google Scholar] [CrossRef]
- Kaya, S.; Kararmaz, A.; Gedik, R.; Turhanoğlu, S. Magnesium sulfate reduces postoperative morphine requirement after remifentanil-based anesthesia. Med. Sci. Monit. 2009, 15, PI5–PI9. [Google Scholar]
- Wilder-Smith, O.H.G.; Arendt-Nielsen, L.; Gaumann, D.; Tassonyi, E.; Rifat, K.R. Sensory Changes and Pain After Abdominal Hysterectomy. Anesth. Analg. 1998, 86, 95–101. [Google Scholar] [CrossRef]
- Tsaoui, G.; Nikopoulou, A.; Pezikoglou, I.; Birba, V.; Grosomanidis, V. Implementation of magnesium sulphate as an adjunct to multinodal analgesic approach for perioperative pain control in lumbar laminectomy surgery: A randomised placebo-controlled clinical trial. Clin. Neurol. Neurosurg. 2020, 197, 106091. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Rosado, G.M.; Neto, J.D.S.; Guimarães, G.M.; Ashmawi, H.A. Magnesium sulfate improves postoperative analgesia in laparoscopic gynecologic surgeries: A double-blind randomized controlled trial. J. Clin. Anesth. 2016, 34, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.N.; Dhengle, Y. Magnesium sulfate for postoperative analgesia after surgery under spinal anesthesia. Acta Anaesthesiol. Taiwanica 2016, 54, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Tramèr, M.R.; Glynn, C.J. An Evaluation of a Single Dose of Magnesium to Supplement Analgesia After Ambulatory Surgery: Randomized Controlled Trial. Anesth. Analg. 2007, 104, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Verki, M.M.; Porozan, S.; Motamed, H.; Fahimi, M.A.; Aryan, A. Comparison the analgesic effect of magnesium sulphate and Ketorolac in the treatment of renal colic patients: Double-blind clinical trial study. Am. J. Emerg. Med. 2019, 37, 1033–1036. [Google Scholar] [CrossRef]
- Sadrabad, A.Z.; Abarghouei, S.A.; Rad, R.F.; Salimi, Y. Intravenous magnesium sulfate vs. morphine sulphate in relieving renal colic: A randomised clinical trial. Am. J. Emerg. 2020, 35, S0735–S6757. [Google Scholar]
- El Sayed, Z.M.; Abouzeid, A.E.; El Sood, A.I.A. Evaluating Effectiveness of Intravenous Magnesium Sulfate as a Treatment in Acute Renal Colic Patients Attending Suez Canal University Hospital Emergency Department. Med. J. Cairo Univ. 2019, 87, 4021–4025. [Google Scholar] [CrossRef][Green Version]
- Jokar, A.; Cyrus, A.; Babaei, M.; Taheri, M.; Almasi-Hashiani, A.; Behzadinia, E.; Yazdanbakhsh, A. The Effect of Magnesium Sulfate on Renal Colic Pain Relief; a Randomized Clinical Trial. Emerg. Tehran. Iran 2017, 5, e25. [Google Scholar]
- Majidi, A.; Derakhshani, F. Intravenous Magnesium Sulfate for Pain Management in Patients with Acute Renal Colic; a Randomized Clinical Trial. Arch. Acad. Emerg. Med. 2019, 8, e5. [Google Scholar] [PubMed]
- Baratloo, A.; Mirbaha, S.; Kasmaei, H.D.; Payandemehr, P.; Elmaraezy, A.; Negida, A. Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J. Pain 2017, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kandil, M.; Jaber, S.; Desai, D.; Cruz, S.N.; Lomotan, N.; Ahmad, U.; Cirone, M.; Burkins, J.; McDowell, M. MAGraine: Magnesium compared to conventional therapy for treatment of migraines. Am. J. Emerg. Med. 2021, 39, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.R.; Olson, C.M.; Shuler, K.B.; Gharib, S.F. Intravenous magnesium for acute benign headache in the emergency department: A randomized double-blind placebo-controlled trial. Can. J. Emerg. Med. 2004, 6, 327–332. [Google Scholar] [CrossRef]
- Ginder, S.; Oatman, B.; Pollack, M. A prospective study of i.v. magnesium and i.v. prochlorperazine in the treatment of headaches. J. Emerg. Med. 2000, 18, 311–315. [Google Scholar] [CrossRef]
- Cete, Y.; Dora, B.; Ertan, C.; Ozdemir, C.; Oktay, C. A Randomized Prospective Placebo-Controlled Study of Intravenous Magnesium Sulphate vs. Metoclopramide in the Management of Acute Migraine Attacks in the Emergency Department. Cephalalgia 2005, 25, 199–204. [Google Scholar] [CrossRef]
- Shahrami, A.; Assarzadegan, F.; Hatamabadi, H.R.; Asgarzadeh, M.; Sarehbandi, B.; Asgarzadeh, S. Comparison of Therapeutic Effects of Magnesium Sulfate vs. Dexamethasone/Metoclopramide on Alleviating Acute Migraine Headache. J. Emerg. Med. 2015, 48, 69–76. [Google Scholar] [CrossRef]
- Demirkaya, S.; Vural, O.; Dora, B.; Topcuoglu, M.A. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache J. Head Face Pain 2001, 41, 171–177. [Google Scholar] [CrossRef]
- Corbo, J.; Esses, D.; Bijur, P.E.; Iannaccone, R.; Gallagher, E. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann. Emerg. Med. 2001, 38, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bigal, E.M.; Bordini, C.A.; Tepper, S.J.; Speciali, J.G. Intravenous Magnesium Sulphate in the Acute Treatment of Migraine Without Aura and Migraine with Aura. A Randomized, Double-Blind, Placebo-Controlled Study. Cephalalgia 2002, 22, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.; Sedgwick, P.M.; Hamann, W.; Di Vadi, P.P. Efficacy of intravenous magnesium in neuropathic pain. Br. J. Anaesth. 2002, 89, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.A.; Al-Deeb, A.E. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia 2013, 68, 260–266. [Google Scholar] [CrossRef]
- Karimi, N.; Razian, A.; Heidari, M. The efficacy of magnesium oxide and sodium valproate in prevention of migraine headache: A randomized, controlled, double-blind, crossover study. Acta Neurol. Belg. 2021, 121, 167–173. [Google Scholar] [CrossRef]
- Köseoglu, E.; Talaslioglu, A.; Gönül, A.S.; Kula, M. The effects of magnesium prophylaxis in migraine without aura. Magnes. Res. 2008, 21, 101–108. [Google Scholar]
- Facchinetti, F.; Sances, G.; Borella, P.; Genazzani, A.R.; Nappi, G. Magnesium Prophylaxis of Menstrual Migraine: Effects on Intracellular Magnesium. Headache J. Head Face Pain 1991, 31, 298–301. [Google Scholar] [CrossRef]
- Wang, F.; Eeden, S.K.V.D.; Ackerson, L.M.; Salk, S.E.; Reince, R.H.; Elin, R.J. Oral Magnesium Oxide Prophylaxis of Frequent Migrainous Headache in Children: A Randomized, Double-Blind, Placebo-Controlled Trial. Headache J. Head Face Pain 2003, 43, 601–610. [Google Scholar] [CrossRef]
- Grazzi, L.; Andrasik, F.; Usai, S.; Bussone, G. Magnesium as a preventive treatment for paediatric episodic tension-type headache: Results at 1-year follow-up. Neurol. Sci. 2007, 28, 148–150. [Google Scholar] [CrossRef]
- Esfanjani, A.T.; Mahdavi, R.; Mameghani, M.E.; Talebi, M.; Nikniaz, Z.; Safaiyan, A. The Effects of Magnesium, l-Carnitine, and Concurrent Magnesium–l-Carnitine Supplementation in Migraine Prophylaxis. Biol. Trace Elem. Res. 2012, 150, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Peikert, A.; Wilimzig, C.; Köhne-Volland, R. Prophylaxis of Migraine with Oral Magnesium: Results from A Prospective, Multi-Center, Placebo-Controlled and Double-Blind Randomized Study. Cephalalgia 1996, 16, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pfaffenrath, V.; Wessely, P.; Meyer, C.; Isler, H.R.; Evers, S.; Grotemeyer, K.H.; Taneri, Z.; Soyka, D.; Bel, G.H.; Fischer, M. Magnesium in the Prophylaxis of Migraine—A Double-Blind, Placebo-Controlled Study. Cephalalgia 1996, 16, 436–440. [Google Scholar] [CrossRef]
- Maizels, M.; Blumenfeld, A.; Burchette, R. A Combination of Riboflavin, Magnesium, and Feverfew for Migraine Prophylaxis: A Randomized Trial. Headache J. Head Face Pain 2004, 44, 885–890. [Google Scholar] [CrossRef]
- Van Der Plas, A.A.; Schilder, J.C.; Marinus, J.; Van Hilten, J.J. An Explanatory Study Evaluating the Muscle Relaxant Effects of Intramuscular Magnesium Sulphate for Dystonia in Complex Regional Pain Syndrome. J. Pain 2013, 14, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Morel, V.; Simen, E.; Cardot, J.-M.; Moustafa, F.; Delage, N.; Picard, P.; Eschalier, S.; Boulliau, S.; DuBray, C. Oral magnesium treatment in patients with neuropathic pain: A randomized clinical trial. Magnes. Res. 2011, 24, 28–35. [Google Scholar] [CrossRef]
- Russell, I.J.; Michalek, E.J.; Flechas, J.D.; Abraham, E.G. Treatment of fibromyalgia syndrome with Super Malic: A randomized, double blind, placebo controlled, crossover pilot study. J. Rheumatol. 1995, 22, 953–958. [Google Scholar] [PubMed]
- Bagis, S.; Karabiber, M.; As, I.; Tamer, L.; Erdogan, C.; Atalay, A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol. Int. 2012, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Gueux, E.; Tressol, J.C.; Mazur, A.; Rayssiguier, Y. Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes. Res. 2005, 18, 215–223. [Google Scholar]
- Uysal, N.; Kizildag, S.; Yuce, Z.; Guvendi, G.; Kandis, S.; Koc, B.; Karakilic, A.; Camsari, U.M.; Ates, M. Timeline (Bioavailability) of Magnesium Compounds in Hours: Which Magnesium Compound Works Best? Biol. Trace Elem. Res. 2019, 187, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.S.; Zobitz, M.M.; Poindexter, J.R.; Pak, C.Y. Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr. 1990, 9, 48–55. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes. Res. 2003, 16, 183–191. [Google Scholar]
- Brilli, E.; Khadge, S.; Fabiano, A.; Zambito, Y.; Williams, T.; Tarantino, G. Magnesium bioavailability after administration of sucrosomial® magnesium: Results of an ex-vivo study and a comparative, double-blinded, cross-over study in healthy subjects. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, L.; Vervaet, C.; Derave, W. Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients 2019, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Duale, C.; Cardot, J.-M.; Joanny, F.; Trzeciakiewicz, A.; Martin, E.; Pickering, G.; DuBray, C. An Advanced Formulation of a Magnesium Dietary Supplement Adapted for a Long-Term Use Supplementation Improves Magnesium Bioavailability: In Vitro and Clinical Comparative Studies. Biol. Trace Elem. Res. 2018, 186, 1–8. [Google Scholar] [CrossRef]
- Ranade, V.V.; Somberg, J.C. Bioavailability and Pharmacokinetics of Magnesium after Administration of Magnesium Salts to Humans. Am. J. Ther. 2001, 8, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Magnesium, n.d. Available online: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ (accessed on 27 November 2020).
- Nieves, J.W. Osteoporosis: The role of micronutrients. Am. J. Clin. Nutr. 2005, 81, 1232S–1239S. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef]
- Alfrey, A.C.; Miller, N.L. Bone Magnesium Pools in Uremia. J. Clin. Investig. 1973, 52, 3019–3027. [Google Scholar] [CrossRef]
- Huang, E.P. Synaptic plasticity: Going through phases with LTP. Curr. Biol. 1998, 8, R350–R352. [Google Scholar] [CrossRef]
- Inagaki, T.; Begum, T.; Reza, F.; Horibe, S.; Inaba, M.; Yoshimura, Y.; Komatsu, Y. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci. Res. 2008, 61, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Morel, V.; Joly, D.; Villatte, C.; DuBray, C.; Durando, X.; Daulhac, L.; Coudert, C.; Roux, D.; Pereira, B.; Pickering, G. Memantine before Mastectomy Prevents Post-Surgery Pain: A Randomized, Blinded Clinical Trial in Surgical Patients. PLoS ONE 2016, 11, e0152741. [Google Scholar] [CrossRef]
- Morel, V.; Joly, D.; Villatte, C.; Pereira, B.; Pickering, G. Preventive effect of oral magnesium in postmastectomy pain: Protocol for a randomised, double-blind, controlled clinical trial. BMJ Open 2018, 8, e017986. [Google Scholar] [CrossRef]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Curr. Med Res. Opin. 2003, 20, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, E.; Nolden, C. Langzeit-HRV-Analyse zeigt Stressreduktion durch Magnesiumzufuhr. MMW Fortschr. Med. 2016, 158, 12–16. [Google Scholar] [CrossRef] [PubMed]
| Indications | Authors | n | Mg | Pain Diminution | Analgesics Consumption Diminution | |
|---|---|---|---|---|---|---|
| Bolus | Infusion | |||||
| Post-surgery Pain | [33] | 100 | / | 30 mg/kg * | p < 0.05 | p < 0.05 |
| [34] | 40 | / | 15 mg/kg/h | p = 0.0001 | p = 0.0001 | |
| [35] | 60 | / | 8 mg/kg/h | p < 0.01 | p < 0.01 | |
| [36] | 60 | / | 7.5 mg/kg * | p < 0.05 | p < 0.001 | |
| / | 5 mg/kg | p > 0.05 | p > 0.05 | |||
| [37] | 24 | / | 50 mg/kg–30 min | p < 0.05 | p < 0.05 | |
| [38] | 75 | / | 50 mg/kg–30 min * | p > 0.05 | p < 0.05 | |
| [39] | 70 | / | 50 mg/kg–30 min | ND | p < 0.001 | |
| [40] | 30 | / | 50 mg/kg–20 min | ND | p < 0.001 | |
| [41] | 50 | / | 50 mg/kg–15 min | p < 0.05 | p < 0.001 | |
| [42] | 60 | / | 150 mg * | p > 0.05 | p > 0.05 | |
| [43] | 38 | / | 65 mg/kg | p < 0.001 | ND | |
| [44] | 50 | / | 50 mg/kg | p > 0.05 | p < 0.01 | |
| [45] | 40 | / | 50 mg/kg | p < 0.05 | p = 0.0001 | |
| [46] | 57 | / | 50 mg/kg | p = 0.034 | p = 0.043 | |
| [47] | 83 | / | 50 mg/kg | p < 0.05 | p > 0.05 | |
| [48] | 120 | / | 30 mg/kg | ND | p < 0.001 | |
| [49] | 120 | 50 mg/kg | 25 mg/kg/h | p < 0.05 | p < 0.001 | |
| [50] | 58 | 50 mg/kg | 15 mg/kg/h | p > 0.05 | p > 0.05 | |
| [51] | 40 | 50 mg/kg | 15 mg/kg/h * | p < 0.001 | p < 0.001 | |
| [52] | 50 | 50 mg/kg | 15 mg/kg/h | p = 0.011 | p = 0.005 | |
| [53] | 74 | 50 mg/kg | 15 mg/kg/h | p = 0.009 | ND | |
| [54] | 50 | 50 mg/kg | 15 mg/kg/h | p < 0.05 | p = 0.07 | |
| [55] | 44 | 50 mg/kg | 15 mg/kg/h | p = 0.001 | p = 0.014 | |
| [56] | 62 | 50 mg/kg | 15 mg/kg/h | p > 0.05 | p = 0.042 | |
| [57] | 40 | 50 mg/kg | 10 mg/kg/h | p > 0.05 | p > 0.05 | |
| [58] | 60 | 50 mg/kg | 10 mg/kg/h | p < 0.05 | p < 0.006 | |
| [59] | 30 | 50 mg/kg | 8 mg/kg/h | p < 0.0001 | p < 0.05 | |
| [60] | 120 | 50 mg/kg | 8 mg/kg/h | ND | p < 0.05 | |
| [61] | 60 | 50 mg/kg | 8 mg/kg/h | p > 0.05 | p < 0.05 | |
| [62] | 46 | 50 mg/kg | 8 mg/kg/h | p > 0.05 | p < 0.05 | |
| [63] | 50 | 50 mg/kg | 8 mg/kg/h | p < 0.05 | ND | |
| [64] | 48 | 50 mg/kg | 500 mg/h | p < 0.05 | p = 0.0002 | |
| [65] | 80 | 40 mg/kg | 20 mg/k/h | ND | p < 0.001 | |
| 10 mg/kg/h | ND | p < 0.001 | ||||
| [66] | 40 | 40 mg/kg | 10 mg/kg/h | p > 0.05 | p = 0.52 | |
| [67] | 60 | 40 mg/kg | 10 mg/kg/h | p = 0.024 | p = 0.048 | |
| [68] | 80 | 30 mg/kg | 20 mg/kg/24 h | p = 0.001 | p = 0.001 | |
| [69] | 50 | 30 mg/kg | 10 mg/kg/h * | p < 0.05 | p < 0.05 | |
| [70] | 96 | 30 mg/kg | 10 mg/kg/h | p > 0.05 | p > 0.05 | |
| [71] | 70 | 30 mg/kg | 10 mg/kg/h | p < 0.001 | p < 0.001 | |
| [72] | 100 | 30 mg/kg | 10 mg/kg/h | p = 0.29 | ND | |
| [73] | 84 | 30 mg/kg | 10 mg/kg/h | p > 0.05 | p > 0.05 | |
| [74] | 294 | 30 mg/kg | 9 mg/kg/h | p < 0.0001 | p < 0.0001 | |
| [75] | 42 | 30 mg/kg | 6 mg/kg/h | p > 0.05 | p < 0.05 | |
| [76] | 40 | 30 mg/kg | 500 mg/h | p < 0.05 | p < 0.05 | |
| [77] | 45 | 20 mg/kg | 10 mg/kg–30 min * vs. fentanyl and ketamine | p > 0.05 | p > 0.05 | |
| [78] | 74 | 20 mg/kg | 20 mg/kg/h | p = 0.005 | p = 0.001 | |
| [79] | 36 | 20 mg/kg | 2 mg/kg/h | p < 0.01 | p = 0.001 | |
| [80] | 108 | 250 mg | 20 mg/kg/h | p = 0.001 | p = 0.033 | |
| [81] | 200 | 4 g | / | p > 0.05 | p > 0.05 | |
| Renal Pain | [82] | 87 | / | 50 mg/kg | p = 0.232 | ND |
| [83] | 80 | / | 50 mg/kg–20 min vs. morphine | p > 0.05 | ND | |
| [84] | 96 | / | 15 mg/kg–15 min vs standard treatment | p < 0.05 | ND | |
| [85] | 100 | / | 15 mg/kg–15 min | p = 0.001 | p = 0.043 | |
| [86] | 90 | / | 2 cc–15 min vs morphine | p = 0.799 | ND | |
| Migraine | [87] | 70 | / | 2 g–10 min * vs. caffeine | p < 0.05 | ND |
| [88] | 157 | / | 2 g–20 min vs. prochlorperazine/ metoclopramide | p > 0.05 | p > 0.05 | |
| [89] | 42 | / | 2 g–10 min | p = 0.63 | ND | |
| [90] | 36 | / | 2 g–10 min vs. prochlorperazine | p > 0.05 | p > 0.05 | |
| [91] | 113 | / | 2 g–10 min | p > 0.05 | p < 0.05 | |
| [92] | 70 | / | 1 g–15 min vs. dexamethasone/ metoclopramide | p < 0.0001 | ND | |
| [93] | 30 | / | 1 g–15 min * | p < 0.0001 | ND | |
| [94] | 44 | 2 g | / | p > 0.05 | p > 0.05 | |
| [95] | 60 | 1 g | / | p < 0.05 | p < 0.05 | |
| Chronic Pain | [96] | 7 | / | 30 mg/kg–30 min; CrO | p = 0.016 | ND |
| [15] | 60 | / | 3 g–30 min; CrO | p = 0.296 | ND | |
| [97] | 80 | / | 1 g–4 h | p = 0.034 | ND | |
| [18] | 10 | 0.16 mmol/kg | 0.16 mmol/kg/h | p = 0.084 | ND | |
| Authors | n | Type of Study | Inorganic Mg Salts | Organic Mg Salts | Combination of Mg Salts | Conclusions |
|---|---|---|---|---|---|---|
| [113] | 17 | P | Mg oxide (60% Mg element: 15 mmol) | Mg citrate (16% Mg element: 4 mmol) | / | Mg citrate is more soluble than Mg Oxide in water (55% vs. 0.8%, p < 0.05), less ph-dependent with lesser ionic concentrations. |
| [114] | 46 | DBP | Mg oxide (60% Mg element: 180 mg) | Mg citrate (16% Mg element: 48 mg); Mg amino-acid chelate: 300 mg (% Mg element: ND) | / | Mg citrate then amino-acid chelate are more bioavailable than Mg oxide (p < 0.02). |
| [115] | 10 | DBCrO | Mg oxide (60% Mg element: 210 mg)/Mg oxide with a sucrester matrix (210 mg) | Mg citrate (16% mg element: 56 mg); Mg bisglycinate (20% Mg element: 70 mg) | / | Mg oxide with a sucrester matrix has a higher Mg bioavailability (p < 0.05). |
| [117] | 20 | DBCrO | Mg oxide (60% Mg element: 241.3, 300, 400, 450, 500 mg); Mg carbonate (40% Mg element: 100 mg); Mg chloride (12% Mg element: 71.5 mg) | Mg citrate (16% Mg element: 19 mg; 100 and 200 mg) | Mg oxide (60% Mg element: 149 mg) + glycerophosphate (12.37% Mg element: 47 mg); Mg citrate (16% Mg element) + Mg bis hydrogen-L-glutamate (Mg element: ND): 40 mg; Mg orotate dihydrate: 32.8 mg (% mg element: ND); Mg glycinate lysinate chelate (20% Mg element: 100 mg) | Higher bioavailability when Mg oxide is combined (p < 0.005) |
| [116] | 20 | CrO | Mg chloride with a novel matrix: 100 mg Mg element) vs. Mg carbonate (3 × 100 mg Mg element) | / | / | Mg chloride with a novel matrix has a better bioavailability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morel, V.; Pickering, M.-E.; Goubayon, J.; Djobo, M.; Macian, N.; Pickering, G. Magnesium for Pain Treatment in 2021? State of the Art. Nutrients 2021, 13, 1397. https://doi.org/10.3390/nu13051397
Morel V, Pickering M-E, Goubayon J, Djobo M, Macian N, Pickering G. Magnesium for Pain Treatment in 2021? State of the Art. Nutrients. 2021; 13(5):1397. https://doi.org/10.3390/nu13051397
Chicago/Turabian StyleMorel, Véronique, Marie-Eva Pickering, Jonathan Goubayon, Marguérite Djobo, Nicolas Macian, and Gisèle Pickering. 2021. "Magnesium for Pain Treatment in 2021? State of the Art" Nutrients 13, no. 5: 1397. https://doi.org/10.3390/nu13051397
APA StyleMorel, V., Pickering, M.-E., Goubayon, J., Djobo, M., Macian, N., & Pickering, G. (2021). Magnesium for Pain Treatment in 2021? State of the Art. Nutrients, 13(5), 1397. https://doi.org/10.3390/nu13051397






