Using Oral Microbiota Data to Design a Short Sucrose Intake Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Recording of Food Intake and Food Preferences
2.3. Recording of Lifestyle Factors
2.4. Microbiota Analysis
2.5. Prediction of Oral Microbiota Functions from the 16S rRNA Gene Sequences
2.6. Replication Cohort
2.7. Caries Scoring
2.8. Ethical Approval
2.9. Data Handling and Statistical Analysis
3. Results
3.1. Derivation Cohort and Oral Microbiota Characteristics
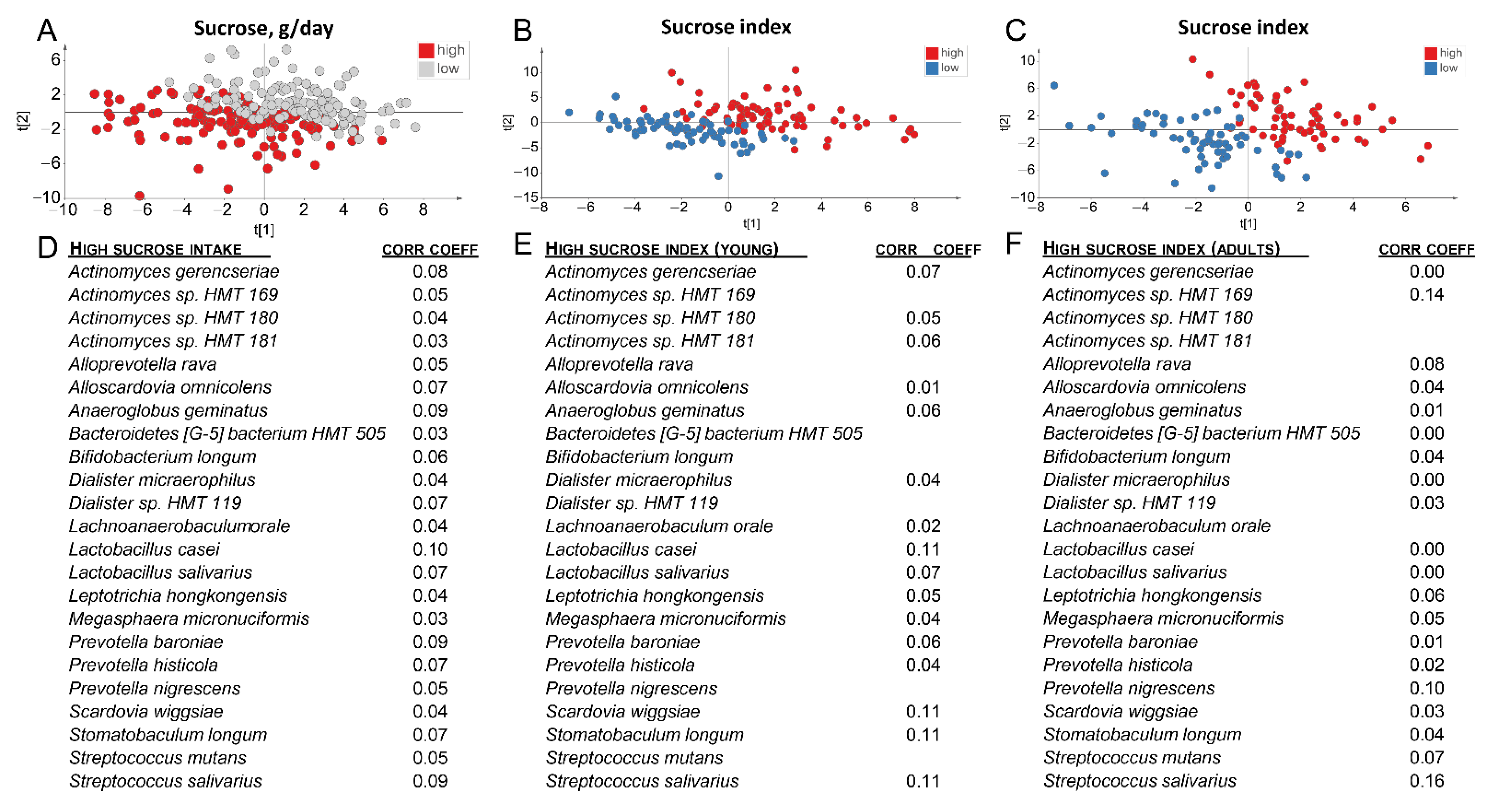
3.2. Predicted Function of the Species Profile in Cluster 4 Versus 2
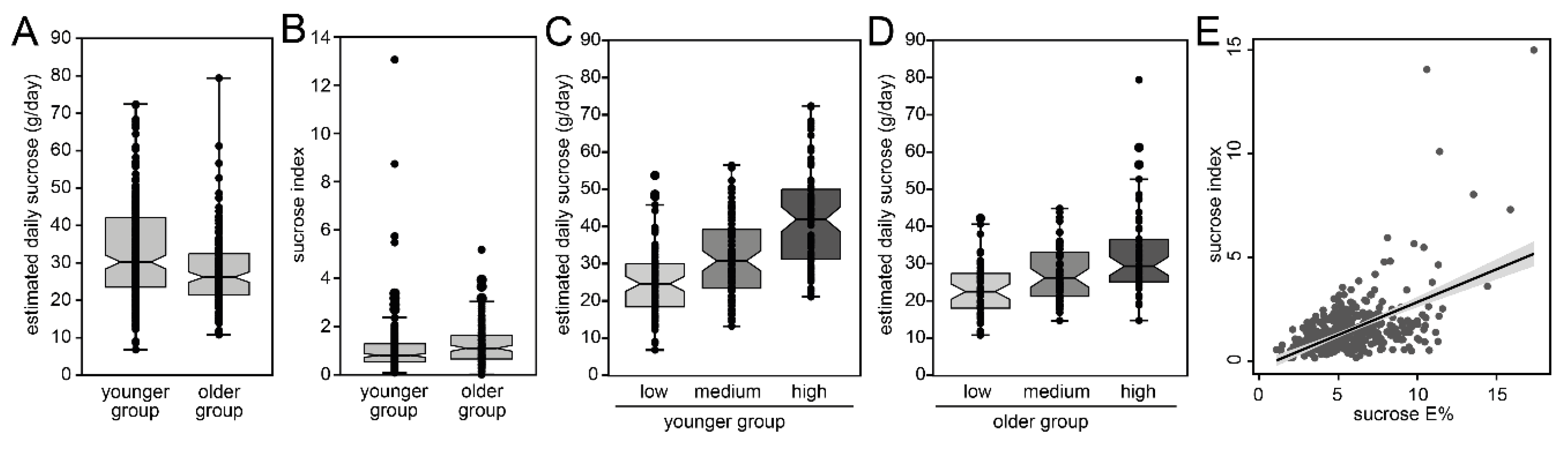
3.3. Discovery of a Short Index for Sucrose
3.4. Evaluation of the Sucrose Index in an Independent Adult Replication Cohort
3.5. Sucrose Score and Caries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Hills, J.R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2020, 1–11. [Google Scholar] [CrossRef]
- Cheng, Z.; Do, T.; Mankia, K.; Meade, J.; Hunt, L.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Emery, P.; Devine, D. Dysbiosis in the oral microbiomes of anti-CCP positive individuals at risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological perspectives. J. Dent. Res. 2010, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Genet. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P. The role of dietary sugars in health: Molecular composition or just calories? Eur. J. Clin. Nutr. 2019, 73, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.J.; Gray, S.R.; Welsh, P.; Mackay, D.F.; Celis-Morales, C.A.; Lyall, D.M.; Forbes, J.; Sattar, N.; Gill, J.M.R.; Pell, J.P. The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: A prospective cohort study. BMC Med. 2020, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ramne, S.; Dias, J.A.; González-Padilla, E.; Olsson, K.; Lindahl, B.; Engström, G.; Ericson, U.; Johansson, I.; Sonestedt, E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population–based prospective cohorts. Am. J. Clin. Nutr. 2018, 109, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- Amoutzopoulos, B.; Steer, T.; Roberts, C.; Collins, D.; Page, P. Free and Added Sugar Consumption and Adherence to Guidelines: The UK National Diet and Nutrition Survey (2014/15–2015/16). Nutrients 2020, 12, 393. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Louie, J.C.Y. Objective Biomarkers for Total Added Sugar Intake—Are We on a Wild Goose Chase? Adv. Nutr. 2020, 11, 1429–1436. [Google Scholar] [CrossRef]
- Esberg, A.; Haworth, S.; Hasslöf, P.; Holgerson, P.L.; Johansson, I. Oral Microbiota Profile Associates with Sugar Intake and Taste Preference Genes. Nutrients 2020, 12, 681. [Google Scholar] [CrossRef] [Green Version]
- Johansson, I.; Hallmans, G.; Wikman, Å.; Riboli, E.; Kaaks, R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Esberg, A.; Haworth, S.; Holgerson, P.L.; Johansson, I. Allelic Variation in Taste Genes Is Associated with Taste and Diet Preferences and Dental Caries. Nutrients 2019, 11, 1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010, 2010, baq013. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Norberg, M.; Wall, S.; Boman, K.; Weinehall, L. The Vasterbotten Intervention Programme: Background, design and im-plications. Glob. Health Action 2010, 3. [Google Scholar] [CrossRef]
- Weinehall, C.-G.H.L. Reduction of selection bias in primary prevention of cardiovascular disease through involvement of primary health care. Scand. J. Prim. Health Care 1998, 16, 171–176. [Google Scholar] [CrossRef]
- Akesson, M.L.; Warnberg Gerdin, E.; Soderstrom, U.; Lindahl, B.; Johansson, I. Health-related quality of life and prospective caries development. BMC Oral Health 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. S1), 5–41. [Google Scholar]
- Johansson, I.; Esberg, A.; Nilsson, L.M.; Jansson, J.-H.; Wennberg, P.; Winkvist, A. Dairy Product Intake and Cardiometabolic Diseases in Northern Sweden: A 33-Year Prospective Cohort Study. Nutrients 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Ö.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, X.; Jiang, W.; Wang, S.; Xu, L.; Tu, Y.; Zheng, P.; Wang, Y.; Lin, X.; et al. Profiling of Oral Microbiota in Early Childhood Caries Using Single-Molecule Real-Time Sequencing. Front. Microbiol. 2017, 8, 2244. [Google Scholar] [CrossRef] [PubMed]
- Svensäter, G.; Larsson, U.-B.; Greif, E.C.G.; Cvitkovitch, D.G.; Hamilton, I.R. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 1997, 12, 266–273. [Google Scholar] [CrossRef]
- Kameda, M.; Abiko, Y.; Washio, J.; Tanner, A.C.R.; Kressirer, C.A.; Mizoguchi, I.; Takahashi, N. Sugar Metabolism of Scardovia wiggsiae, a Novel Caries-Associated Bacterium. Front. Microbiol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Nyvad, B.; Takahashi, N. Integrated hypothesis of dental caries and periodontal diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [Green Version]
- Ramne, S.; Brunkwall, L.; Ericson, U.; Gray, N.; Kuhnle, G.G.C.; Nilsson, P.M.; Orho-Melander, M.; Sonestedt, E. Gut microbiota composition in relation to intake of added sugar, sugar-sweetened beverages and artificially sweetened beverages in the Malmo Offspring Study. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Anderson, C.A.; Curzon, M.E.; Van Loveren, C.; Tatsi, C.; Duggal, M.S. Sucrose and dental caries: A review of the evidence. Obes. Rev. 2009, 10 (Suppl. 1), 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.I.; Weinert, C.H.; Egert, B.; Ferrario, P.G.; Bub, A.; Hoffmann, I.; Watzl, B.; Daniel, H.; Kulling, S.E. The complex human urinary sugar profile: Determinants revealed in the cross-sectional KarMeN study. Am. J. Clin. Nutr. 2018, 108, 502–516. [Google Scholar] [CrossRef]
- Mack, C.I.; Ferrario, P.G.; Weinert, C.H.; Egert, B.; Hoefle, A.S.; Lee, Y.-M.; Skurk, T.; Kulling, S.E.; Daniel, H. Exploring the Diversity of Sugar Compounds in Healthy, Prediabetic, and Diabetic Volunteers. Mol. Nutr. Food Res. 2020, 64, e1901190. [Google Scholar] [CrossRef] [PubMed]
- Ramne, S.; Gray, N.; Hellstrand, S.; Brunkwall, L.; Enhörning, S.; Nilsson, P.M.; Engström, G.; Orho-Melander, M.; Ericson, U.; Kuhnle, G.G.C.; et al. Comparing Self-Reported Sugar Intake with the Sucrose and Fructose Biomarker From Overnight Urine Samples in Relation to Cardiometabolic Risk Factors. Front. Nutr. 2020, 7, 62. [Google Scholar] [CrossRef]
- Shanita, N.S.; Norimah, A.K.; Abu Hanifah, S. Development and validation of a Food Frequency Questionnaire (FFQ) for assessing sugar consumption among adults in Klang Valley, Malaysia. Malays. J. Nutr. 2012, 18, 283–293. [Google Scholar]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is Sweet Taste Perception Associated with Sweet Food Liking and Intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashi, H.; Campus, G.; Forslund, H.B.; Hafiz, W.; Ahmed, N.; Lingström, P. The Influence of Sweet Taste Perception on Dietary Intake in Relation to Dental Caries and BMI in Saudi Arabian Schoolchildren. Int. J. Dent. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Johnson, S.T.; Oldman, S.M.; Duffy, V.B. Pediatric Adapted Liking Survey: A Novel, Feasible and Reliable Dietary Screening in Clinical Practice. Caries Res. 2019, 53, 153–159. [Google Scholar] [CrossRef]
- Opal, S.; Garg, S.; Jain, J.; Walia, I. Genetic factors affecting dental caries risk. Aust. Dent. J. 2015, 60, 2–11. [Google Scholar] [CrossRef]
- Vieira, K.A.; Rosa-Júnior, L.S.; Souza, M.A.V.; Santos, N.B.; Florêncio, T.M.M.T.; Bussadori, S.K. Chronic malnutrition and oral health status in children aged 1 to 5 years. Medicine 2020, 99, e19595. [Google Scholar] [CrossRef]
- Johnston, L.; Vieira, A.R. Caries experience and overall health status. Oral Health Prev. Dent. 2014, 12, 163–170. [Google Scholar]
- Haworth, S.; Esberg, A.; Holgerson, P.L.; Kuja-Halkola, R.; Timpson, N.; Magnusson, P.; Franks, P.; Johansson, I. Heritability of Caries Scores, Trajectories, and Disease Subtypes. J. Dent. Res. 2020, 99, 264–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divaris, K. The Era of the Genome and Dental Medicine. J. Dent. Res. 2019, 98, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, M.N.; Schoeller, D.A. Traditional Self-Reported Dietary Instruments Are Prone to Inaccuracies and New Ap-proaches Are Needed. Front. Nutr. 2020, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Landry, D.; Little, J.; Minelli, C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med Res. Methodol. 2017, 17, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leme, A.F.P.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The Role of Sucrose in Cariogenic Dental Biofilm Formation—New Insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | All Study | Clusters Groups | |||||
|---|---|---|---|---|---|---|---|
| Participants | C1 | C2 | C3 | C4 | C5 | pgroups | |
| Number 1 | 427 1 | 54 | 179 | 45 | 40 | 98 | |
| Sex, % females | 61.6 | 61.1 | 64.2 | 71.1 | 45.0 | 60.2 | 0.136 |
| Age, years, mean (SD) | 29.6 (15.9) | 31.7 (17.9) | 33.1 (16.9) | 31.5 (14.6) | 32.4 (17.2) | 18.6 (1.0) | <0.001 |
| BMI 2, kg/m2, mean (SD) | 23.1 (3.2) | 23.1 (3.5) | 22.7 (3.0) | 23.2 (2.7) | 23.8 (3.5) | 23.7 (3.4) | 0.081 |
| Overweight, % with BMI ≥ 25 | 24.3 | 26.9 | 19.2 | 26.2 | 42.5 | 24.0 | 0.042 |
| Current or past smoker, % | 6.4 | 7.7 | 6.5 | 2.4 | 10.0 | 6.2 | 0.588 |
| Current or past snuff user, % | 12.9 | 15.4 | 9.4 | 16.7 | 17.5 | 13.6 | 0.732 |
| Education 3, % highest level for age | 46.6 | 45.1 | 53.0 | 50.0 | 35.9 | 46.7 | 0.378 |
| Diabetes in close family, % | 13.3 | 11.5 | 18.2 | 19.0 | 10.3 | 2.1 | 0.003 |
| Physically inactive, % | 33.4 | 46.2 | 28.1 | 26.2 | 32.5 | 39.6 | 0.071 |
| Tooth brushing daily, % | 85.9 | 82.7 | 97.6 | 100.0 | 85.0 | 76.8 | <0.001 |
| Gum bleeding on brushing, % | 18.7 | 26.9 | 11.8 | 21.4 | 27.5 | 25.0 | 0.034 |
| Protein 2, g/day, mean (SD) | 83 (19) | 81 (21) | 83 (17) | 84 (24) | 79 (16) | 83 (21) | 0.517 |
| Fat 2, g/day, mean (SD) | 106 (22) | 109 (22) | 108 (23) | 103 (21) | 106 (23) | 102 (20) | 0.063 |
| Carbohydrates 2, g/day, mean (SD) | 217 (40) | 213 (38) | 213 (41) | 215 (33) | 224 (43) | 223 (40) | 0.175 |
| Sucrose 2, g/day, mean (SD) | 31 (12) | 31.1 (14) | 29.7 (13) | 30.2 (9) | 36.9 (14) | 31.3 (11) | 0.028 |
| Characteristics | Replication Cohort |
|---|---|
| Number 1 | 105,520 |
| Screening period | 1991–2016 |
| Sex, % females | 51.0 |
| Age, years (mean (SD)) | 46.6 (9.0) |
| BMI 2, kg/m2 (mean (SD)) | 26.0 (4.3) |
| Overweight, % with BMI ≥ 25 | 53.8 |
| University, % | 29.9 |
| Diabetes in close family, % | 20.0 |
| Physically inactive, % | 64.1 |
| Protein 2, g/day (mean (SD)) | 83.1 (15.1) |
| Fat 2, g/day (mean (SD)) | 88.5 (22.2) |
| Carbohydrates 2, g/day (mean (SD)) | 263.2 (45.6) |
| Sucrose 2, g/day (mean (SD)) | 34.0 (16.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esberg, A.; Eriksson, L.; Hasslöf, P.; Haworth, S.; Holgerson, P.L.; Johansson, I. Using Oral Microbiota Data to Design a Short Sucrose Intake Index. Nutrients 2021, 13, 1400. https://doi.org/10.3390/nu13051400
Esberg A, Eriksson L, Hasslöf P, Haworth S, Holgerson PL, Johansson I. Using Oral Microbiota Data to Design a Short Sucrose Intake Index. Nutrients. 2021; 13(5):1400. https://doi.org/10.3390/nu13051400
Chicago/Turabian StyleEsberg, Anders, Linda Eriksson, Pamela Hasslöf, Simon Haworth, Pernilla Lif Holgerson, and Ingegerd Johansson. 2021. "Using Oral Microbiota Data to Design a Short Sucrose Intake Index" Nutrients 13, no. 5: 1400. https://doi.org/10.3390/nu13051400
APA StyleEsberg, A., Eriksson, L., Hasslöf, P., Haworth, S., Holgerson, P. L., & Johansson, I. (2021). Using Oral Microbiota Data to Design a Short Sucrose Intake Index. Nutrients, 13(5), 1400. https://doi.org/10.3390/nu13051400






