Anti-Inflammatory and Anti-Oxidative Synergistic Effect of Vitamin D and Nutritional Complex on Retinal Pigment Epithelial and Endothelial Cell Lines against Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stable Cell Line Phenotypic Characterization
2.3. Experimental Design
2.3.1. Oxidative Stress and Inflammatory-Like Conditions
2.3.2. Treatments with the Nutritional Antioxidant Complex (Nutrof Total®) and Vitamin D
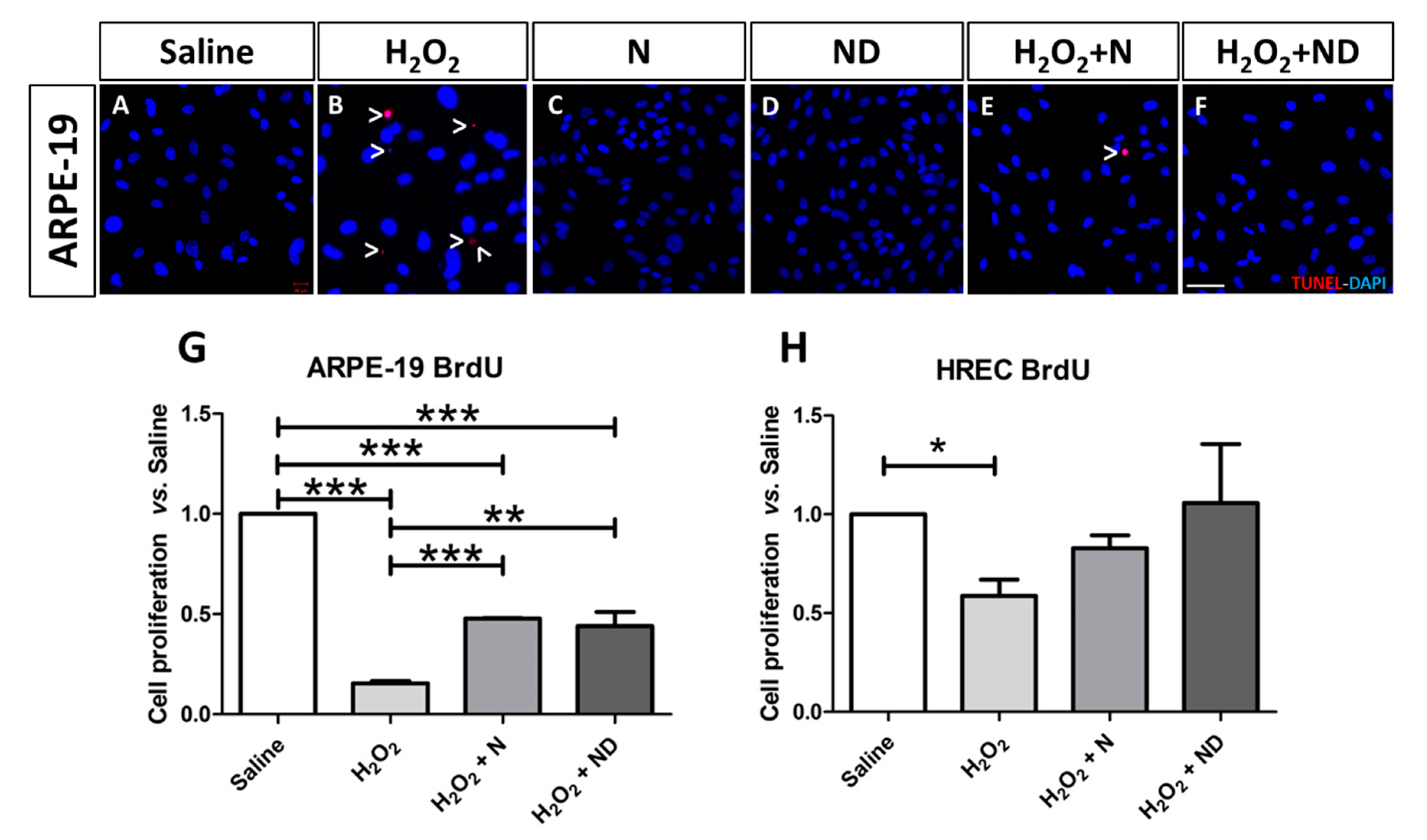
2.4. Cell Viability/Cytotoxicity Assay (MTT), and Proliferation Assay (Bromodeoxyuridine, BrdU)
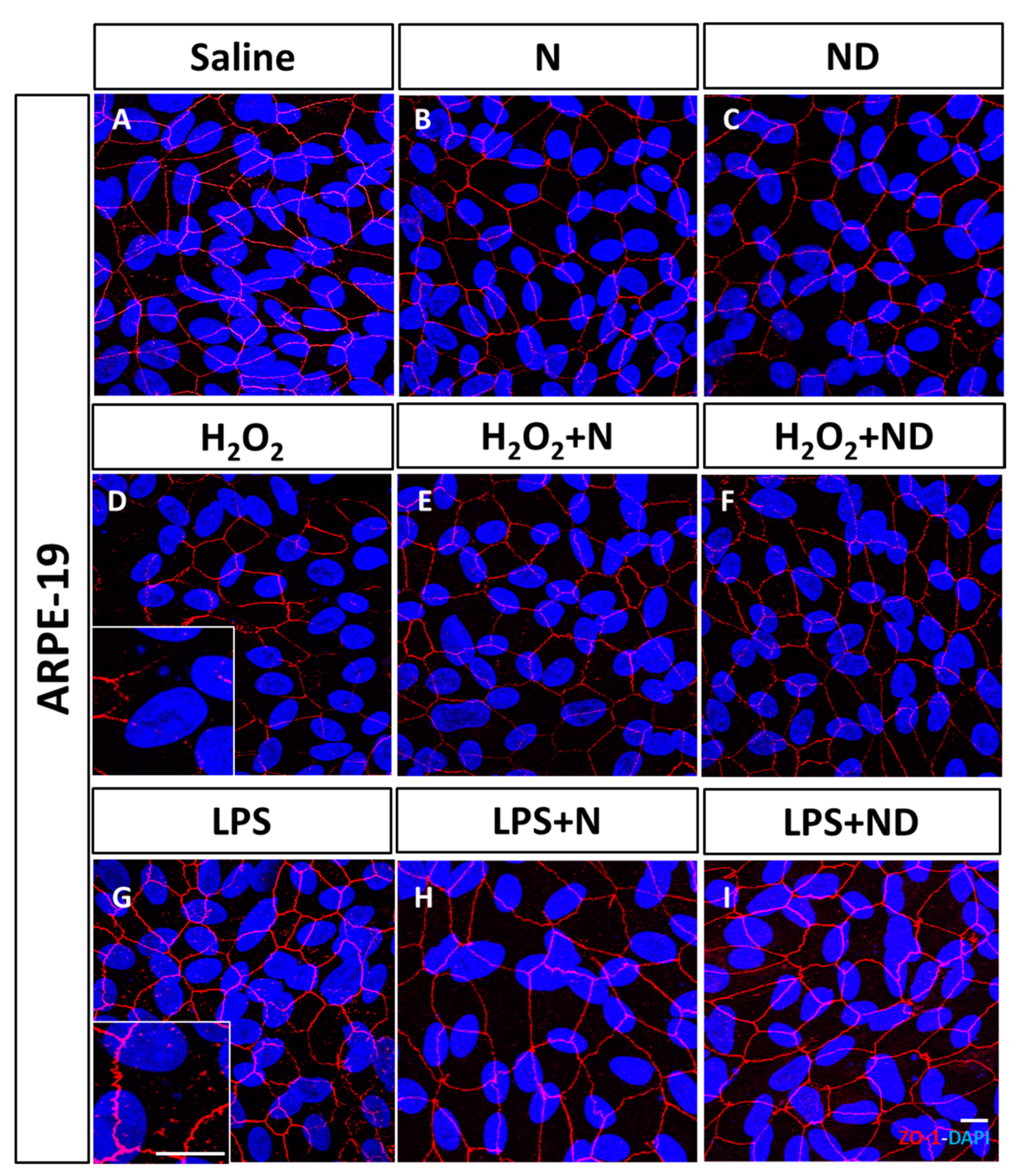
2.5. Zonula Occludens (ZO-1) Immunofluorescence for Cell Structure and Integrity
2.6. Cell Apoptosis Assay
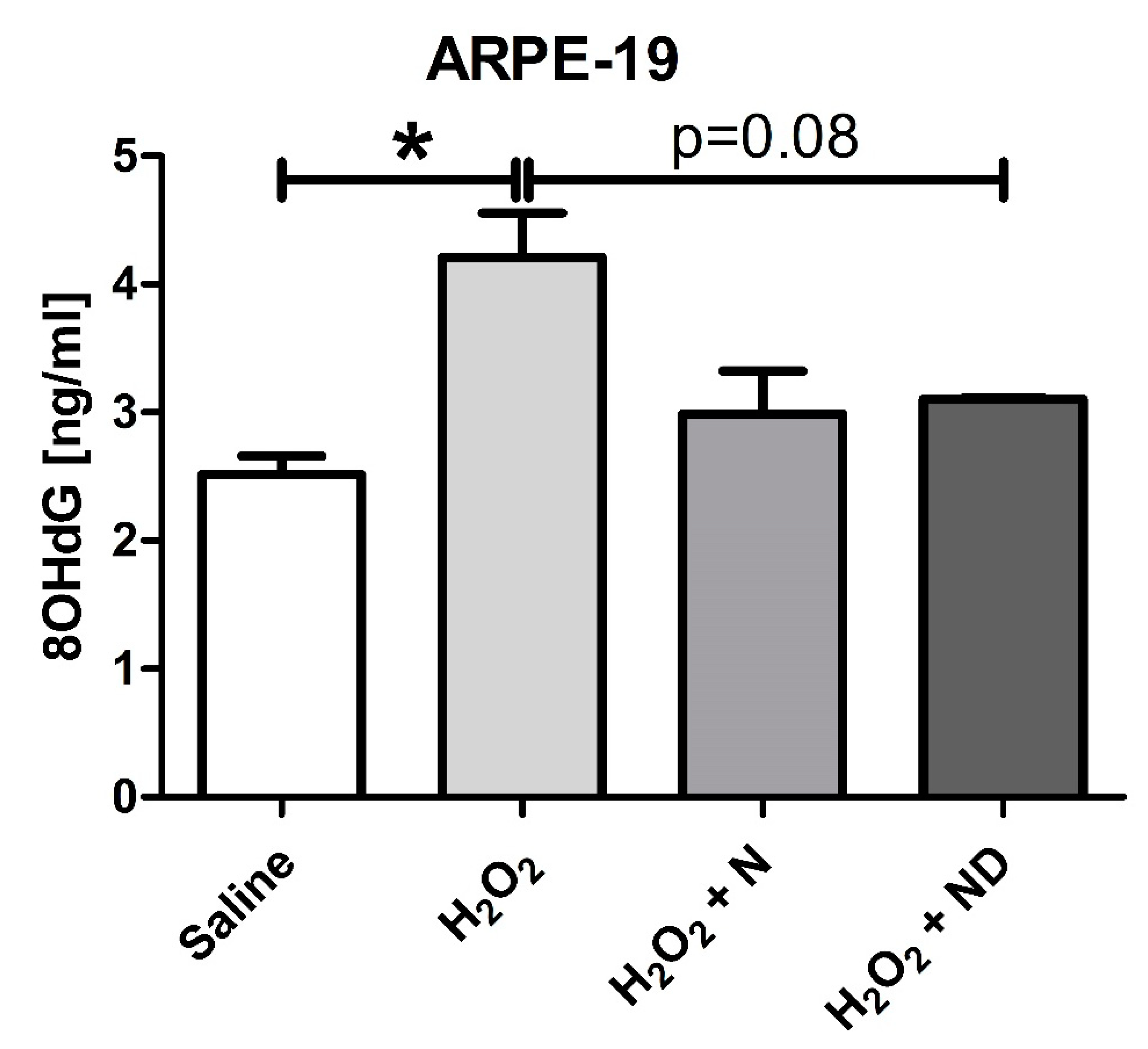
2.7. Evaluation of Oxidative DNA Damage by 8-Hydroxidioguanosine (8-OHdG)
2.8. Cytokine Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of N and ND Treatments on Junctional Integrity
3.2. Effect of N and ND treatments on Apoptosis and Cell Proliferation
3.3. Antioxidant properties of N and ND treatments
3.4. Regulation of Inflammatory Cytokines by N and ND Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Gheorghe, A.; Mahdi, L.; Musat, O. Age-related macular degeneration. Rom. J. Ophthalmol. 2015, 59, 74–77. [Google Scholar] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Klein, R.; Cruickshanks, K.J.; Nash, S.D.; Krantz, E.M.; Nieto, F.J.; Huang, G.H.; Pankow, J.S.; Klein, B.E. The prevalence of age-related macular degeneration and associated risk factors. Arch. Ophthalmol. 2010, 128, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104. [Google Scholar] [CrossRef]
- Mitchell, P.; Wang, J.J.; Smith, W.; Leeder, S.R. Smoking and the 5-year incidence of age-related maculopathy: The Blue Mountains Eye Study. Arch. Ophthalmol. 2002, 120, 1357–1363. [Google Scholar] [CrossRef]
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef]
- Klein, M.L.; Mauldin, W.M.; Stoumbos, V.D. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch. Ophthalmol. 1994, 112, 932–937. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; VandenLangenberg, G.M.; Klein, B.E.; Palta, M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995, 113, 743–748. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J. Nutr. 2002, 132, 518s–524s. [Google Scholar] [CrossRef]
- Mares, J. Carotenoids and eye disease: Epidemiological evidence. In Krinsky NI Mayne ST Sies Heds. Carotenoids in Health and Disease; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Seddon, J.M.; Rosner, B.; Sperduto, R.D.; Yannuzzi, L.; Haller, J.A.; Blair, N.P.; Willett, W. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001, 119, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Khandhadia, S.; Lotery, A. Oxidation and age-related macular degeneration: Insights from molecular biology. Expert Rev. Mol. Med. 2010, 12, e34. [Google Scholar] [CrossRef] [PubMed]
- Layana, A.G.; Minnella, A.M.; Garhöfer, G.; Aslam, T.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C.; Souied, E.; Seddon, J.M. Vitamin D and Age-Related Macular Degeneration. Nutrients 2017, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Mammadzada, P.; André, H. The Different Facades of Retinal and Choroidal Endothelial Cells in Response to Hypoxia. Int. J. Mol. Sci. 2018, 19, 3846. [Google Scholar] [CrossRef]
- Carmeliet, P.; De Smet, F.; Loges, S.; Mazzone, M. Branching morphogenesis and antiangiogenesis candidates: Tip cells lead the way. Nat. Rev. Clin. Oncol. 2009, 6, 315–326. [Google Scholar] [CrossRef] [PubMed]
- A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [CrossRef]
- Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [CrossRef]
- Parekh, N.; Chappell, R.J.; Millen, A.E.; Albert, D.M.; Mares, J.A. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch. Ophthalmol. 2007, 125, 661–669. [Google Scholar] [CrossRef]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef]
- Annweiler, C.; Drouet, M.; Duval, G.T.; Paré, P.Y.; Leruez, S.; Dinomais, M.; Milea, D. Circulating vitamin D concentration and age-related macular degeneration: Systematic review and meta-analysis. Maturitas 2016, 88, 101–112. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Silver, R.E.; Rosner, B.; Seddon, J.M. Associations Between Vitamin D Intake and Progression to Incident Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4569–4578. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Kan, E.K.; Yücel, Ö.E. The Possible Link Between Vitamin D Levels and Exudative Age-related Macular Degeneration. Oman. Med. J. 2020, 35, e83. [Google Scholar] [CrossRef]
- Millen, A.E.; Nie, J.; Mares, J.A.; Lutsey, P.L.; LaMonte, M.J.; Meuer, S.M.; Sahli, M.W.; Andrews, C.A.; Klein, B.E.K.; Klein, R. Serum 25-Hydroxyvitamin D Concentrations and Incidence of Age-Related Macular Degeneration: The Atherosclerosis Risk in Communities Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1362–1371. [Google Scholar] [CrossRef]
- Millen, A.E.; Nie, J.; Sahli, M.W.; Mares, J.A.; Meyers, K.J.; Klein, B.E.K.; LaMonte, M.J.; Lutsey, P.L.; Andrews, C.A.; Klein, R. Vitamin D Status and Prevalent Early Age-Related Macular Degeneration in African Americans and Caucasians: The Atherosclerosis Risk in Communities (ARIC) Study. J. Nutr. Health Aging 2017, 21, 772–780. [Google Scholar] [CrossRef]
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 Fatty Acid, α-Tocopherol, Zinc, vitamin D, vitamin C, and β-carotene are Associated with Age-Related Macular Degeneration in Japan. Sci. Rep. 2016, 6, 20723. [Google Scholar] [CrossRef]
- Bae, S.H.; Shin, Y.J.; Kim, H.K.; Hyon, J.Y.; Wee, W.R.; Park, S.G. Vitamin D Supplementation for Patients with Dry Eye Syndrome Refractory to Conventional Treatment. Sci. Rep. 2016, 6, 33083. [Google Scholar] [CrossRef] [PubMed]
- Karaca, E.E.; Kemer, Ö.E.; Özek, D.; Berker, D.; Imga, N.N. Clinical outcomes of ocular surface in patients treated with vitamin D oral replacement. Arq. Bras. Oftalmol. 2020, 83, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, J.A.; Patel, D.; Susarla, R.; Coca-Prados, M.; Bland, R.; Walker, E.A.; Rauz, S.; Wallace, G.R. Characterization of vitamin D production by human ocular barrier cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Reins, R.Y.; McDermott, A.M. Vitamin D: Implications for ocular disease and therapeutic potential. Exp. Eye Res. 2015, 134, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, M.A.; Kazmin, D.; Hu, P.; McDonnell, D.P.; Malek, G. Research resource: Nuclear receptor atlas of human retinal pigment epithelial cells: Potential relevance to age-related macular degeneration. Mol. Endocrinol. 2011, 25, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D and regulation of vascular cell function. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef] [PubMed]
- Tohari, A.M.; Alhasani, R.H.; Biswas, L.; Patnaik, S.R.; Reilly, J.; Zeng, Z.; Shu, X. Vitamin D Attenuates Oxidative Damage and Inflammation in Retinal Pigment Epithelial Cells. Antioxidants 2019, 8, 341. [Google Scholar] [CrossRef]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Campbell, R.J.; Kumar, R. Immuno-localization of the calcitriol receptor, calbindin-D28k and the plasma membrane calcium pump in the human eye. Curr. Eye Res. 1995, 14, 101–108. [Google Scholar] [CrossRef]
- Morrison, M.A.; Silveira, A.C.; Huynh, N.; Jun, G.; Smith, S.E.; Zacharaki, F.; Sato, H.; Loomis, S.; Andreoli, M.T.; Adams, S.M.; et al. Systems biology-based analysis implicates a novel role for vitamin D metabolism in the pathogenesis of age-related macular degeneration. Hum. Genom. 2011, 5, 538–568. [Google Scholar] [CrossRef]
- Millen, A.E.; Voland, R.; Sondel, S.A.; Parekh, N.; Horst, R.L.; Wallace, R.B.; Hageman, G.S.; Chappell, R.; Blodi, B.A.; Klein, M.L.; et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch. Ophthalmol. 2011, 129, 481–489. [Google Scholar] [CrossRef]
- Golan, S.; Shalev, V.; Treister, G.; Chodick, G.; Loewenstein, A. Reconsidering the connection between vitamin D levels and age-related macular degeneration. Eye (London) 2011, 25, 1122–1129. [Google Scholar] [CrossRef]
- Day, S.; Acquah, K.; Platt, A.; Lee, P.P.; Mruthyunjaya, P.; Sloan, F.A. Association of vitamin D deficiency and age-related macular degeneration in medicare beneficiaries. Arch. Ophthalmol. 2012, 130, 1070–1071. [Google Scholar] [CrossRef]
- Cougnard-Grégoire, A.; Merle, B.M.; Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Féart, C.; Le Goff, M.; Dartigues, J.F.; Barberger-Gateau, P.; Delcourt, C. Vitamin D Deficiency in Community-Dwelling Elderly Is Not Associated with Age-Related Macular Degeneration. J. Nutr. 2015, 145, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Han, K.; Jee, D. Inverse relationship between high blood 25-hydroxyvitamin D and late stage of age-related macular degeneration in a representative Korean population. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.L.; Wang, G.F.; Li, D.D.; Chen, J.X.; Zhang, C.L.; Yu, Y.Z.; Zhou, W.J.; Zou, Y.P.; Rao, B.Q. Protection of tight junction between RPE cells with tissue factor targeting peptide. Int. J. Ophthalmol. 2018, 11, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Murugeswari, P.; Firoz, A.; Murali, S.; Vinekar, A.; Krishna, L.; Anandula, V.R.; Jeyabalan, N.; Chevour, P.; Jayadev, C.; Shetty, R.; et al. Vitamin-D3 (α-1, 25(OH) 2D3) Protects Retinal Pigment Epithelium From Hyperoxic Insults. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Marrs, J.A.; Andersson-Fisone, C.; Jeong, M.C.; Cohen-Gould, L.; Zurzolo, C.; Nabi, I.R.; Rodriguez-Boulan, E.; Nelson, W.J. Plasticity in epithelial cell phenotype: Modulation by expression of different cadherin cell adhesion molecules. J. Cell Biol. 1995, 129, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Totan, Y.; Yağci, R.; Bardak, Y.; Ozyurt, H.; Kendir, F.; Yilmaz, G.; Sahin, S.; Sahin Tiğ, U. Oxidative macromolecular damage in age-related macular degeneration. Curr. Eye Res. 2009, 34, 1089–1093. [Google Scholar] [CrossRef]
- Abokyi, S.; To, C.H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxid. Med. Cell Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Li, J.; Jiang, H.; Kong, J. Klotho Levels are Decreased and Associated with Enhanced Oxidative Stress and Inflammation in the Aqueous Humor in Patients with Exudative Age-related Macular Degeneration. Ocul. Immunol. Inflamm. 2020, 1–8. [Google Scholar] [CrossRef]
- Longo-Mbenza, B.; Mvitu Muaka, M.; Masamba, W.; Muizila Kini, L.; Longo Phemba, I.; Kibokela Ndembe, D.; Tulomba Mona, D. Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: A new phenotype in Central Africa? Int. J. Ophthalmol. 2014, 7, 293–301. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Damghanian, P.; Javanbakht, M.H.; Mohammadzadeh Honarvar, N.; Yousefirad, E.; Mohammadi, H.; Zarei, M.; Djalalli, M. Effects of Vitamin D supplementation on 8-hydroxydeoxy guanosine and 3-nitrotyrosine in patients with type 2 diabetes: A randomized clinical trial. Prog. Nutr. 2019, 21, 138–146. [Google Scholar] [CrossRef]
- Tohari, A.M.; Zhou, X.; Shu, X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 2016, 34, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Antioxidants suppress apoptosis. J. Nutr. 2004, 134, 3179s–3180s. [Google Scholar] [CrossRef]
- Jin, G.F.; Hurst, J.S.; Godley, B.F. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr. Eye Res. 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.R.; Wang, R.X.; Tuo, J.; Chan, C.C. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef]
- Tohari, A.M.; Almarhoun, M.; Alhasani, R.H.; Biswas, L.; Zhou, X.; Reilly, J.; Zeng, Z.; Shu, X. Protection by vitamin D against high-glucose-induced damage in retinal pigment epithelial cells. Exp. Cell Res. 2020, 392, 112023. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D(3) Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef]
- Vilema-Enríquez, G.; Arroyo, A.; Grijalva, M.; Amador-Zafra, R.I.; Camacho, J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxid. Med. Cell Longev. 2016, 2016, 1908164. [Google Scholar] [CrossRef]
- Aryan, N.; Betts-Obregon, B.S.; Perry, G.; Tsin, A.T. Oxidative Stress Induces Senescence in Cultured RPE Cells. Open Neurol. J. 2016, 10, 83–87. [Google Scholar] [CrossRef]
- Chen, X.D.; Su, M.Y.; Chen, T.T.; Hong, H.Y.; Han, A.D.; Li, W.S. Oxidative stress affects retinal pigment epithelial cell survival through epidermal growth factor receptor/AKT signaling pathway. Int. J. Ophthalmol. 2017, 10, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Barszczewska, G.; Gralewska, P.; Kaarniranta, K. Oxidative stress induces mitochondrial dysfunction and autophagy in ARPE-19 cells. Acta. Ophthalmol. 2019, 97. [Google Scholar] [CrossRef]
- Antunes, F.; Brito, P.M. Quantitative biology of hydrogen peroxide signaling. Redox Biol. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Groeger, G.; Quiney, C.; Cotter, T.G. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid Redox Signal 2009, 11, 2655–2671. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- An, E.; Gordish-Dressman, H.; Hathout, Y. Effect of TNF-alpha on human ARPE-19-secreted proteins. Mol. Vis. 2008, 14, 2292–2303. [Google Scholar]
- Kutty, R.K.; Samuel, W.; Boyce, K.; Cherukuri, A.; Duncan, T.; Jaworski, C.; Nagineni, C.N.; Redmond, T.M. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol. Vis. 2016, 22, 1156–1168. [Google Scholar]
- Zhao, M.; Bai, Y.; Xie, W.; Shi, X.; Li, F.; Yang, F.; Sun, Y.; Huang, L.; Li, X. Interleukin-1β Level Is Increased in Vitreous of Patients with Neovascular Age-Related Macular Degeneration (nAMD) and Polypoidal Choroidal Vasculopathy (PCV). PLoS ONE 2015, 10, e0125150. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Goverdhan, S.V.; Ennis, S.; Hannan, S.R.; Madhusudhana, K.C.; Cree, A.J.; Luff, A.J.; Lotery, A.J. Interleukin-8 promoter polymorphism -251A/T is a risk factor for age-related macular degeneration. Br J. Ophthalmol. 2008, 92, 537–540. [Google Scholar] [CrossRef]
- Yildirim, Z.; Ucgun, N.I.; Yildirim, F.; Sepici-Dincel, A. Choroidal Neovascular Membrane in Age-Related Macular Degeneration is Associated with Increased Interleukin-6. Int. J. Gerontol. 2012, 6, 101–104. [Google Scholar] [CrossRef]
- Nassar, K.; Grisanti, S.; Elfar, E.; Lüke, J.; Lüke, M.; Grisanti, S. Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Krogh Nielsen, M.; Subhi, Y.; Molbech, C.R.; Falk, M.K.; Nissen, M.H.; Sørensen, T.L. Systemic Levels of Interleukin-6 Correlate With Progression Rate of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Detrick, B.; Hooks, J.J. The RPE Cell and the Immune System. In Retinal Pigment Epithelium in Health and Disease; Klettner, A., Dithmar, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Cascella, R.; Ragazzo, M.; Strafella, C.; Missiroli, F.; Borgiani, P.; Angelucci, F.; Marsella, L.T.; Cusumano, A.; Novelli, G.; Ricci, F.; et al. Age-related macular degeneration: Insights into inflammatory genes. J. Ophthalmol. 2014, 2014, 582842. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Joint Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Doyle, S.L.; Campbell, M.; Ozaki, E.; Salomon, R.G.; Mori, A.; Kenna, P.F.; Farrar, G.J.; Kiang, A.S.; Humphries, M.M.; Lavelle, E.C.; et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat. Med. 2012, 18, 791–798. [Google Scholar] [CrossRef]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye (London) 2018, 32, 491–505. [Google Scholar] [CrossRef]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Sommer, A.; Fabri, M. Vitamin D regulates cytokine patterns secreted by dendritic cells to promote differentiation of IL-22-producing T cells. PLoS ONE 2015, 10, e0130395. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Canning, M.O.; Grotenhuis, K.; de Wit, H.; Ruwhof, C.; Drexhage, H.A. 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur. J. Endocrinol. 2001, 145, 351–357. [Google Scholar] [CrossRef]
- Lightman, S.; Calder, V. Is IL-10 a good target to inhibit choroidal neovascularisation in age-related macular disease? PLoS Med. 2006, 3, e364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ji, M.; Wang, X. IL-10 inhibits retinal pigment epithelium cell proliferation and migration through regulation of VEGF in rhegmatogenous retinal detachment. Mol. Med. Rep. 2018, 17, 7301–7306. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Copland, D.A.; Liu, J.; Wu, J.; Gardner, P.J.; Ozaki, E.; Doyle, S.L.; Campbell, M.; Dick, A.D. Interleukin-33 regulates tissue remodelling and inhibits angiogenesis in the eye. J. Pathol. 2017, 241, 45–56. [Google Scholar] [CrossRef]
- Barbour, M.; Allan, D.; Xu, H.; Pei, C.; Chen, M.; Niedbala, W.; Fukada, S.Y.; Besnard, A.G.; Alves-Filho, J.C.; Tong, X.; et al. IL-33 attenuates the development of experimental autoimmune uveitis. Eur. J. Immunol. 2014, 44, 3320–3329. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, M.; Recalde, S.; González-Zamora, J.; Bilbao-Malavé, V.; Sáenz de Viteri, M.; Bezunartea, J.; Moreno-Orduña, M.; Belza, I.; Barrio-Barrio, J.; Fernandez-Robredo, P.; et al. Anti-Inflammatory and Anti-Oxidative Synergistic Effect of Vitamin D and Nutritional Complex on Retinal Pigment Epithelial and Endothelial Cell Lines against Age-Related Macular Degeneration. Nutrients 2021, 13, 1423. https://doi.org/10.3390/nu13051423
Hernandez M, Recalde S, González-Zamora J, Bilbao-Malavé V, Sáenz de Viteri M, Bezunartea J, Moreno-Orduña M, Belza I, Barrio-Barrio J, Fernandez-Robredo P, et al. Anti-Inflammatory and Anti-Oxidative Synergistic Effect of Vitamin D and Nutritional Complex on Retinal Pigment Epithelial and Endothelial Cell Lines against Age-Related Macular Degeneration. Nutrients. 2021; 13(5):1423. https://doi.org/10.3390/nu13051423
Chicago/Turabian StyleHernandez, Maria, Sergio Recalde, Jorge González-Zamora, Valentina Bilbao-Malavé, Manuel Sáenz de Viteri, Jaione Bezunartea, Maite Moreno-Orduña, Idoia Belza, Jesús Barrio-Barrio, Patricia Fernandez-Robredo, and et al. 2021. "Anti-Inflammatory and Anti-Oxidative Synergistic Effect of Vitamin D and Nutritional Complex on Retinal Pigment Epithelial and Endothelial Cell Lines against Age-Related Macular Degeneration" Nutrients 13, no. 5: 1423. https://doi.org/10.3390/nu13051423





