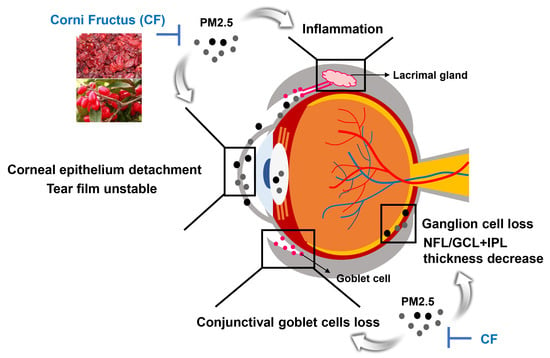
The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PM2.5 and Treatments
2.2. Animals and Experimental Procedures
2.3. Hematological and Biochemical Analysis
2.4. Tear Production
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Periodic Acid-Schiff (PAS) Staining
2.7. Immunohistochemistry
2.8. Statistical Analysis
3. Results
3.1. Effect of CFW on the Physiological Changes in PM2.5-Exposed Sprague-Dawley (SD) Rats
3.2. Effect of CFW on the Changes of Hematological, Biochemical, and Lipid Profiles in PM2.5- Exposed SD Rats
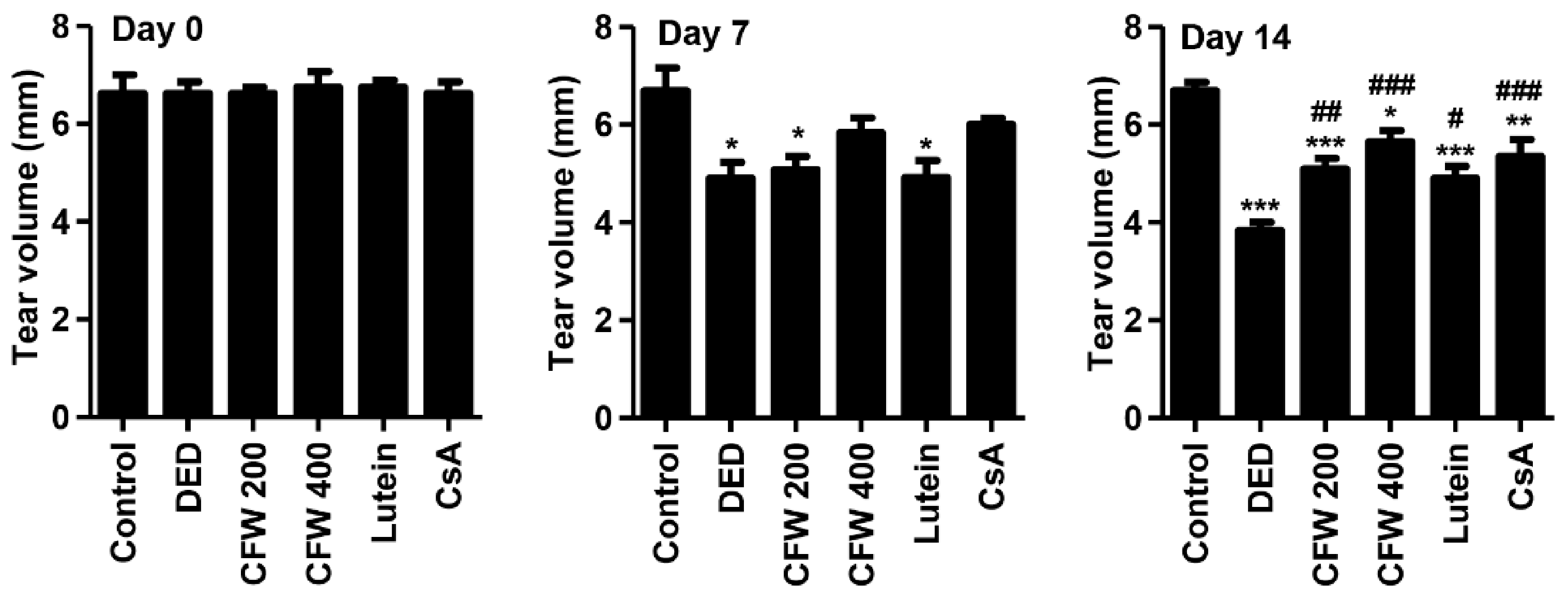
3.3. Effect of CFW on Tear Secretion after Topical Exposure to PM2.5 in SD Rats
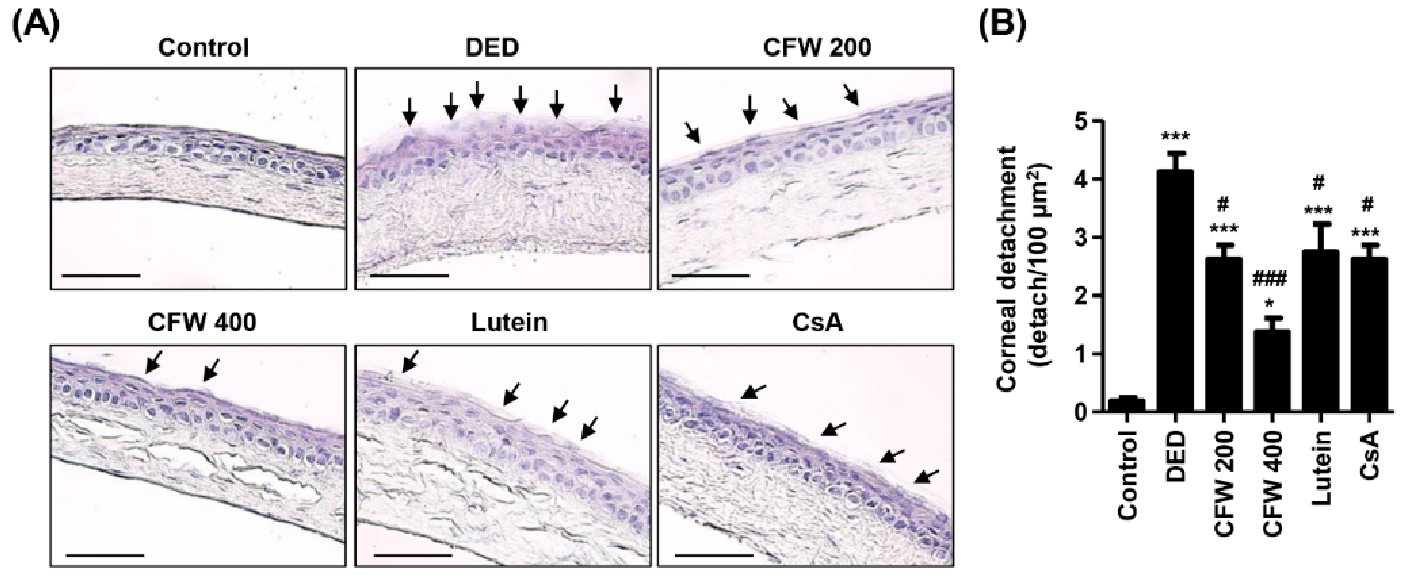
3.4. Effect of CFW on Detachment of Corneal Epithelium in PM2.5-Induced DED Rat Model
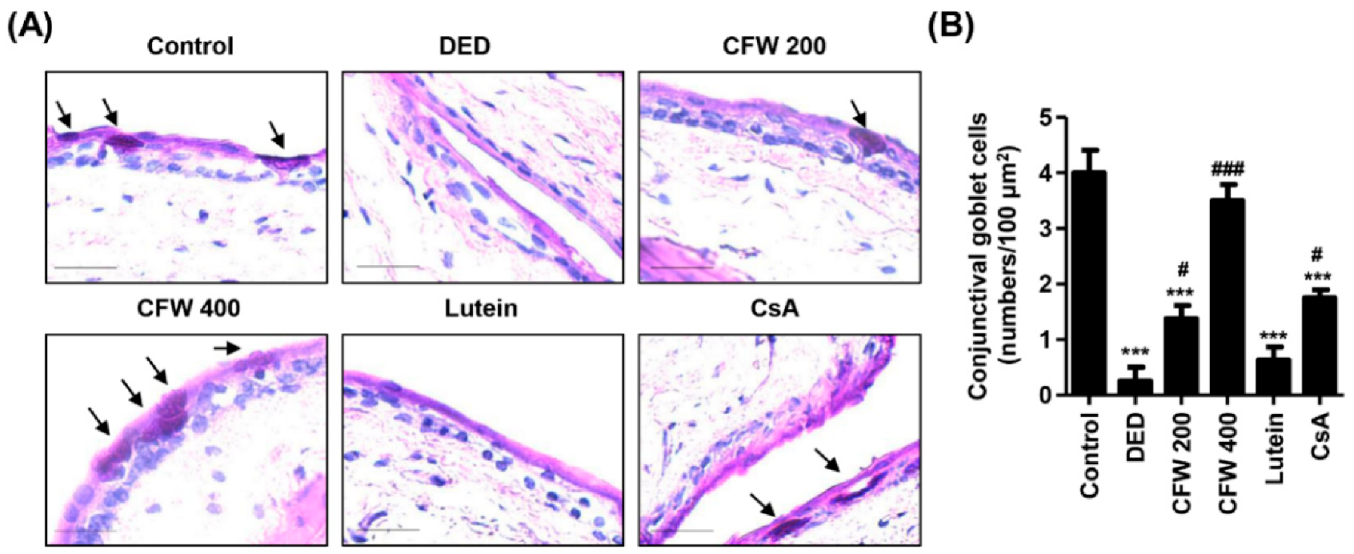
3.5. Effect of CFW on Conjunctival Goblet Cell Population in PM2.5-Induced DED Rat Model
3.6. Effect of CFW on Inflammation of Lacrimal Gland in PM2.5-Induced DED Rats
3.7. Effect of CFW on Histological Changes of the Retina after Topical Exposure to PM2.5 in SD Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bo, Y.; Chang, L.Y.; Guo, C.; Lin, C.; Lau, A.K.H.; Tam, T.; Lao, X.Q. Reduced ambient PM2.5, better lung function, and decreased risk of chronic obstructive pulmonary disease. Environ. Int. 2021, 156, 106706. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez-Andrade, M.; Chirino, Y.I.; González-Ramírez, I.; Sánchez-Pérez, Y.; García-Cuellar, C.M. Deciphering the code between air pollution and disease: The effect of particulate matter on cancer hallmarks. Int. J. Mol. Sci. 2019, 21, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, M.; Han, S.; Seo, J.W.; Jeon, K.J.; Lee, H.S. Traffic-related particulate matter aggravates ocular allergic inflammation by mediating dendritic cell maturation. J. Toxicol. Environ. Health A 2021, 84, 661–673. [Google Scholar] [CrossRef]
- Kang, W.S.; Choi, H.; Jang, G.; Lee, K.H.; Kim, E.; Kim, K.J.; Jeong, G.Y.; Kim, J.S.; Na, C.S.; Kim, S. Long-term exposure to urban particulate matter on the ocular surface and the incidence of deleterious changes in the cornea, conjunctiva and retina in rats. Int. J. Mol. Sci. 2020, 21, 4976. [Google Scholar] [CrossRef]
- Yang, Q.; Li, K.; Li, D.; Zhang, Y.; Liu, X.; Wu, K. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharm. 2019, 117, 109177. [Google Scholar] [CrossRef]
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- De Paiva, C.S. Effects of aging in dry eye. Int. Ophthalmol. Clin. 2017, 57, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014, 9, 240–250. [Google Scholar]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Fu, Q.; Mo, Z.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Lou, X.; et al. Air pollution and outpatient visits for conjunctivitis: A case-crossover study in Hangzhou, China. Environ. Pollut. 2017, 231 Pt 2, 1344–1350. [Google Scholar] [CrossRef]
- Mo, Z.; Fu, Q.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Wang, X.; et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ. Pollut. 2019, 246, 183–189. [Google Scholar] [CrossRef]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.C.; Bao, J.; Li, C.; Tan, G.; Wu, A.H.; Ye, L.; Ye, L.H.; Zhou, Q.; Shao, Y. A murine model of dry eye induced by topical administration of erlotinib eye drops. Int. J. Mol. Med. 2018, 41, 1427–1436. [Google Scholar] [CrossRef]
- Song, S.J.; Hyun, S.W.; Lee, T.G.; Park, B.; Jo, K.; Kim, C.S. New application for assessment of dry eye syndrome induced by particulate matter exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111125. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Iizuka, Y. Effect and underlying mechanisms of airborne particulate matter 2.5 (PM2.5) on cultured human corneal epithelial cells. Sci. Rep. 2020, 10, 19516. [Google Scholar] [CrossRef]
- Niu, L.; Li, L.; Xing, C.; Luo, B.; Hu, C.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM2.5) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol. Environ. Saf. 2021, 207, 111306. [Google Scholar] [CrossRef]
- Hyun, S.W.; Song, S.J.; Park, B.; Lee, T.G.; Kim, C.S. Toxicological effects of urban particulate matter on corneal and conjunctival epithelial cells. Toxicol. Res. 2020, 36, 311–318. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Z.L.; Chen, H.B.; Wang, F.S.; Lu, J.H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethanopharmacoloy, phytochemistry, and pharmacology of Cornus officinalis Sieb. Et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef]
- Chang, J.S.; Chiang, L.C.; Hsu, F.F.; Lin, C.C. Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro. Am. J. Chin. Med. 2004, 32, 717–725. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. Antiproliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [PubMed] [Green Version]
- Kang, D.G.; Moon, M.K.; Lee, A.S.; Kwon, T.O.; Kim, J.S.; Lee, H.S. Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol. Pharm. Bull. 2007, 30, 1796–1799. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.L.; Chen, X.G.; Zhu, H.B.; Hou, J.; Tian, J.W. Cornuside attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Pharmacology 2009, 84, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.J.; Song, J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement Altern. Med. 2016, 16, 196. [Google Scholar] [CrossRef] [Green Version]
- Hwangbo, H.; Kwon, D.H.; Choi, E.O.; Kim, M.Y.; Ahn, K.I.; Ji, S.Y.; Kim, J.S.; Kim, K.I.; Park, N.; Kim, B.H.; et al. Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats. Nutr. Res. Pract. 2018, 12, 378–386. [Google Scholar] [CrossRef]
- Fernando, P.D.S.M.; Piao, M.J.; Zhen, A.X.; Ahn, M.J.; Yi, J.M.; Choi, Y.H.; Hyun, J.W. Extract of Cornus officinalis Protects Keratinocytes from Particulate Matter-induced Oxidative Stress. Int. J. Med. Sci. 2020, 17, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Cha, H.J.; Lee, H.; Kim, G.Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]
- Cao, G.; Cai, H.; Cai, B.; Tu, S. Effect of 5-hydroxymethylfurfural derived from processed Cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chem. 2013, 140, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Pi, W.X.; Feng, X.P.; Ye, L.H.; Cai, B.C. Combination of morroniside and diosgenin prevents high glucose-induced cardiomyocytes apoptosis. Molecules 2017, 22, 163. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Lee, H.; Kwon, C.Y.; Kim, G.Y.; Jeong, J.W.; Kim, S.O.; Choi, S.H.; Jeong, S.J.; Noh, J.S.; Choi, Y.H. Loganin inhibits lipopolysaccharide-induced inflammation and oxidative response through the activation of the Nrf2/HO-1 signaling pathway in RAW264.7 macrophages. Biol. Pharm. Bull. 2021, 44, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein supplementation for eye diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef]
- Matossian, C.; Trattler, W.; Loh, J. Dry eye treatment with topical cyclosporine 0.1% in chondroitin sulfate ophthalmic emulsion. Clin. Ophthalmol. 2021, 15, 1979–1984. [Google Scholar] [CrossRef]
- Lin, X.; Wu, Y.; Tang, L.; Ouyang, W.; Yang, Y.; Liu, Z.; Wu, J.; Zheng, X.; Huang, C.; Zhou, Y.; et al. Comparison of treatment effect and tolerance of the topical application of Mizoribine and Cyclosporine A in a mouse dry eye model. Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef]
- Kang, M.J.; Gong, J.E.; Kim, J.E.; Choi, H.J.; Bae, S.J.; Choi, Y.J.; Lee, S.J.; Seo, M.S.; Kim, K.S.; Jung, Y.S.; et al. Influence of three BALB/c substrain backgrounds on the skin tumor induction efficacy to DMBA and TPA cotreatment. Lab. Anim. Res. 2020, 36, 30. [Google Scholar] [CrossRef]
- Lee, T.G.; Hyun, S.W.; Jo, K.; Park, B.; Lee, I.S.; Song, S.J.; Kim, C.S. Achyranthis radix extract improves urban particulate matter-induced dry eye disease. Int. J. Environ. Res. Public Health 2019, 16, 3229. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.Y.; Kim, J.H.; Lee, G.; Choi, S.; Kim, S.R.; Hong, Y.C.; Park, S.M. Exposure to ambient fine particulate matter is associated with changes in fasting glucose and lipid profiles: A nationwide cohort study. BMC Public Health 2020, 20, 430. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Li, S.; Wang, C.; Liu, Y.; Li, N.; Liu, F.; Huang, S.; Liu, S.; Lu, Y.; Mao, Z.; et al. Is long-term PM1 exposure associated with blood lipids and dyslipidemias in a Chinese rural population? Environ. Int. 2020, 138, 105637. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Cho, E.J.; Yokozawa, T. Protection against hypercholesterolemia by Corni fructus extract and its related protective mechanism. J. Med. Food 2009, 12, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Sui, Y.; Liu, X.X.; Fu, C.Y.; Qiao, Y.H.; Liu, W.J.; Li, Z.Z.; Li, X.Q.; Cao, W. Structures and anti-atherosclerotic effects of 1,6-α-glucans from Fructus Corni. Int. J. Biol. Macromol. 2020, 161, 1346–1357. [Google Scholar] [CrossRef]
- Park, C.H.; Tanaka, T.; Yokozawa, T. Evaluation of 7-O-galloyl-D-sedoheptulose, isolated from Corni Fructus, in the adipose tissue of type 2 diabetic db/db mice. Fitoterapia 2013, 89, 131–142. [Google Scholar] [CrossRef]
- Yamabe, N.; Noh, J.S.; Park, C.H.; Kang, K.S.; Shibahara, N.; Tanaka, T.; Yokozawa, T. Evaluation of loganin, iridoid glycoside from Corni Fructus, on hepatic and renal glucolipotoxicity and inflammation in type 2 diabetic db/db mice. Eur. J. Pharmacol. 2010, 648, 179–187. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar]
- Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of air pollution and weather on dry eye. J. Clin. Med. 2020, 9, 3740. [Google Scholar] [CrossRef]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta 2016, 1858, 2421–2430. [Google Scholar] [CrossRef]
- King-Smith, P.E.; Fink, B.A.; Hill, R.M.; Koelling, K.W.; Tiffany, J.M. The thickness of the tear film. Curr. Eye Res. 2004, 29, 357–368. [Google Scholar] [CrossRef]
- Inatomi, T.; Spurr-Michaud, S.; Tisdale, A.S.; Zhan, Q.; Feldman, S.T.; Gipson, I.K. Expression of secretory mucin genes by human conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1684–1692. [Google Scholar]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020, 78, 100842. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A.; Kato, H.; Komuro, A.; Sonomura, Y.; Sotozono, C.; Tsubota, K.; Kinoshita, S. Classification of Fluorescein Breakup Patterns: A Novel Method of Differential Diagnosis for Dry Eye. Am. J. Ophthalmol. 2017, 180, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Inflammatory response in dry eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef] [Green Version]
- Belfort, R., Jr.; Mendes, N.F. Identification of T and B lymphocytes in the human conjunctiva and lacrimal gland in ocular diseases. Br. J. Ophthalmol. 1980, 64, 217–219. [Google Scholar] [CrossRef] [Green Version]
- Dana, M.R.; Hamrah, P. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv. Exp. Med. Biol. 2002, 506 Pt B, 729–738. [Google Scholar]
- Korn, T.; Reddy, J.; Gao, W.; Bettelli, E.; Awasthi, A.; Petersen, T.R.; Bäckström, B.T.; Sobel, R.A.; Wucherpfennig, K.W.; Strom, T.B.; et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007, 13, 423–431. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J. Th17 cell differentiation: The long and winding road. Immunity 2008, 28, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Han, S.; Seo, J.W.; Jeon, K.J. Exposure to traffic-related particulate matter 2.5 triggers Th2-dominant ocular immune response in a murine model. Int. J. Environ. Res. Public Health 2020, 17, 2965. [Google Scholar] [CrossRef]
- Hyun, S.W.; Kim, J.; Park, B.; Jo, K.; Lee, T.G.; Kim, J.S.; Kim, C.S. Apricot Kernel extract and amygdalin inhibit urban particulate matter-induced keratoconjunctivitis sicca. Molecules 2019, 24, 650. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef]
- Chua, S.Y.L.; Warwick, A.; Peto, T.; Balaskas, K.; Moore, A.T.; Reisman, C.; Desai, P.; Lotery, A.J.; Dhillon, B.; Khaw, P.T.; et al. Association of ambient air pollution with age-related macular degeneration and retinal thickness in UK Biobank. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Park, H.; Joung, B.; Kim, E. The acute respiratory exposure by intratracheal instillation of Sprague-Dawley rats with diesel particulate matter induces retinal thickening. Cutan. Ocul. Toxicol. 2016, 35, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Louwies, T.; Vuegen, C.; Panis, L.I.; Cox, B.; Vrijens, K.; Nawrot, T.S.; De Boever, P. miRNA expression profiles and retinal blood vessel calibers are associated with short-term particulate matter air pollution exposure. Environ. Res. 2016, 147, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Park, C.; Hong, S.H.; Kim, G.Y.; Song, K.S.; Hyun, J.W.; et al. Diesel particulate matter 2.5 promotes epithelial-mesenchymal transition of human retinal pigment epithelial cells via generation of reactive oxygen species. Environ. Pollut. 2020, 262, 114301. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, D.H.; Kim, J.H.; Park, S.K.; Jeong, J.W.; Kim, M.Y.; Hong, S.H.; Song, K.S.; Kim, G.Y.; Hyun, J.W.; et al. Urban aerosol particulate matter promotes necrosis and autophagy via reactive oxygen species-mediated cellular disorders that are accompanied by cell cycle arrest in retinal pigment epithelial cells. Antioxidants 2021, 10, 149. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Podet, E.J.; Patsch, W.P.; Harati, Y.; Appel, V.; Gotto, A.M., Jr.; Young, J.B. Effects of cyclosporine therapy on plasma lipoprotein levels. JAMA 1989, 262, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Kuster, G.M.; Drexel, H.; Bleisch, J.A.; Rentsch, K.; Pei, P.; Binswanger, U.; Amann, F.W. Relation of cyclosporine blood levels to adverse effects on lipoproteins. Transplantation 1994, 57, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef]
- Richer, S.; Devenport, J.; Lang, J.C. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry 2007, 78, 213–219. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–230. [Google Scholar] [CrossRef]
- Matossian, C.; McDonald, M.; Donaldson, K.E.; Nichols, K.K.; MacIver, S.; Gupta, P.K. Dry eye disease: Consideration for women’s health. J. Womens Health 2019, 28, 502–514. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- McClellan, A.J.; Volpe, E.A.; Zhang, X.; Darlington, G.J.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am. J. Pathol. 2014, 184, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Song, S.J.; Hyun, S.W.; Lee, T.G.; Park, B.; Jo, K.; Lee, I.S.; Kim, C.S. Topical application of Liriope platyphylla extract attenuates dry eye syndrome induced by particulate matter. J. Ophthalmol. 2019, 2019, 1429548. [Google Scholar] [CrossRef] [Green Version]


| Organ Weight (g) | Group | |||||
|---|---|---|---|---|---|---|
| Control | DED | CFW 200 | CFW 400 | Lutein | CsA | |
| BW gain | 31.92 ± 9.35 | 28.11 ± 9.03 | 31.13 ± 6.94 | 30.05 ± 8.07 | 30.48 ± 6.77 | 34.03 ± 10.04 |
| Thymus | 0.40 ± 0.08 | 0.39 ± 0.07 | 0.41 ± 0.06 | 0.42 ± 0.10 | 0.36 ± 0.04 | 0.38 ± 0.09 |
| Heart | 0.69 ± 0.04 | 0.68 ± 0.03 | 0.67 ± 0.04 | 0.65 ± 0.06 | 0.66 ± 0.02 | 0.67 ± 0.06 |
| Lung | 1.08 ± 0.09 | 1.02 ± 0.07 | 1.01 ±0.06 | 0.98 ±0.08 | 0.99 ± 0.06 | 1.01 ± 0.07 |
| Liver | 6.43 ± 0.47 | 6.47 ± 0.59 | 6.33 ± 0.68 | 6.28 ± 0.59 | 6.42 ± 0.88 | 6.44 ± 0.67 |
| Kidney | 1.52 ± 0.18 | 1.47± 0.11 | 1.49 ± 0.09 | 1.51 ± 0.14 | 1.45 ± 0.06 | 1.49 ± 0.12 |
| Spleen | 0.53 ± 0.04 | 0.52 ± 0.07 | 0.52 ± 0.07 | 0.53 ± 0.05 | 0.51 ± 0.08 | 0.54 ± 0.04 |
| Uterus and Ovary | 0.51 ± 0.10 | 0.48 ± 0.08 | 0.49 ± 0.11 | 0.52 ± 0.10 | 0.59 ± 0.18 | 0.58 ± 0.14 |
| Group | ||||||
|---|---|---|---|---|---|---|
| Control | DED | CFW 200 | CFW 400 | Lutein | CsA | |
| RBC (106/μL) | 8.15 ± 0.16 | 8.20 ± 0.12 | 8.11 ± 0.24 | 8.03± 0.35 | 8.06 ± 0.15 | 7.91 ± 0.30 |
| WBC (103/μL) | 5.10 ± 0.73 | 4.55 ± 1.23 | 4.82 ± 0.86 | 4.32 ± 0.98 | 4.41 ± 1.11 | 4.09 ± 1.37 |
| Hematocrit (%) | 50.04 ± 1.65 | 49.58 ± 1.25 | 49.96 ± 2.08 | 49.42 ± 2.65 | 50.56 ± 1.24 | 49.62 ± 2.21 |
| Hemoglobin(g/dL) | 15.46 ± 0.21 | 15.32 ± 0.17 | 15.25 ± 0.82 | 15.08 ± 0.95 | 15.06 ± 0.69 | 15.04 ± 0.51 |
| MCV (fL) | 61.42 ± 1.71 | 60.32 ± 0.97 | 60.87 ± 1.11 | 61.52 ± 1.40 | 61.20 ± 0.82 | 61.20 ± 2.24 |
| MCH (pg) | 19.00 ± 0.32 | 18.72 ± 0.34 | 18.89 ± 0.63 | 18.76 ± 0.62 | 19.04 ± 0.45 | 19.20 ± 0.71 |
| MCHC (g/dL) | 30.98 ± 0.57 | 31.02 ± 0.27 | 30.44 ± 0.61 | 30.50 ± 0.44 | 30.38 ± 0.73 | 30.96 ± 0.47 |
| Platelet (103/μL) | 839.60 ± 81.15 | 912.67 ± 63.07 | 885.71 ± 54.83 | 873.40 ± 62.96 | 870.80 ± 45.92 | 898.00 ± 48.46 |
| AST (U/L) | 141.26 ± 24.35 | 150.88 ± 20.75 | 142.18 ± 19.48 | 138.04 ± 23.77 | 143.28 ± 15.23 | 139.30 ± 21.43 |
| ALT (U/L) | 20.94 ± 2.49 | 22.93 ± 3.68 | 21.53 ± 3.05 | 22.48 ± 4.14 | 21.42 ± 1.57 | 20.54 ± 2.55 |
| ALP (U/L) | 428.24 ± 41.58 | 428.87 ± 63.12 | 431.17 ± 48.49 | 421.12 ± 62.66 | 439.28 ± 38.65 | 428.76 ± 79.26 |
| BUN (mg/dL) | 14.19 ± 0.94 | 14.40 ± 1.15 | 14.38 ± 1.02 | 14.89 ± 0.75 | 14.22 ± 1.66 | 14.43 ± 1.30 |
| Creatinine (mg/dL) | 0.48 ± 0.03 | 0.48 ± 0.04 | 0.48 ± 0.02 | 0.47 ± 0.03 | 0.48 ± 0.02 | 0.48 ± 0.03 |
| TC (mg/dL) | 57.98 ± 3.37 | 74.47 ± 5.95 ** | 66.18 ± 3.35 | 62.20 ± 3.77 # | 65.38 ± 8.59 | 60.65 ± 4.17 # |
| TG (mg/dL) | 48.44 ± 5.13 | 48.00 ± 7.94 | 45.77 ± 5.85 | 43.64 ± 6.62 | 45.26 ± 8.72 | 46.58 ± 8.63 |
| HDL-C (mg/dL) | 29.50 ± 3.26 | 28.35 ± 3.97 | 28.97 ± 3.49 | 29.18 ± 3.32 | 28.56 ± 3.33 | 29.32 ± 4.25 |
| LDL-C (mg/dL) | 6.08 ± 0.39 | 9.68 ± 1.28 *** | 7.78 ± 0.65 # | 7.18 ± 0.91 ## | 8.45 ± 0.21 ** | 8.47 ± 0.90 ** |
| FFA (uEq/L) | 665.00 ± 27.81 | 720.17 ± 82.88 | 688.61 ± 49.04 | 683.60 ± 43.76 | 676.20 ± 102.10 | 695.00 ± 56.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, M.Y.; Ji, S.Y.; Kim, D.H.; Kim, S.Y.; Hwangbo, H.; Park, C.; Hong, S.H.; Kim, G.-Y.; Choi, Y.H. The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5. Nutrients 2021, 13, 2986. https://doi.org/10.3390/nu13092986
Lee H, Kim MY, Ji SY, Kim DH, Kim SY, Hwangbo H, Park C, Hong SH, Kim G-Y, Choi YH. The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5. Nutrients. 2021; 13(9):2986. https://doi.org/10.3390/nu13092986
Chicago/Turabian StyleLee, Hyesook, Min Yeong Kim, Seon Yeong Ji, Da Hye Kim, So Young Kim, Hyun Hwangbo, Cheol Park, Su Hyun Hong, Gi-Young Kim, and Yung Hyun Choi. 2021. "The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5" Nutrients 13, no. 9: 2986. https://doi.org/10.3390/nu13092986
APA StyleLee, H., Kim, M. Y., Ji, S. Y., Kim, D. H., Kim, S. Y., Hwangbo, H., Park, C., Hong, S. H., Kim, G.-Y., & Choi, Y. H. (2021). The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5. Nutrients, 13(9), 2986. https://doi.org/10.3390/nu13092986