Abstract
The coronavirus disease 2019 (COVID-19) pandemic has had significant morbidity, mortality, social and financial implications for the global population. Despite this knowledge, we still know very little about how COVID-19 infection affects quality of life resulting from changes in nutritional behaviour and, conversely, how nutrition could modulate the epidemiology of COVID-19. In addition, the social isolation most have experienced due to the regulations imposed by governments during the COVID-19 pandemic may have also had effects on our nutritional behaviour. It is possible that nutritional interventions may have effects on the incidence of COVID-19 infection and mortality rates. The purpose of this review is to evaluate the current status of research on the topic of nutrition as it relates to the COVID-19 pandemic.
1. Introduction
As identified in the Global Burden of Disease Study 2016, dietary habits represent the second most important risk factor for determining mortality and disability-adjusted life-years in the world [1]. Any disruption in nutrition, therefore, will have a significant immediate and long-term impact on health. Conversely, improvements in nutritional behaviour can have a beneficial therapeutic effect in populations both with and without clinical symptoms.
At the termination of 2019 and throughout 2020, the spread of the coronavirus disease 2019 (COVID-19) virus from first identification in China across the world has had a major health and financial impact that the world has not seen for at least a century. This is expected to continue well into 2021 and probably 2022 as well. The potential impact of COVID-19 on nutritional habits and, inversely, the impact of nutrition on the epidemiology of the COVID-19 pandemic is worthy of study considering the positive and negative associations of nutrition with morbidity and mortality. The purpose of this review is to evaluate nutritional research nutrition as it relates to the COVID-19 pandemic.
2. Material and Methods
A systematic review was performed. The literature search when it was relevant to COVID-19 was limited to the period of COVID-19 infection to date (2020 until February 2021). The literature search was conducted through electronic databases including PubMed, Google Scholar and Web of Science. All case reports, reviews and retrospective and prospective studies on the topic were reviewed. Keywords used to search the literature database were “COVID-19” or “COVID” or “H1N1” or “Ebola” or “influenza” and “nutrition”, or “food”, or “diet” or “food security”. Because research on the topic and actual data was limited, all available papers were reviewed without exclusion as long as they were reported in a peer reviewed journal in the English language. Government reports (i.e., Centers for Disease Control and Prevention or World Health Organization) on the topic were also reviewed as appropriate to the topic. Two independent researchers searched the databases to avoid risk of bias.
3. Predictions from Past Pandemics
Our past history with global pandemics would strongly suggest that nutritional status would have important implications for population health even decades after the current COVID-19 viral pandemic has been controlled. The 1918 flu pandemic had significant effects on markers of nutritional status assessed in individuals 75 years and older after being exposed to the virus in utero, during infancy and early childhood. Using knee height as a marker of nutritional status, a significant depression in growth was found which was exaggerated by increasing severity of the flu symptoms [2]. Women were more affected than men. The depressed growth was not minor. The Dutch population took 40 years to gain a similar magnitude of growth in non-pandemic times as was lost by those infected by the 1918 flu [2]. This marker of nutritional status may associate with the incidence of disease. Prenatal exposure to the 1918 flu virus was associated with an increased incidence (>20%) of ischemic heart disease much later in the century in people aged 60 to 82 years old [3]. This time, men were more strongly affected than women [3].
The 1918 influenza pandemic is not isolated when considering the impact of nutritional status on disease outbreaks. More recent pandemics including those from H1N1 influenza, Swine flu, and the Ebola and Nipah viruses have all been impacted by questionable food security practices including distribution and food availability concerns [4]. This is particularly true in African countries stricken with Ebola virus where food security was negatively impacted by the pandemic, and governments appeared unprepared to address the nutritional problems facing their populations and to guide them nutritionally during the pandemic [5,6,7,8]. The result was malnutrition, particularly in the children of these regions [9]. The capacity for a variety of nutraceuticals and nutritional supplements to confer protection from and survival after infection by H1N1 has been demonstrated in animal studies [10,11,12]. Because of this, sales of these products have increased but suspicions have arisen regarding their actual efficacy against H1N1 in humans [13,14]. In trials of patients with Ebola virus infection, multivitamin therapy was associated with lower mortality rates [15]. In view of these results following past pandemics, it is prudent to consider the possibility that the current COVID-19 virus may have affected today’s population in a similar manner. The distinct possibility that the COVID-19 pandemic in any way altered dietary habits, or if nutritional alterations may impact COVID-19 transmission, morbidity or mortality are valuable questions that will be discussed in this review.
5. Food Security in the COVID-19 Pandemic
The discussion of the impact of nutrition on COVID-19 infection and mortality rates, as discussed previously in this manuscript is one valid approach to the issue but the opposite is also worthy of study. It is very possible that the pandemic has influenced the world’s food security. Maintaining the security of our food supply and having ready access to appropriate amounts of safe food is of paramount importance to all peoples. According to the US Department of Agriculture there are four categories of food security (Table 1). It is possible through the various lines of evidence obtained in several surveys and studies conducted throughout the world to begin to understand the impact that the pandemic has had on nutritional behaviour and food security globally.

Table 1.
US Department of Agriculture categories of food security.
Several studies have investigated food security as it relates to COVID-19. First and foremost, there is no evidence that viral transmission of COVID-19 has occurred via food packaging [45,46,47]. The virus remains viable under controlled laboratory conditions for 24–72 h on plastic, stainless steel and cardboard matrices. This offers the remote potential for transmission of the virus via food packaging. However, no evidence for such transmission exists to date. Food workers practice conventional hygienic methods like the regular use of personal protective equipment (masks, eye wear, disposable gloves), hand washing, disinfectants, maintaining physical distance, frequent viral testing and refraining from entering the workplace with any symptoms of respiratory illness. These practices are no different to what would be expected to occur in any workplace.
Similarly, there is no evidence that viral transmission of COVID-19 has occurred through foods [47]. Although the COVID-19 virus was suspected to be transmitted to humans from bats, the CDC has stated that the risk of COVID-19 transmission from animals to humans is considered low [48]. Coronaviruses can only multiply in live animals and humans. Food provides an inadequate environment for the virus to live. Similarly, the risk of contracting live COVID-19 virus from ingesting food products like cooked meats is extremely unlikely.
Food security during a pandemic involves the stability of food availability in countries and communities as well. In Brazil, additional measures were implemented to increase food availability including a Basic Emergency Income program, a Food Acquisition Program and an emergency food donation program [49]. Nutritional inadequacies were reported in post-operative bariatric patients during the COVID-19 quarantine [50]. In Uganda, the pandemic caused delays to food distribution, poorer food quality, the use of mobile cash transfers to reduce corruption and provide economic assistance to families that were struggling with food insecurity [51]. In Jordan, surveys of more than 3000 citizens revealed 41% of the participants exhibited food security but 36% were moderately food insecure and 23% were severely insecure [52]. In Nepal, food prices increased and food quality and quality decreased significantly (Figure 1) during the pandemic [53]. Moderate food insecurity was associated with a monthly per capita income below the poverty line whereas a younger adult age (18–30 years old) and living in a rented house was significantly associated with severe food insecurity [52].
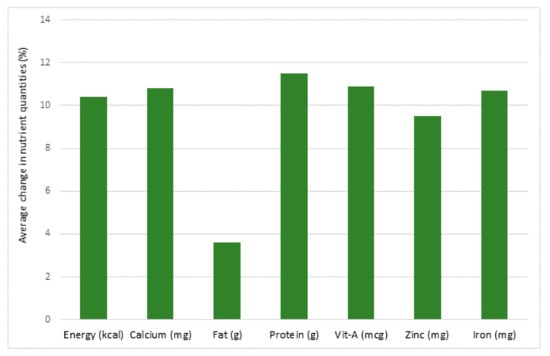
Figure 1.
Average decrease in nutrient quantities (%) for school meals in Nepal between June 2019 (pre-coronavirus disease 2019 (COVID-19)) and May/June 2020 (post-COVID-19), due to food price inflation. (mg: milligram; mcg:microgram; g:gram; kcal:kilocalorie) [53].
The nutritional response to the pandemic has been quite different in Europe and North America. In the US, the prevalence of food insecurity increased by one third from before to during the pandemic [54,55]. Even in the USA, disadvantaged communities have shown signs of severe food insecurity during the COVID-19 pandemic [56]. This appears to have been experienced across a wide age range of adults, from college students to the elderly [54].
6. Changes in Dietary Behaviour during the Pandemic and Their Consequences
In a Canadian study, the nutrient and caloric intake of university students were significantly reduced during the pandemic while alcohol intake increased significantly [57]. The frequency of consuming food groups across the board were decreased during the pandemic [57]. Low physical activity and increased sedentary activities were also observed. Conversely, in a Dutch study, overeating, primarily through snacking, was reported in 20–32% of respondents during the pandemic whereas 7–15% reported behaviour predisposed to undernutrition by skipping warm meals [58]. These changes in nutritional behaviour may be dependent upon the country examined. In Croatia, more than 4000 participants in a study revealed cooking frequency increased during the COVID-19 pandemic lockdown with an increased consumption of vegetables, legumes and seafood [59].
Social isolation during the pandemic-induced lockdown or quarantine resulted in more severe effects on nutritional status [58]. In a Belgian study, 10% of participants often or sometimes could not afford to eat a healthy diet during confinement [59]. Tendencies for fruit and vegetable consumption decreased and the consumption of soft drinks, sweets, bread and salty snacks increased [60,61,62]. Exercise in quarantine led to better nutritional choices which in turn had a beneficial effect on mental state as well [63]. However, it is important to emphasize that exercise frequency has been repeatedly reported to decrease during the pandemic [57,59] The poor nutritional behaviour and lack of exercise have resulted in predictable and unhealthy increases in weight gain during the pandemic [64,65]. These changes in dietary habits have important implications for the health impact of the pandemic. In countries across the world that had COVID-19 disease statistics collected by the WHO, infection and mortality rates due to COVID-19 were positively correlated with higher intake of fruits and sugar-sweetened beverages and negatively correlated with bean and legume consumption [54].
7. Conclusions
These and other studies taken together allow for the following conclusions (Figure 2) regarding the direct and indirect impact of the COVID-19 pandemic on food security:
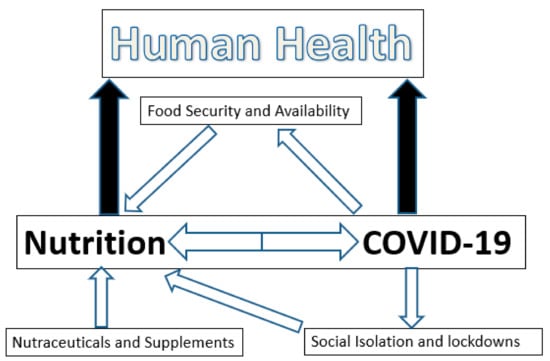
Figure 2.
The interactions of nutrition and COVID-19 infection.
- The COVID-19 pandemic has caused changes in food supply. Special programs have been implemented in many countries to increase food availability and safety. Nonetheless, alterations in food availability due to the pandemic have occurred throughout the world. The severity depends upon the affluence and the economic status of the country. This has more serious implications particularly in third world countries as delays in food distribution, loss of food quality and quantity, impairments in food access and losses of income to purchase food have occurred. This has serious health implications acutely and, in view of the deleterious impacts that past pandemics have had on human growth and health, it is reasonable to predict that the current COVID-19 pandemic will induce nutritional deficiencies across the globe that will have a long-lasting negative impact on human health.
- There is no evidence that the COVID-19 virus has been transmitted through foods or food packaging. This does not negate the importance of using appropriate precautionary measures in the food industry with, for example, the use of appropriate personal protective equipment, hand hygiene and disinfectants.
- The nutritional status of a person can modulate infectious disease and the inflammatory processes associated with it positively or negatively by altering the immune system. Malnutrition in disadvantaged populations and in the elderly clearly leaves these populations more susceptible to COVID-19 infections and more severe clinical symptoms and outcomes. However, although infectivity rates and the severity of the clinical symptoms associated with a coronavirus infection may be modulated, it is highly unlikely that strong viral transmission can be fully prevented by following a healthy diet or supplementing the diet with nutraceuticals. The impact of vitamin D and zinc status in COVID-19 patients regarding viral transmission and its clinical symptoms remains unclear and requires further research.
- COVID-19 has had a significant impact in some populations through altered eating behaviors. The impact of social isolation and lockdowns on eating behaviors during the COVID-19 pandemic should not be underestimated as it has already had acute effects and will likely produce long-term deleterious effects on population health as well. Poor nutritional choices sustained over extended periods of time will have increased plasma risk factors for cardiovascular disease, diabetes and cancer.
Author Contributions
Conceptualization, D.R.-L. and G.N.P.; methodology, G.N.P.; validation, D.R.-L. and G.N.P.; formal analysis, G.N.P.; investigation, D.R.-L. and G.N.P.; resources, G.N.P.; writing—original draft preparation, G.N.P.; writing—review and editing, D.R.-L. and G.N.P.; project administration, G.N.P.; funding acquisition, G.N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes for Health Research, a Foundation grant to G.N.P.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef]
- Palloni, A.; McEniry, M.; Huangfu, Y.; Beltran-Sanchez, H. Impacts of the 1918 flu on survivors’ nutritional status: A double quasi-natural experiment. PLoS ONE 2020, 15, e0232805. [Google Scholar] [CrossRef]
- Mazumder, B.; Almond, D.; Park, K.; Crimmins, E.M.; Finch, C.E. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J. Dev. Orig. Health Dis. 2010, 1, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, A.; Pingali, P. Pandemics and food systems–towards a proactive food safety approach to disease prevention and management. Food Secur. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Kodish, S.R.; Roher, F.; Beauliere, J.-M.; Daffe, M.; Ayoya, M.A.; Wirth, J.P.; Ngnie-Teta, I. Implications of the Ebola virus disease outbreak in Guinea: Qualitative findings to inform future health and nutrition-related responses. PLoS ONE 2018, 13, e0202468. [Google Scholar]
- Kodish, S.R.; Simen-Kapeu, A.; Beauliere, J.-M.; Ngnie-Teta, I.; Jalloh, M.B.; Pyne-Bailey, S.; Schwartz, H.; Wirth, J.P. Consensus building around nutrition lessons from the 2014-16 Ebola virus disease outbreak in Guinea and Sierra Leone. Health Policy Plan. 2019, 34, 83–91. [Google Scholar] [CrossRef]
- Ververs, M.; Gabra, M. Nutritional care for patients with Ebola virus disease. Emerg. Infect. Dis. 2020, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ververs, M.; Vorfeld, C. Guidance materials from 2014-2019 on nutritional care for Ebola patients in Ebola treatment units: An analysis. Public Health Nutr. 2021, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kamara, M.H.; Najjemba, R.; van Griensven, J.; Yorpoi, D.; Jimissa, A.S.; Chan, A.K.; Mishra, S. Increase in acute malnutrition in children following the 2014-2015 Ebola outbreak in rural Sierra Leone. Public Health Action 2017, 7 (Suppl. 1), S27–S33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Jung, Y.-J.; Kim, K.-H.; Kwon, Y.; Kim, Y.-J.; Zhang, Z.; Kang, H.-S.; Wang, B.-Z.; Quan, F.-S.; Kang, S.-M. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses 2018, 1, 471. [Google Scholar] [CrossRef]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 9, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Tanaka, K.; Hirota, Y. Immune response to influenza vaccine in healthy adults and the elderly: Association with nutritional status. Vaccine 2005, 23, 1457–1463. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; COVID-19 Review Consortium; Greene, C.S. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. mSystems 2021, 6, e00122-21. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Meydani, S.N.; Vandermause, M.; Shay, D.K.; Coleman, L.A. Vitamin E, vitamin A, and zinc status are not related to serologic response to influenza vaccine in older adults: An observational prospective cohort study. Nutr. Res. 2014, 34, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yam, D.; Aluisio, A.R.; Perera, S.M.; Peters, J.L.; Cho, D.K.; Kennedy, S.B.; Massaquoi, M.; Sahr, F.; Smit, M.A.; Locks, L.; et al. Association between multivitamin supplementation and mortality among patients with Ebola virus disease: An international multisite cohort study. Afr. J. Emerg. Med. 2020, 10, 23–29. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Aman, F.; Masood, S. How nutrition can help to fight against COVID-19 pandemic. Pak. J. Med. Sci. 2020, 36, S121–S123. [Google Scholar] [CrossRef] [PubMed]
- Bold, J.; Harris, M.; Fellows, L.; Chouchane, M. Nutrition, the digestive system and immunity in COVID-19 infection. Gastroenterol. Hepatol. Bed Bench. 2020, 13, 331–340. [Google Scholar]
- Virgens, I.P.A.; Santana, N.M.; Lima, S.C.V.C.; Fayh, A.P.T. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br. J. Nutr. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Mossink, J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.J.; Ibrahim, S.A. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- McAuliffe, S.; Ray, S.; Fallon, E.; Bradfield, J.; Eden, T.; Kohlmeier, M. Dietary micronutrients in the wake of COVID-19: An appraisal of evidence with a focus on high-risk groups and preventative healthcare. BMJ Nutr. Prev. Health 2020, 3, 93–99. [Google Scholar] [CrossRef]
- Paoli, A.; Gorini, S.; Caprio, M. The dark side of the spoon glucose, ketones and COVID-19: A possible role for ketogenic diet? J. Transl. Med. 2020, 20, 441. [Google Scholar] [CrossRef]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Orhan, C.; Uckun, F.M.; Sahin, K. Clinical impact potential of supplemental nutrients as adjuncts of therapy in high-risk COVID-19 for obese patients. Front. Nutr. 2020, 7, 580504. [Google Scholar] [CrossRef]
- Butters, D.; Whitehouse, M. COVID-19 and nutraceutical therapies, especially using zinc to supplement antimicrobials. Inflammopharmacology 2020, 16, 1–5. [Google Scholar]
- Chowdhury, P.; Barooah, A.K. Tea bioactive modulate innate immunity: In perception to COVID-19 pandemic. Front. Immunol. 2020, 11, 590716. [Google Scholar] [CrossRef] [PubMed]
- De Araujo Morais, A.H.; Passos, T.S.; de Lima Vale, S.H.; da Silva Maia, J.K.; Maciel, B.L. Obesity and the increased risk for COVID-19: Mechanisms and nutritional management. Nutr. Res. Rev. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites in the pathobiology of (coronavirus disease 2019) COVID-19. Nutrition 2021, 82, 111052. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, F.; Sessa, F.; Valenzano, A.; Polito, R.; Monda, V.; Cibelli, G.; Villano, I.; Pisanelli, D.; Perrella, M.; Daniele, A.; et al. COVID-19: Role of nutrition and supplementation. Nutrients 2021, 13, 976. [Google Scholar] [CrossRef]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Formisano, E.; Di Maio, P.; Ivaldi, C.; Sferrazzo, E.; Arieta, L.; Bongiovanni, S.; Panizzi, L.; Valentino, E.; Pasta, A.; Giudice, M.; et al. Nutritional therapy for patients with coronavirus disease 2019 (COVID-19): Practical protocol from a single center highly affected by outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nutrition 2021, 82, 111048. [Google Scholar] [CrossRef] [PubMed]
- Damayanthi, H.D.W.T.; Prabani, K.I.P. Nutritional determinants and COVID-19 outcomes of older patients with COVID-19: A systematic review. Arch. Gerontol. Geriatr. 2021, 95, 104411. [Google Scholar] [CrossRef] [PubMed]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Tekin, O.M.; Yazihan, N.; Sahin, D. The relationship between trace element status (zinc, copper, magnesium) and clinical outcomes in COVID-19 infection during pregnancy. Biol. Trace Elem. Res. 2020, 24, 1–10. [Google Scholar]
- Huang, F.; Zhang, C.; Liu, Q.; Zhao, Y.; Zhang, Y.; Qin, Y.; Li, X.; Li, C.; Zhou, C.; Jin, N.; et al. Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. PLoS Pathog. 2020, 16, e1008341. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-review on the roles of Vitamin C, Vitamin D, and selenium in the immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin. Med. 2021, 21, e48–e51. [Google Scholar] [CrossRef]
- Lanham-New, S.A.; Webb, A.R.; Cashman, K.D.; Buttriss, J.L.; Fallowfield, J.L.; Masud, T.; Hewison, M.; Mathers, J.C.; Kiely, M.; A Welch, A.; et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 106–110. [Google Scholar] [CrossRef]
- Goncalves, T.J.M.; Goncalves, S.E.A.B.; Guarnieri, A.; Risegato, R.C.; Guimaraes, M.P.; Cabral de Freitas, D.; Razuk-Filho, A.; Junior, P.B.B.; Parrillo, E.F. Prevalence of obesity and hypovitaminosis D in elderly with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Nutr. 2020, 40, 110–114. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Klersy, C.; Lobascio, F.; Masi, S.; Crotti, S.; De Stefano, L.; Bruno, R.; Corsico, A.G.; Di Sabatino, A.; et al. Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin. Nutr. 2020, 40, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Avoidance of vitamin D deficiency to slow the Covid-19 pandemic. BMJ Nutr. Prev. Health 2020, 3, e000096. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Bogliolo, L.; Lobascio, F.; Barichella, M.; Zecchinelli, A.L.; Pezzoli, G.; Caccialanza, R. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition 2021, 82, 111055. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Definitions of Food Security. Economic Research Service. 2020. Available online: https://ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security.aspx (accessed on 18 January 2021).
- World Health Organization; Food and Agriculture Organization of the United Nations. COVID-19 and Food Safety: Guidance for Competent Authorities Responsible for National Food Safety Control Systems; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United Nations. COVID-19 and Food Safety: Guidance for Food Business; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Mardones, F.O.; Rich, K.M.; Boden, L.A.; Moreno-Switt, A.I.; Caipo, M.L.; Zimin-Veselkoff, N.; Alateeqi, A.M.; Baltenweck, I. The COVID-19 pandemic and global food security. Front. Vet. Sci. 2020, 7, 578508. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 and Animals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html (accessed on 18 January 2021).
- Gurgel Ado, M.; Silva Dos Santos, C.C.; de Souza Alves, K.P.; Araujo, J.M.; Leal, V.S. Government strategies to ensure the human right to adequate and healthy food facing the COVID-19 pandemic in Brazil. Cien Saude Colet. 2020, 25, 4945–4956. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Esteves, G.P.; Genario, R.; Santo, M.A.; de Cleva, R.; Gualano, B.; Roschel, H. Nutritional inadequacies among post-bariatric patients during COVID-19 quarantine in Sao Paulo, Brazil. Obes. Surg. 2020, 24, 1–5. [Google Scholar]
- Nathan, I.; Benon, M. COVID-19 relief food distribution: Impact and lessons for Uganda. Pan. Afr. Med. J. 2020, 35 (Suppl. 2), 142. [Google Scholar] [CrossRef]
- Elsahoryi, N.; Al-Sayyed, H.; Odeh, M.; McGrattan, A.; Hammad, F. Effect of COVID-19 on food security: A cross-sectional survey. Clin. Nutr. 2020, 40, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nourozi, S.; Acharya, L.; Thapa, S. Estimating the potential effects of COVID-19 pandemic on food commodity prices and nutrition security in Nepal. J. Nutr. Sci. 2020, 9, e51. [Google Scholar] [CrossRef] [PubMed]
- Soldavini, J.; Andrew, H.; Berner, M. Characteristics associated with changes in food security status among college students during the COVID-19 pandemic. Transl. Behav. Med. 2021, 11, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Niles, M.T.; Bertmann, F.; Belarmino, E.H.; Wentworth, T.; Biehl, E.; Neff, R. The early food insecurity impacts of COVID-19. Nutrients 2020, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Zack, R.M.; Weil, R.; Babbin, M.; Lynn, C.D.; Velez, D.S.; Travis, L.; Taitelbaum, D.J.; Fiechtner, L. An overburdened charitable food system: Making the case for increased government support during COVID-19 crisis. Am. J. Public Health 2021, 111, 804–807. [Google Scholar] [CrossRef]
- Bertrand, L.; Shaw, K.A.; Ko, J.; Deprez, D.; Chilibeck, P.D.; Zello, G.A. The impact of the coronavirus disease 2019 (COVID-19) pandemic on university students’ dietary intake, physical activity, and sedentary behaviour. Appl. Physiol. Nutr. Metab. 2021, 15, 1–8. [Google Scholar]
- Visser, M.; Schaap, L.A.; Wijnhoven, H.A.H. Self-reported impact of the COVID-19 pandemic on nutrition and physical activity behaviour in Dutch older adults living independently. Nutrients 2020, 12, 3708. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, D.; Rešetar, J.; Kljusurić, J.G.; Krbavčić, I.P.; Bender, D.V.; Rodríguez-Pérez, C.; Ruíz-López, M.D.; Šatalić, Z. Cooking at home and adherence to the Mediterranean diet during COVID-19 confinement: The experience from the Croatian COVIDiet Study. Front. Nutr. 2021, 8, 617721. [Google Scholar] [CrossRef]
- Vandevijvere, S.; De Ridder, S.; Drieskens, S.; Charafeddine, R.; Berete, F.; Demarest, S. Food insecurity and its association with changes in nutritional habits among adults during the COVID-19 confinement measures in Belgium. Public Health Nutr. 2020, 9, 1–12. [Google Scholar]
- Huber, B.C.; Steffen, J.; Schichtiger, J.; Brunner, S. Altered nutrition behaviour during COVID-19 pandemic lockdown in young adults. Eur. J. Nutr. 2020, 1, 1–10. [Google Scholar]
- Zarah, A.B.; Enriquez-Marulanda, J.; Andrade, J.M. Relationship between dietary habits, food attitudes and food security status among adults living within the United States three months post-mandated quarantine: A cross-sectional study. Nutrients 2020, 12, 3468. [Google Scholar] [CrossRef] [PubMed]
- Amatori, S.; Zeppa, S.D.; Preti, A.; Gervasi, M.; Gobbi, E.; Ferrini, F.; Rocchi, M.B.L.; Baldari, C.; Perroni, F.; Piccoli, G.; et al. Dietary Habits and Psychological States during COVID-19 Home Isolation in Italian College Students: The Role of Physical Exercise. Nutrients 2020, 12, 3660. [Google Scholar] [CrossRef]
- Galali, Y. The impact of COVID-19 confinement on the eating habits and lifestyle changes: A cross sectional study. Food Sci. Nutr. 2021, 9, 2105–2113. [Google Scholar] [CrossRef]
- Sulejmani, E.; Hyseni, A.; Xhabiri, G.; Rodríguez-Pérez, C. Relationship in dietary habits variations during COVID-19 lockdown in Kosovo: The COVIDiet study. Appetite 2021, 164, 105244. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).