Relation of Serum Copper Status to Survival in COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serum Copper Analysis
2.3. CP Quantification by ELISA
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients
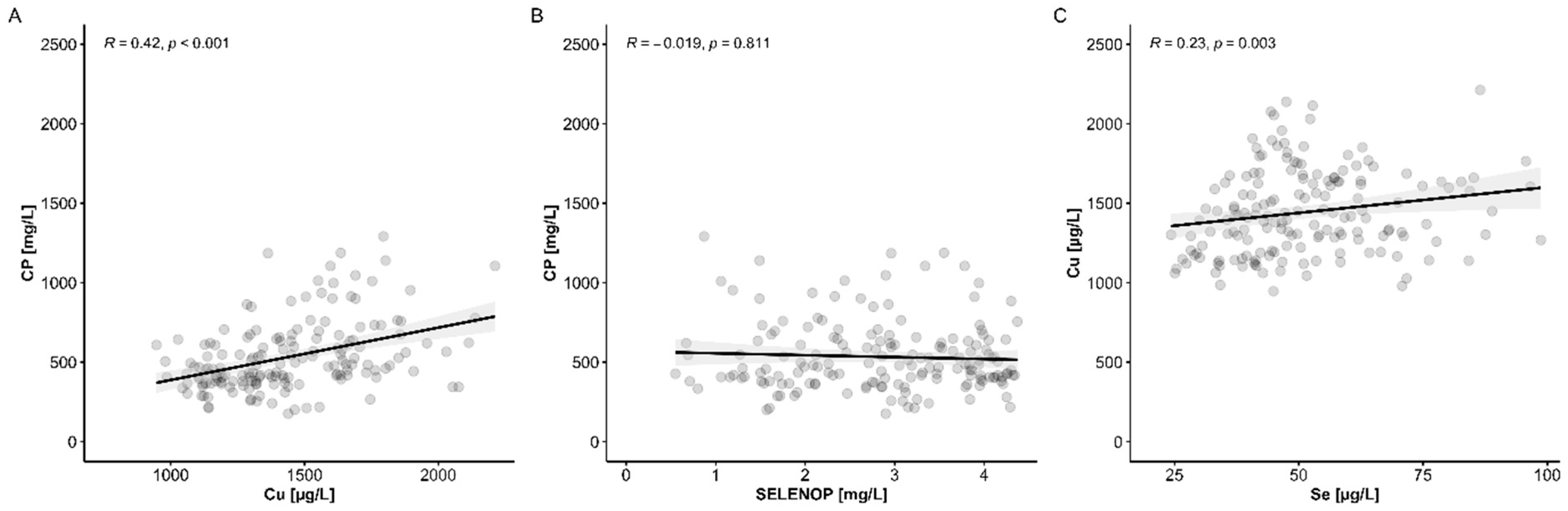
3.2. Copper (Cu) Status Analysis and Comparison to Serum Se Status
3.3. Cu Status of COVID-19 Patients in Relation to Survival
3.4. Dynamics of Serum Cu Status in COVID-19 in Comparison to Se Status and in Relation to Survival
3.5. Predictive Value of Compound Biomarkers inclucing the Cu Status for Surviving COVID-19
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Galmes, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R. Sorting Out Whether Vitamin D Deficiency Raises COVID-19 Risk. JAMA 2021, 325, 329–330. [Google Scholar] [CrossRef]
- Weir, E.K.; Thenappan, T.; Bhargava, M.; Chen, Y.J. Does vitamin D deficiency increase the severity of COVID-19? Clin. Med. 2020, 20, E107–E108. [Google Scholar] [CrossRef]
- Ferrari, D.; Locatelli, M.; Briguglio, M.; Lombardi, G. Is there a link between vitamin D status, SARS-CoV-2 infection risk and COVID-19 severity? Cell Biochem. Funct. 2021, 39, 35–47. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Di Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2020, 44, 765–771. [Google Scholar] [CrossRef]
- Vickaryous, N.; Garjani, A.; Jacobs, B.; Jitlal, M.; Middleton, R.; Das Nair, R.; Tuite-Dalton, K.; Hunter, R.; Chandran, S.; Macdougall, N. Vitamin D levels in the UK MS population and COVID-19 susceptibility. Mult. Scler. J. 2020, 26, 55. [Google Scholar]
- Bennouar, S.; Cherif, A.B.; Kessira, A.; Bennouar, D.E.; Abdi, S. Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J. Am. Coll. Nutr. 2020, 40, 104–110. [Google Scholar] [CrossRef]
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: Mixing up cause and consequence. Metabolism 2021, 115, 154434. [Google Scholar] [CrossRef]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Keflie, T.S.; Biesalski, H.K. Micronutrients and bioactive substances: Their potential roles in combating COVID-19. Nutrition 2020, 84, 111103. [Google Scholar] [CrossRef]
- Grober, U.; Holick, M.F. The coronavirus disease (COVID-19)—A supportive approach with selected micronutrients. Int. J. Vitam. Nutr. Res. 2021, 1–22. [Google Scholar] [CrossRef]
- Ma, C.; Hoffmann, P.R. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. The immune system and the impact of zinc during aging. Immun. Ageing 2009, 6, 9. [Google Scholar] [CrossRef]
- Hardy, G.; Hardy, I.; Manzanares, W. Selenium supplementation in the critically ill. Nutr. Clin. Pract. 2012, 27, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Joachimiak, M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021, 15, e0008895. [Google Scholar] [CrossRef]
- Haase, H.; Schomburg, L. You’d Better Zinc-Trace Element Homeostasis in Infection and Inflammation. Nutrients 2019, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Stoedter, M.; Renko, K.; Hog, A.; Schomburg, L. Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem. J. 2010, 429, 43–51. [Google Scholar] [CrossRef]
- Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Rink, L.; Mariani, E.; Fulop, T.; Dedoussis, G.; Herbein, G.; Provinciali, M.; et al. Main biomarkers associated with age-related plasma zinc decrease and copper/zinc ratio in healthy elderly from ZincAge study. Eur. J. Nutr. 2017, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I. Interactions between nutrition and immune function: Using inflammation biomarkers to interpret micronutrient status. Proc. Nutr. Soc. 2014, 73, 1–8. [Google Scholar] [CrossRef]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Sak, S.; Barut, M.; Celik, H.; Incebiyik, A.; Agacayak, E.; Uyanikoglu, H.; Kirmit, A.; Sak, M. Copper and ceruloplasmin levels are closely related to the severity of preeclampsia. J. Matern. Fetal Neonatal Med. 2020, 33, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Royer, A.; Shah, N.J. Biochemistry, Ceruloplasmin; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hackler, J.; Wisniewska, M.; Greifenstein-Wiehe, L.; Minich, W.B.; Cremer, M.; Buhrer, C.; Schomburg, L. Copper and selenium status as biomarkers of neonatal infections. J. Trace Elem. Med. Biol. 2020, 58, 126437. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Beck, M.A.; Handy, J.; Levander, O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004, 12, 417–423. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Tekin, O.M.; Yazihan, N.; Sahin, D. The Relation Between Trace Element Status (Zinc, Copper, Magnesium) and Clinical Outcomes in COVID-19 Infection During Pregnancy. Biol. Trace Elem. Res. 2020, 1–10. [Google Scholar] [CrossRef]
- Camakaris, J.; Voskoboinik, I.; Mercer, J.F. Molecular mechanisms of copper homeostasis. Biochem. Biophys. Res. Commun. 1999, 261, 225–232. [Google Scholar] [CrossRef]
- Lalioti, V.; Muruais, G.; Tsuchiya, Y.; Pulido, D.; Sandoval, I.V. Molecular mechanisms of copper homeostasis. Front. Biosci. 2009, 14, 4878–4903. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, J.; Varona, S.; Canes, L.; Galan, M.; Briones, A.M.; Cachofeiro, V.; Rodriguez, C. Emerging Roles of Lysyl Oxidases in the Cardiovascular System: New Concepts and Therapeutic Challenges. Biomolecules 2019, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Washington-Hughes, C.; Ralle, M.; Schmidt, K. Copper and the brain noradrenergic system. J. Biol. Inorg. Chem. 2019, 24, 1179–1188. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Wildman, R.E.C. Newer findings on a unified perspective of copper restriction and cardiomyopathy. Proc. Soc. Exp. Biol. Med. 1997, 215, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Altarelli, M.; Ben-Hamouda, N.; Schneider, A.; Berger, M.M. Copper Deficiency: Causes, Manifestations, and Treatment. Nutr. Clin. Pract. 2019, 34, 504–513. [Google Scholar] [CrossRef]
- Tan, J.C.; Burns, D.L.; Jones, H.R. Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. J. Parenter. Enter. Nutr. 2006, 30, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Btaiche, I.F.; Yeh, A.Y.; Wu, I.J.; Khalidi, N. Neurologic dysfunction and pancytopenia secondary to acquired copper deficiency following duodenal switch: Case report and review of the literature. Nutr. Clin. Pract. 2011, 26, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kaler, S.G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011, 7, 15–29. [Google Scholar] [CrossRef]
- Hammadah, M.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H. Prognostic value of elevated serum ceruloplasmin levels in patients with heart failure. J. Card. Fail. 2014, 20, 946–952. [Google Scholar] [CrossRef]
- Wiehe, L.; Cremer, M.; Wisniewska, M.; Becker, N.P.; Rijntjes, E.; Martitz, J.; Hybsier, S.; Renko, K.; Buhrer, C.; Schomburg, L. Selenium status in neonates with connatal infection. Br. J. Nutr. 2016, 116, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M.; Cremer, M.; Wiehe, L.; Becker, N.P.; Rijntjes, E.; Martitz, J.; Renko, K.; Buhrer, C.; Schomburg, L. Copper to Zinc Ratio as Disease Biomarker in Neonates with Early-Onset Congenital Infections. Nutrients 2017, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Jenab, M.; Freisling, H.; Becker, N.P.; Czuban, M.; Tjonneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.C.; Mancini, F.R.; et al. Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Carcinogenesis 2017, 38, 699–707. [Google Scholar] [CrossRef]
- Stepien, M.; Hughes, D.J.; Hybsier, S.; Bamia, C.; Tjonneland, A.; Overvad, K.; Affret, A.; His, M.; Boutron-Ruault, M.C.; Katzke, V.; et al. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br. J. Cancer 2017, 116, 688–696. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer Inc.: New York, NY, USA, 2009. [Google Scholar]
- Zhang, Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef]
- Akaike, H. Likelihood of a model and information criteria. J. Econom. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Sato, M.; Gitlin, J.D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J. Biol. Chem. 1991, 266, 5128–5134. [Google Scholar] [CrossRef]
- Middleton, R.B.; Linder, M.C. Synthesis and turnover of ceruloplasmin in rats treated with 17 beta-estradiol. Arch. Biochem. Biophys. 1993, 302, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Galloway, P.; McMillan, D.C.; Sattar, N. Effect of the inflammatory response on trace element and vitamin status. Ann. Clin. Biochem. 2000, 37 Pt 3, 289–297. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef]
- Renko, K.; Hofmann, P.J.; Stoedter, M.; Hollenbach, B.; Behrends, T.; Kohrle, J.; Schweizer, U.; Schomburg, L. Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J. 2009, 23, 1758–1765. [Google Scholar] [CrossRef]
- Nichol, C.; Herdman, J.; Sattar, N.; O’Dwyer, P.J.; O’Reilly, D.S.J.; Littlejohn, D.; Fell, G. Changes in the concentrations of plasma selenium and selenoproteins after minor elective surgery: Further evidence for a negative acute phase response? Clin. Chem. 1998, 44, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.L.; Yang, Q.; Yuan, P.; Wang, X.; Cheng, L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021, 35, e21392. [Google Scholar] [CrossRef]
- Lee, C.; Choi, W.J. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch. Pharm. Res. 2021, 44, 99–116. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Van Damme, J.; Rieder, H.; Meyer zum Buschenfelde, K.H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur. J. Immunol. 1988, 18, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, N.L.; Willmore, W.G. Regulation of iron pathways in response to hypoxia. Free Radic. Biol. Med. 2011, 50, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Martitz, J.; Becker, N.P.; Renko, K.; Stoedter, M.; Hybsier, S.; Schomburg, L. Gene-specific regulation of hepatic selenoprotein expression by interleukin-6. Metallomics 2015, 7, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.P.; Martitz, J.; Renko, K.; Stoedter, M.; Hybsier, S.; Cramer, T.; Schomburg, L. Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics 2014, 6, 1079–1086. [Google Scholar] [CrossRef]
- Mittag, J.; Behrends, T.; Nordstrom, K.; Anselmo, J.; Vennstrom, B.; Schomburg, L. Serum copper as a novel biomarker for resistance to thyroid hormone. Biochem. J. 2012, 443, 103–109. [Google Scholar] [CrossRef]
- Mittag, J.; Behrends, T.; Hoefig, C.S.; Vennstrom, B.; Schomburg, L. Thyroid hormones regulate selenoprotein expression and selenium status in mice. PLoS ONE 2010, 5, e12931. [Google Scholar] [CrossRef]
- Schwarz, Y.; Percik, R.; Oberman, B.; Yaffe, D.; Zimlichman, E.; Tirosh, A. Sick Euthyroid Syndrome on Presentation of Patients With COVID-19: A Potential Marker for Disease Severity. Endocr. Pract. 2021, 27, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Parohan, M.; Yaghoubi, S.; Seraji, A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol. Res. 2020, 50, 924–935. [Google Scholar] [CrossRef]



| Death | Discharge | Total | Samples | |
|---|---|---|---|---|
| Sex | ||||
| Female | 5 (71.4%) | 15 (53.6%) | 20 (57.1%) | 116 (67.1%) |
| Male | 2 (28.6%) | 13 (46.4%) | 15 (42.9%) | 57 (32.9%) |
| Age | ||||
| Median (IQR) | 89 (81, 94) | 69 (38, 91) | 77 (38, 94) | |
| Time to discharge or death [d] | ||||
| Median (IQR) | 8 (3, 33) | 20 (4, 47) | 16 (3, 47) |
| Age | Cu | SELENOP | Age + Cu + SELENOP | |
|---|---|---|---|---|
| Age | −3.59 * | −6.79 *** | ||
| [−6.44, −0.74] | [−9.49, −4.09] | |||
| Cu | 0.70 ** | 0.97 * | ||
| [0.24, 1.15] | [0.27, 1.66] | |||
| SELENOP | 1.09 *** | 1.61 *** | ||
| [0.64, 1.54] | [0.75, 2.46] | |||
| N | 35 | 167 | 167 | 167 |
| AIC | 23.3 | 164.9 | 147.5 | 82.8 |
| BIC | 26.4 | 171.1 | 153.8 | 98.4 |
| Pseudo R2 | 0.57 | 0.10 | 0.24 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients 2021, 13, 1898. https://doi.org/10.3390/nu13061898
Hackler J, Heller RA, Sun Q, Schwarzer M, Diegmann J, Bachmann M, Moghaddam A, Schomburg L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients. 2021; 13(6):1898. https://doi.org/10.3390/nu13061898
Chicago/Turabian StyleHackler, Julian, Raban Arved Heller, Qian Sun, Marco Schwarzer, Joachim Diegmann, Manuel Bachmann, Arash Moghaddam, and Lutz Schomburg. 2021. "Relation of Serum Copper Status to Survival in COVID-19" Nutrients 13, no. 6: 1898. https://doi.org/10.3390/nu13061898
APA StyleHackler, J., Heller, R. A., Sun, Q., Schwarzer, M., Diegmann, J., Bachmann, M., Moghaddam, A., & Schomburg, L. (2021). Relation of Serum Copper Status to Survival in COVID-19. Nutrients, 13(6), 1898. https://doi.org/10.3390/nu13061898







