Association of Carbohydrate and Fat Intake with Prevalence of Metabolic Syndrome Can Be Modified by Physical Activity and Physical Environment in Ecuadorian Adults: The ENSANUT-ECU Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. General Characteristics
2.4. Physical Environmental Conditions
2.5. Health-Related Lifestyles
2.6. Dietary Assessment
2.7. Metabolic Syndrome
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- IDF Communications. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2006; pp. 4–7. [Google Scholar]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, R.M.; Brown, T.M.; Muntner, P. Epidemiology of Obesity, the Metabolic Syndrome, and Chronic Kidney Disease. Curr. Hypertens. Rep. 2012, 14, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Galarza, J.; Baldeón, L.; Franco, O.H.; Muka, T.; Drexhage, H.A.; Voortman, T.; Freire, W.B. Prevalence of overweight and metabolic syndrome, and associated sociodemographic factors among adult Ecuadorian populations: The ENSANUT-ECU study. J. Endocrinol. Investig. 2021, 44, 63–74. [Google Scholar] [CrossRef]
- National Institute of Statistics and Censuses. Yearbook of Vital Statistics Births and Deaths 2019. Available online: https://www.ecuadorencifras.gob.ec/documentos/webinec/Poblacion_y_Demografia/Nacimientos_Defunciones/2020/Boletin_%20tecnico_%20EDG%202019%20prov.pdf (accessed on 5 June 2020).
- Gosadi, I.M. Assessment of the environmental and genetic factors influencing prevalence of metabolic syndrome in Saudi Arabia. Saudi Med. J. 2016, 37, 12–20. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar]
- Juna, C.F.; Cho, Y.H.; Joung, H. Low Elevation and Physical Inactivity are Associated with a Higher Prevalence of Metabolic Syndrome in Ecuadorian Adults: A National Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2020, 13, 2217–2226. [Google Scholar] [CrossRef]
- Juna, C.F.; Cho, Y.H.; Ham, D.; Joung, H. Associations of Relative Humidity and Lifestyles with Metabolic Syndrome among the Ecuadorian Adult Population: Ecuador National Health and Nutrition Survey (ENSANUT-ECU) 2012. Int. J. Environ. Res. Public Health 2020, 17, 9023. [Google Scholar] [CrossRef]
- Voss, J.D.; Masuoka, P.; Webber, B.J.; I Scher, A.; Atkinson, R.L. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int. J. Obes. 2013, 37, 1407–1412. [Google Scholar] [CrossRef] [Green Version]
- Pico, S.M.; Bergonzoli, G.; Contreras, A. Risk factors associated with the metabolic syndrome in Cali, Colombia (2013): A case-control study. Biomédica 2019, 39, 46–54. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Ambrosini, G.; Huang, R.-C.; Mori, T.; Hands, B.; O’Sullivan, T.; de Klerk, N.; Beilin, L.; Oddy, W. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Ha, K.; Joung, H.; Song, Y. Low-carbohydrate diet and the risk of metabolic syndrome in Korean adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1122–1132. [Google Scholar] [CrossRef]
- Choi, H.; Song, S.; Kim, J.; Chung, J.; Yoon, J.; Paik, H.-Y.; Song, Y. High carbohydrate intake was inversely associated with high-density lipoprotein cholesterol among Korean adults. Nutr. Res. 2012, 32, 100–106. [Google Scholar] [CrossRef]
- Mansoor, N.; Vinknes, K.J.; Veierod, M.B.; Retterstol, K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016, 115, 466–479. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- Naude, C.E.; Schoonees, A.; Senekal, M.; Young, T.; Garner, P.; Volmink, J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: A systematic review and meta-analysis. PLoS ONE 2014, 9, e100652, Erratum in 2018, 13, e0200284. [Google Scholar] [CrossRef]
- Drehmer, M.; Odegaard, A.O.; Schmidt, M.I.; Duncan, B.B.; Cardoso, L.D.O.; Matos, S.M.A.; Molina, M.D.C.B.; Barreto, S.M.; Pereira, M.A. Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol. Metab. Syndr. 2017, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, E.P.; McLellan, K.C.P.; Vaz de Arruda Silveira, L.; Burini, R.C. Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr. J. 2012, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Nabuco, H.C.; Tomeleri, C.M.; Junior, P.S.; Fernandes, R.D.R.; Cavalcante, E.F.; Antunes, M.; Burini, R.C.; Venturini, D.; Barbosa, D.S.; Silva, A.M.; et al. Lower protein and higher carbohydrate intake are related with altering metabolic syndrome components in elderly women: A cross-sectional study. Exp. Gerontol. 2018, 103, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Freire, W.B.; Ramírez-Luzuriaga, M.J.; Belmont, P.; Mendieta, M.J.; Silva-Jaramillo, K.M.; Romero, N.; Saenz, K.; Pineiros, P.; Gomez, L.F.; Monge, R. Encuesta Nacional de Salud y Nutrición de la Población Ecuatoriana de Cero a 59 Años, 1st ed.; ENSANUT-ECU 2012; Ministerio de Salud Pública/Instituto Nacional de Estadísticas y Censos: Quito, Ecuador, 2014. [Google Scholar]
- Terrados-Cepeda, N. Fisiología del Ejercicio en Altitud. In Fisiología de la Actividad Física y del Deporte; Gonzales Gallego, J., Ed.; Interamericana/McGraw-Hill: Barcelona, Spain; New York, NY, USA, 1992; pp. 287–298. [Google Scholar]
- McLean, B.; Buttifant, D.; Gore, C.; White, K.; Liess, C.; Kemp, J. Physiological and performance responses to a preseason altitude-training camp in elite team-sport athletes. Int. J. Sports Physiol. Perform. 2013, 8, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Meteorología e Hidrología de Ecuador. Anuario Meteorológico No. 52–2012. Available online: http://www.serviciometeorologico.gob.ec/wpcontent/uploads/anuarios/meteorologicos/Am%202012.pdf (accessed on 5 January 2020).
- Tyrovolas, S.; Chalkias, C.; Morena, M.; Kalogeropoulos, K.; Tsakountakis, N.; Zeimbekis, A.; Gotsis, E.; Metallinos, G.; Bountziouka, V.; Lionis, C.; et al. High relative environmental humidity is associated with diabetes among elders living in Mediterranean islands. J. Diabetes Metab. Disord. 2014, 13, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonocore, C.; De Vecchi, R.; Scalco, V.; Lamberts, R. Influence of relative air humidity and movement on human thermal perception in classrooms in a hot and humid climate. Build. Environ. 2018, 146, 98–106. [Google Scholar] [CrossRef]
- Ryan, H.; Trosclair, A.; Gfroerer, J. Adult Current Smoking: Differences in Definitions and Prevalence Estimates—NHIS and NSDUH, 2008. J. Environ. Public Health 2012, 2012, 918368. [Google Scholar] [CrossRef] [Green Version]
- US Department of Health and Human Services. Physical Activity Guidelines for Americans; US Department of Health and Human Services: Hyattsville, MD, USA, 2008. Available online: http://www.health.gov/paguidelines (accessed on 5 January 2020).
- Ministerio de Salud Pública del Ecuador y Organización de las Naciones Unidas para la Alimentación y la Agricultura. Documento Técnico de las Guías Alimentarias Basadas en Alimentos (GABA) del Ecuador; GABA-ECU 2018; Government of Ecuador: Quito, Ecuador, 2018.
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- De Onis, M.; Habicht, J.P. Anthropometric reference data for international use: Recommendations from a World Health Organization Expert Committee. Am. J. Clin. Nutr. 1996, 64, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Eblen-Zajjur, M.; Eblen-Zajjur, A. Cálculo de la concentración de colesterol de la lipoproteína de baja densidad: Análisis de regresión versus fórmula de Friedewald. Revista Médica de Chile 2001, 129, 1263–1270. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute Inc. SAS/STAT® 9.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Churuangsuk, C.; Kherouf, M.; Combet, E.; Lean, M. Low-carbohydrate diets for overweight and obesity: A systematic review of the systematic reviews. Obes. Rev. 2018, 19, 1700–1718. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-L.; Liu, W.-J.; Wang, J.-S. Associations of low-carbohydrate and low-fat intakes with all-cause mortality in subjects with prediabetes with and without insulin resistance. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Duda, M.; O’Shea, K.M.; Lei, B.; Barrows, B.R.; Azimzadeh, A.M.; McElfresh, T.E.; Hoit, B.D.; Kop, W.J.; Stanley, W.C. Low-Carbohydrate/High-Fat Diet Attenuates Pressure Overload-Induced Ventricular Remodeling and Dysfunction. J. Card. Fail. 2008, 14, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.; Brinkworth, G.D.; Noakes, M.; Keogh, J.; Clifton, P. Metabolic Effects of Weight Loss on a Very-Low-Carbohydrate Diet Compared With an Isocaloric High-Carbohydrate Diet in Abdominally Obese Subjects. J. Am. Coll. Cardiol. 2008, 51, 59–67. [Google Scholar] [CrossRef]
- Triantafyllidi, H.; Benas, D.; Vlachos, S.; Vlastos, D.; Pavlidis, G.; Schoinas, A.; Varoudi, M.; Birmpa, D.; Moutsatsou, P.; Lekakis, J.; et al. HDL cholesterol levels and endothelial glycocalyx integrity in treated hypertensive patients. J. Clin. Hypertens. 2018, 20, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, C.; Zhang, S.; Li, R.; Zhang, Y.; He, P.; Zhang, Z.; Liu, M.; Zhou, C.; Ye, Z.; et al. Dietary Carbohydrate Intake and New-Onset Hypertension: A Nationwide Cohort Study in China. Hypertension 2021. [Google Scholar] [CrossRef]
- He, D.; Sun, N.; Xiong, S.; Qiao, Y.; Ke, C.; Shen, Y. Association between the proportions of carbohydrate and fat intake and hypertension risk: Findings from the China Health and Nutrition Survey. J. Hypertens. 2021. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thakur, G.; Kumar, B.D.; Mitra, A.; Chakraborty, C. Long-term effects of a carbohydrate-rich diet on fasting blood sugar, lipid profile, and serum insulin values in rural Bengalis. J. Diabetes 2009, 1, 288–295. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Lee, H.-S.; Lee, J.-W. Association of carbohydrate and fat intake with metabolic syndrome. Clin. Nutr. 2018, 37, 746–751. [Google Scholar] [CrossRef]
- Ha, K.; Kim, K.; Chun, O.K.; Joung, H.; Song, Y. Differential association of dietary carbohydrate intake with metabolic syndrome in the US and Korean adults: Data from the 2007–2012 NHANES and KNHANES. Eur. J. Clin. Nutr. 2018, 72, 848–860. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The Effects of High Protein Diets on Thermogenesis, Satiety and Weight Loss: A Critical Review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.N.; Kondrup, J.; Børsheim, E. Health effects of protein intake in healthy adults: A systematic literature review. Food Nutr. Res. 2013, 57, 21245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, R.D.; Cardoso, M.A.; Gimeno, S.G.A.; Ferreira, S.R.G. Dietary Fat Is Associated with Metabolic Syndrome in Japanese Brazilians. Diabetes Care 2005, 28, 1779–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, W.W.; Kim, J.E.; Amankwaah, A.F.; Gordon, S.L.; Weinheimer-Haus, E.M. Higher Total Protein Intake and Change in Total Protein Intake Affect Body Composition but Not Metabolic Syndrome Indexes in Middle-Aged Overweight and Obese Adults Who Perform Resistance and Aerobic Exercise for 36 Weeks. J. Nutr. 2015, 145, 2076–2083. [Google Scholar] [CrossRef] [Green Version]
- Hruby, A.; Jacques, P.F. Dietary protein and changes in markers of cardiometabolic health across 20 years of follow-up in middle-aged Americans. Public Health Nutr. 2018, 21, 2998–3010. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.E.; McGregor, G.R.; Enfield, K.B. Humidity: A review and primer on atmospheric moisture and human health. Environ. Res. 2016, 144, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Sharif Nia, H.; Chan, Y.H.; Froelicher, E.S.; Pahlevan Sharif, S.; Yaghoobzadeh, A.; Jafari, A.; Goudarzian, A.H.; Pourkia, R.; Haghdoost, A.A.; Arefinia, F.; et al. Weather fluctuations: Predictive factors in the prevalence of acute coronary syndrome. Health Promot. Perspect. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Gouveia, N.; Hajat, S.; Armstrong, B. Socioeconomic differentials in the temperature-mortality relationship in Sao Paulo, Brazil. Int. J. Epidemiol. 2003, 32, 390–397. [Google Scholar] [CrossRef] [Green Version]
- López-Pascual, A.; Bes-Rastrollo, M.; Sayon-Orea, C.; Pérez-Cornago, A.; Díaz-Gutiérrez, J.; Pons, J.J.; Martínez-González, M.A.; González-Muniesa, P.; Martínez, J.A. Living at a Geographically Higher Elevation Is Associated with Lower Risk of Metabolic Syndrome: Prospective Analysis of the SUN Cohort. Front. Physiol. 2017, 7, 658. [Google Scholar] [CrossRef] [Green Version]
- López-Pascual, A.; Arévalo, J.; Martínez, J.A.; González-Muniesa, P. Inverse Association Between Metabolic Syndrome and Altitude: A Cross-Sectional Study in an Adult Population of Ecuador. Front. Endocrinol. 2018, 9, 658. [Google Scholar] [CrossRef]
- Hirschler, V. Cardiometabolic risk factors in native populations living at high altitudes. Int. J. Clin. Pr. 2016, 70, 113–118. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Ader, M.; Bergman, R.N. Glucose Homeostasis During Short-term and Prolonged Exposure to High Altitudes. Endocr. Rev. 2015, 36, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Horscroft, J.A.; Kotwica, A.O.; Laner, V.; West, J.A.; Hennis, P.J.; Levett, D.Z.H.; Howard, D.J.; Fernandez, B.O.; Burgess, S.L.; Ament, Z.; et al. Metabolic basis to Sherpa altitude adaptation. Proc. Natl. Acad. Sci. USA 2017, 114, 6382–6387. [Google Scholar] [CrossRef] [Green Version]
- Louis, M.; Punjabi, N.M. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J. Appl. Physiol. 2009, 106, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Gangwar, A.; Pooja; Sharma, M.; Singh, K.; Patyal, A.; Bhaumik, G.; Bhargava, K.; Sethy, N.K. Intermittent normobaric hypoxia facilitates high altitude acclimatization by curtailing hypoxia-induced inflammation and dyslipidemia. Pflugers Arch. Eur. J. Physiol. 2019, 471, 949–959. [Google Scholar] [CrossRef]
- Maughan, R.J.; Otani, H.; Watson, P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2012, 112, 2313–2321. [Google Scholar] [CrossRef]
- Chmura, P.; Konefal, M.; Andrzejewski, M.; Kosowski, J.; Rokita, A.; Chmura, J. Physical activity profile of 2014 FIFA World Cup players, with regard to different ranges of air temperature and relative humidity. Int. J. Biometeorol. 2017, 61, 677–684. [Google Scholar] [CrossRef]
- Rennie, K.L.; McCarthy, N.; Yazdgerdi, S.; Marmot, M.; Brunner, E. Association of the metabolic syndrome with both vigorous and moderate physical activity. Int. J. Epidemiol. 2003, 32, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [Green Version]

| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | LCHF | MCF | HCLF | p-Value a | LCHF | MCF | HCLF | p-Value |
| Number (%) | 330(16.8) | 1410 (71.8) | 224 (11.4) | 797 (19.7) | 2907 (71.6) | 355 (8.7) | ||
| Age(years), N (%) | 0.7632 | 0.1004 | ||||||
| 20–29 | 121 (39.7) | 514 (39.2) | 70 (33.9) | 255 (32.4) | 1051 (37.1) | 115 (33.7) | ||
| 30–39 | 106 (27.6) | 468 (28.5) | 85 (36.0) | 293 (32.9) | 1024 (28.9) | 139 (33.0) | ||
| 40–49 | 80 (21.4) | 317 (20.6) | 50 (17.8) | 222 (27.3) | 691 (23.2) | 79 (19.3) | ||
| 50–59 | 23 (11.3) | 111 (11.7) | 19 (12.3) | 27 (7.4) | 141 (10.8) | 22 (14.0) | ||
| Ethnicity, N (%) | 0.0917 | 0.0486 | ||||||
| Mestizo | 304 (88.9) | 1247 (82.3) | 188 (79.4) | 723 (87.2) | 2562 (83.4) | 298 (77.5) | ||
| Others | 26 (11.1) | 163 (17.7) | 36 (20.6) | 74 (12.8) | 345 (16.6) | 57 (22.5) | ||
| Family economic status b, N (%) | <0.0001 | <0.0001 | ||||||
| Low | 55 (11.7) | 388 (26.7) | 90 (35.7) | 174 (18.1) | 897 (26.8) | 164 (45.9) | ||
| Middle | 133 (37.7) | 695 (47.4) | 105 (51.0) | 350 (39.5) | 1390 (45.5) | 151 (42.9) | ||
| High | 142 (50.6) | 327 (25.9) | 29 (13.3) | 273 (42.4) | 630 (27.7) | 40 (11.2) | ||
| Education level, N (%) | <0.0001 | <0.0001 | ||||||
| Primary school | 59 (18.9) | 354 (23.4) | 94 (43.0) | 173 (21.8) | 828 (27.2) | 130 (41.4) | ||
| Secondary school | 147 (39.1) | 714 (53.2) | 103 (44.5) | 369 (44.7) | 1368 (47.2) | 174 (46.1) | ||
| College or higher | 124 (42.0) | 342 (23.4) | 27 (12.5) | 255 (33.5) | 711 (25.6) | 51 (12.5) | ||
| Current alcohol consumption c, N (%) | 0.8775 | 0.4129 | ||||||
| Yes | 190 (58.7) | 784 (57.8) | 135 (55.7) | 235 (27.2) | 753 (25.6) | 76 (21.7) | ||
| No | 140 (41.3) | 626 (42.2) | 89 (44.3) | 562 (72.8) | 2154 (74.4) | 279 (78.3) | ||
| Current smoking d, N (%) | 0.1374 | 0.1013 | ||||||
| Yes | 125 (33.3) | 467 (29.6) | 61 (22.6) | 56 (7.1) | 165 (6.7) | 11 (2.4) | ||
| No | 205 (66.7) | 943 (70.4) | 163 (77.4) | 741 (92.9) | 2742 (93.3) | 344 (97.6) | ||
| Physical activity e, N (%) | 0.8032 | 0.5826 | ||||||
| Yes | 147 (44.0) | 608 (42.0) | 95 (44.6) | 138 (19.2) | 478 (17.7) | 59 (15.5) | ||
| No | 183 (66.0) | 802 (58.0) | 129 (55.4) | 659 (80.8) | 2429 (82.3) | 296 (84.5) | ||
| Elevation f, N (%) | <0.0001 | <0.0001 | ||||||
| High | 167 (52.8) | 557 (38.3) | 59 (25.4) | 367 (51.3) | 1014 (38.0) | 88 (26.5) | ||
| Low | 163 (47.2) | 853 (61.7) | 165 (74.6) | 430 (48.7) | 1893 (62.0) | 267 (73.5) | ||
| Humidity g, N(%) | 0.1431 | 0.0004 | ||||||
| High | 173 (30.5) | 783 (34.5) | 139 (59.0) | 443 (30.6) | 1843 (38.4) | 249 (47.7) | ||
| Low | 157 (69.5) | 627 (65.5) | 85 (41.0) | 354 (69.4) | 1064 (61.6) | 106 (52.6) | ||
| Variables | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| LCHF | MCF | HCLF | p-Value a | LCHF | MCF | HCLF | p-Value | |
| (N = 330) | (N = 1410) | (N = 224) | (N = 797) | (N = 2907) | (N = 355) | |||
| Anthropometric and biochemical variables (mean ± SE) | ||||||||
| BMI (kg/m2) | 26.3 ± 0.4 | 26.8 ± 0.2 | 26.1 ± 0.4 | 0.0433 | 27.1 ± 0.3 | 27.3 ± 0.1 | 28.1 ± 0.5 | <0.0001 |
| Waist circumference (cm) | 101.9 ± 7.7 | 93.6 ± 1.2 | 93.7 ± 4.7 | <0.0001 | 98.4 ± 5.1 | 94.9 ± 2.9 | 95.9 ± 3.9 | 0.1364 |
| SBP (mmHg) | 132.5 ± 7.9 | 122.3 ± 0.5 | 127.9 ± 1.7 | <0.0001 | 116.3 ± 2.6 | 115.3 ± 0.9 | 120.1 ± 2.9 | 0.0007 |
| DBP (mmHg) | 88.8 ± 8.3 | 77.1 ± 0.6 | 81.9 ± 4.7 | <0.0001 | 73.73 ± 2.7 | 72.6 ± 0.9 | 76.6 ± 2.9 | 0.0047 |
| Fasting glucose (mg/dL) | 94.4 ± 2.0 | 94.3 ± 1.3 | 94.0 ± 1.3 | 0.5412 | 93.0 ± 1.4 | 92.5 ± 0.8 | 95.3 ± 2.0 | 0.0126 |
| Total cholesterol (mg/dL) | 190.8 ± 3.5 | 184.8 ± 1.5 | 187.0 ± 3.8 | 0.5957 | 183.4 ± 2.0 | 179.7 ± 1.2 | 177.1 ± 2.8 | 0.0002 |
| HDL cholesterol (mg/dL) | 41.5 ± 1.1 | 40.8 ± 0.4 | 42.5 ± 0.9 | 0.0012 | 47.4 ± 0.7 | 46.3 ± 0.4 | 45.4 ± 0.9 | <0.0001 |
| LDL cholesterol (mg/dL) | 113.2 ± 2.6 | 111.4 ± 1.3 | 110.4 ± 2.7 | 0.3310 | 112.2 ± 1.8 | 108.1 ± 0.9 | 106.6 ± 2.4 | <0.0001 |
| Triglyceride (mg/dL) | 194.1 ± 15.1 | 171.7 ± 4.5 | 174.6 ± 11.1 | 0.5907 | 119.8 ± 3.7 | 129.1 ± 2.8 | 126.6 ± 6.1 | 0.0008 |
| Macronutrient intake (mean ± SE) | ||||||||
| Energy (kcal) | 2024 ± 34.6 | 2160.6 ± 16.3 | 2142.6 ± 46.7 | <0.0001 | 1846.7 ± 26.8 | 1832.7 ± 11.5 | 1928.4 ± 36.4 | <0.0001 |
| Carbohydrate(g) | 214.4 ± 7.9 | 322.7 ± 2.6 | 373.3 ± 8.0 | <0.0001 | 188.8 ± 7.3 | 271.1 ± 1.8 | 338.7 ± 6.6 | <0.0001 |
| Protein (g) | 78.3 ± 2.8 | 71.3 ± 0.6 | 64.9 ± 1.5 | <0.0001 | 71.9 ± 2.4 | 59.9 ± 0.4 | 57.3 ± 1.1 | <0.0001 |
| Fat (g) | 86.4 ± 3.4 | 65.0 ± 0.6 | 41.6 ± 1.0 | <0.0001 | 81.1 ± 3.1 | 56.7 ± 0.4 | 37.7 ± 0.7 | <0.0001 |
| % Energy from | ||||||||
| Carbohydrate | 42.2 ± 0.6 | 59.8 ± 0.2 | 69.8 ± 0.3 | <0.0001 | 40.7 ± 0.6 | 59.8 ± 0.1 | 70.3 ± 0.2 | <0.0001 |
| Protein | 15.6 ± 0.4 | 13.3 ± 0.1 | 12.2 ± 0.1 | <0.0001 | 15.9 ± 0.4 | 13.2 ± 0.1 | 12.0 ± 0.1 | <0.0001 |
| Fat | 38.3 ± 0.6 | 27.0 ± 0.1 | 17.5 ± 0.2 | <0.0001 | 39.3 ± 0.5 | 27.8 ± 0.2 | 17.6 ± 0.1 | <0.0001 |
| EER% | 72.0 ± 3.6 | 83.3 ± 0.8 | 84.9 ± 2.3 | <0.0001 | 95.1 ± 3.6 | 97.6 ± 0.7 | 103.9 ± 2.1 | <0.0001 |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| LCHF a | MCF b | HCLF c | LCHF | MCF | HCLF | |
| (N = 330) | (N = 1410) | (N = 224) | (N = 797) | (N = 2907) | (N = 355) | |
| Increased waist circumference | ||||||
| Prevalence (%) | 55.8 | 47.28 | 53.55 | 63.86 | 67.13 | 70.38 |
| OR (95% CI) | 1.67 (0.95–2.97) | 1.38 (0.93–2.04) | 1.00 | 0.85 (0.54–1.34) | 0.75 (0.50–1.13) | 1.00 |
| Elevated blood pressure | ||||||
| Prevalence (%) | 25.01 | 28.99 | 30.31 | 10.70 | 13.72 | 24.97 |
| OR (95% CI) | 0.87 (0.50–1.55) | 0.94 (0.61–1.44) | 1.00 | 0.34 (0.19–0.59) | 0.50 (0.32–0.79) | 1.00 |
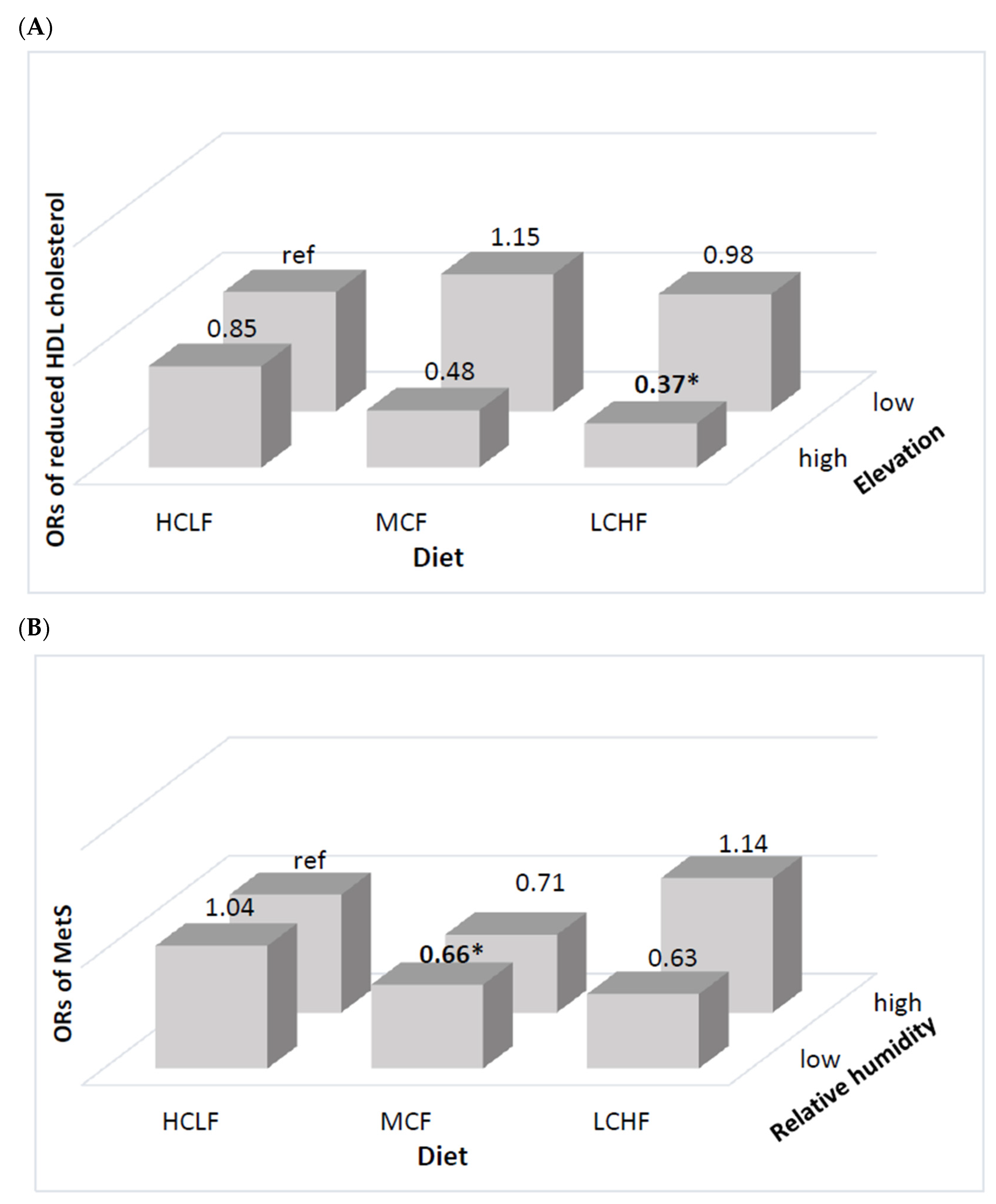
| Reduced HDL cholesterol | ||||||
| Prevalence (%) | 50.44 | 42.25 | 50.73 | 59.73 | 65.34 | 66.13 |
| OR (95% CI) | 1.39 (0.88–2.44) | 1.23 (0.79–1.91) | 1.00 | 0.87 (0.57–1.33) | 1.00 (0.70–1.43) | 1.00 |
| Elevated triglycerides | ||||||
| Prevalence (%) | 48.18 | 44.63 | 44.53 | 24.17 | 26.87 | 26.45 |
| OR (95% CI) | 1.10 (0.61–1.85) | 0.81 (0.52–1.26) | 1.00 | 0.97 (0.61–1.56) | 1.12 (0.75–1.67) | 1.00 |
| Elevated fasting glucose | ||||||
| Prevalence (%) | 14.66 | 15.50 | 23.96 | 14.23 | 13.95 | 24.39 |
| OR (95% CI) | 0.82 (0.43–1.55) | 0.69 (0.40–1.17) | 1.00 | 0.68 (0.40–1.16) | 0.58 (0.37–0.91) | 1.00 |
| Metabolic syndrome | ||||||
| Prevalence (%) | 37.03 | 36.80 | 34.26 | 27.00 | 28.63 | 38.50 |
| OR (95% CI) | 1.17 (0.62–2.18) | 0.94 (0.57–1.54) | 1.00 | 0.77 (0.46–1.29) | 0.71 (0.47–1.07) | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juna, C.F.; Cho, Y.; Ham, D.; Joung, H. Association of Carbohydrate and Fat Intake with Prevalence of Metabolic Syndrome Can Be Modified by Physical Activity and Physical Environment in Ecuadorian Adults: The ENSANUT-ECU Study. Nutrients 2021, 13, 1834. https://doi.org/10.3390/nu13061834
Juna CF, Cho Y, Ham D, Joung H. Association of Carbohydrate and Fat Intake with Prevalence of Metabolic Syndrome Can Be Modified by Physical Activity and Physical Environment in Ecuadorian Adults: The ENSANUT-ECU Study. Nutrients. 2021; 13(6):1834. https://doi.org/10.3390/nu13061834
Chicago/Turabian StyleJuna, Christian F., Yoonhee Cho, Dongwoo Ham, and Hyojee Joung. 2021. "Association of Carbohydrate and Fat Intake with Prevalence of Metabolic Syndrome Can Be Modified by Physical Activity and Physical Environment in Ecuadorian Adults: The ENSANUT-ECU Study" Nutrients 13, no. 6: 1834. https://doi.org/10.3390/nu13061834
APA StyleJuna, C. F., Cho, Y., Ham, D., & Joung, H. (2021). Association of Carbohydrate and Fat Intake with Prevalence of Metabolic Syndrome Can Be Modified by Physical Activity and Physical Environment in Ecuadorian Adults: The ENSANUT-ECU Study. Nutrients, 13(6), 1834. https://doi.org/10.3390/nu13061834








