Dietary Macronutrient Intake May Influence the Effects of TCF7L2 rs7901695 Genetic Variants on Glucose Homeostasis and Obesity-Related Parameters: A Cross-Sectional Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Anthropometric and Body Composition Analysis
2.3. Blood Collection and Biochemical Analysis
2.4. Calculations
2.5. Daily Physical Activity and Dietary Intake Analysis
2.6. Genetic Analyses
2.7. Statistical Analysis
3. Results
3.1. Dietary Assessment
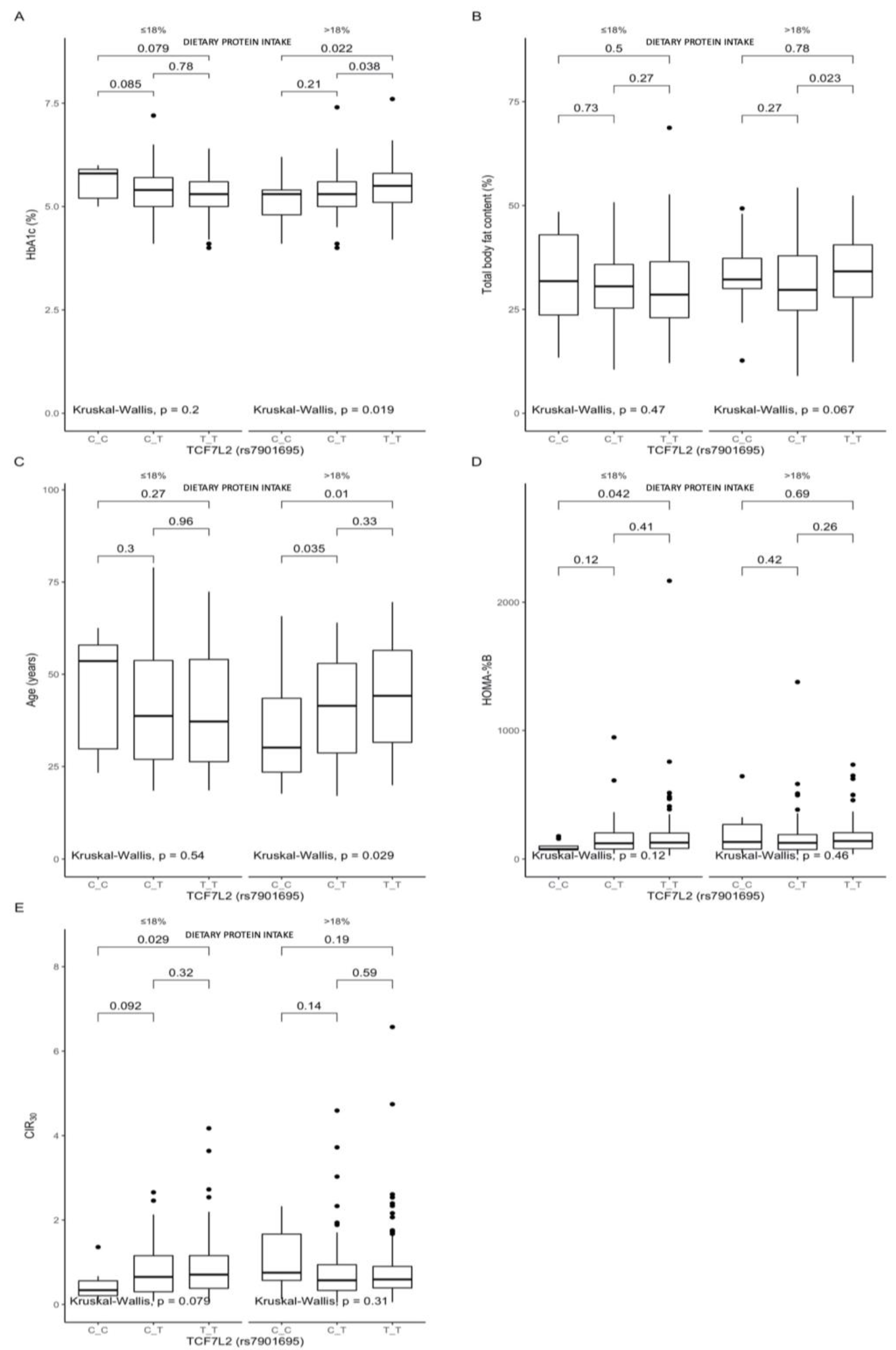
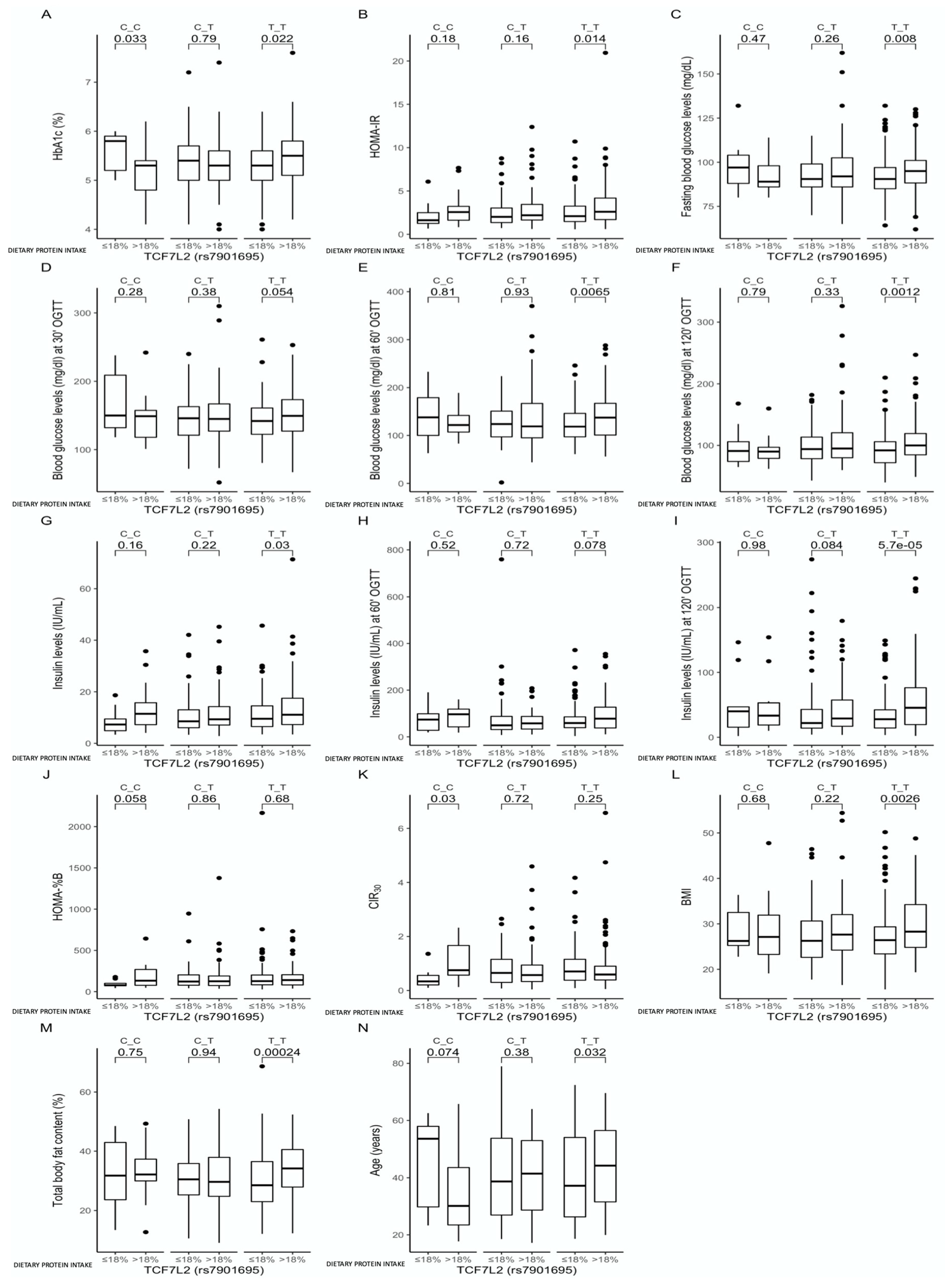
3.2. Associations between the rs7901695 Polymorphism, Glucose Homeostasis, Obesity-Related Parameters, and Dietary Protein Intake
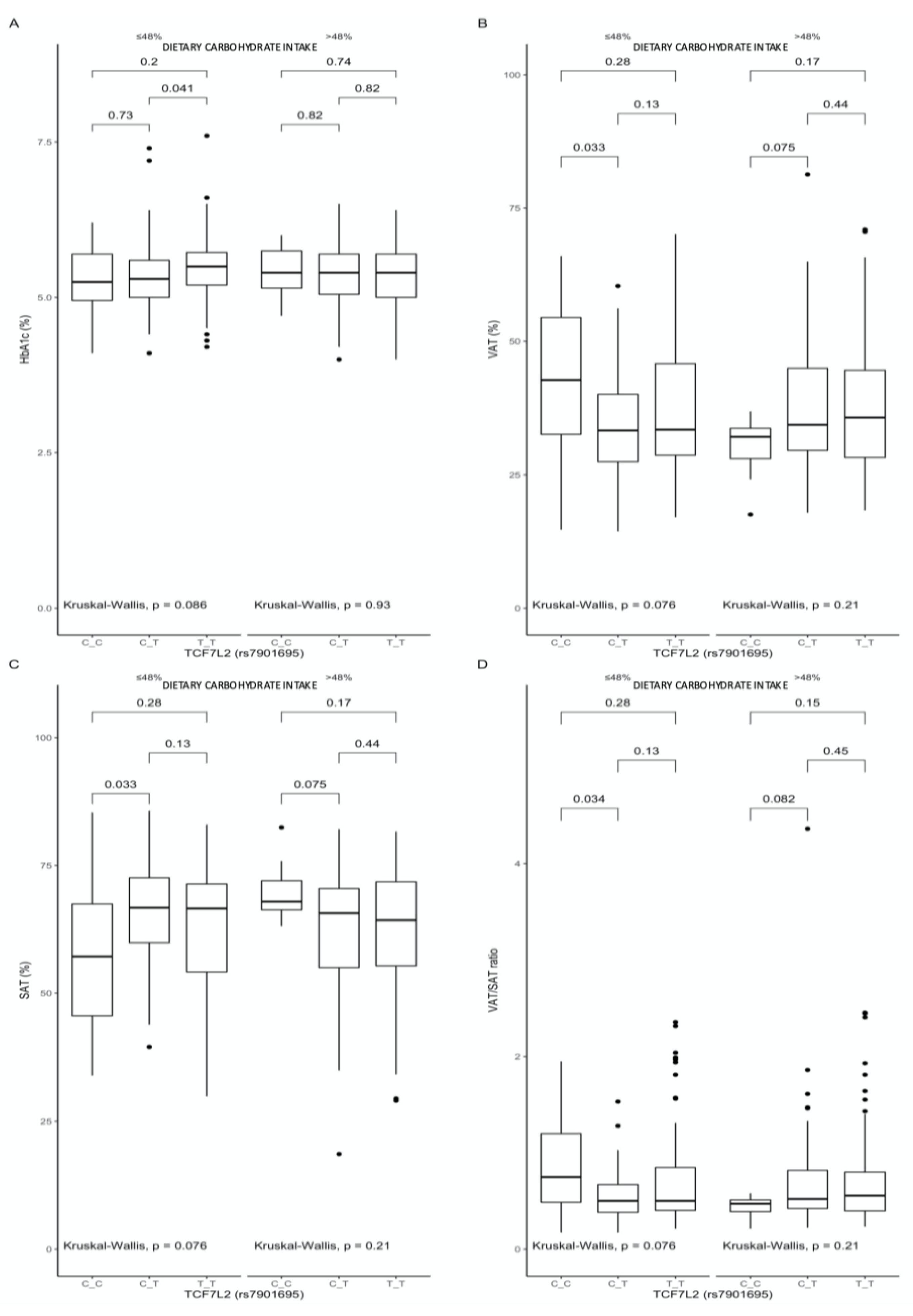
3.3. Associations between the rs7901695 Polymorphism, Glucose Homeostasis, Obesity-Related Parameters, and Dietary Carbohydrate Intake
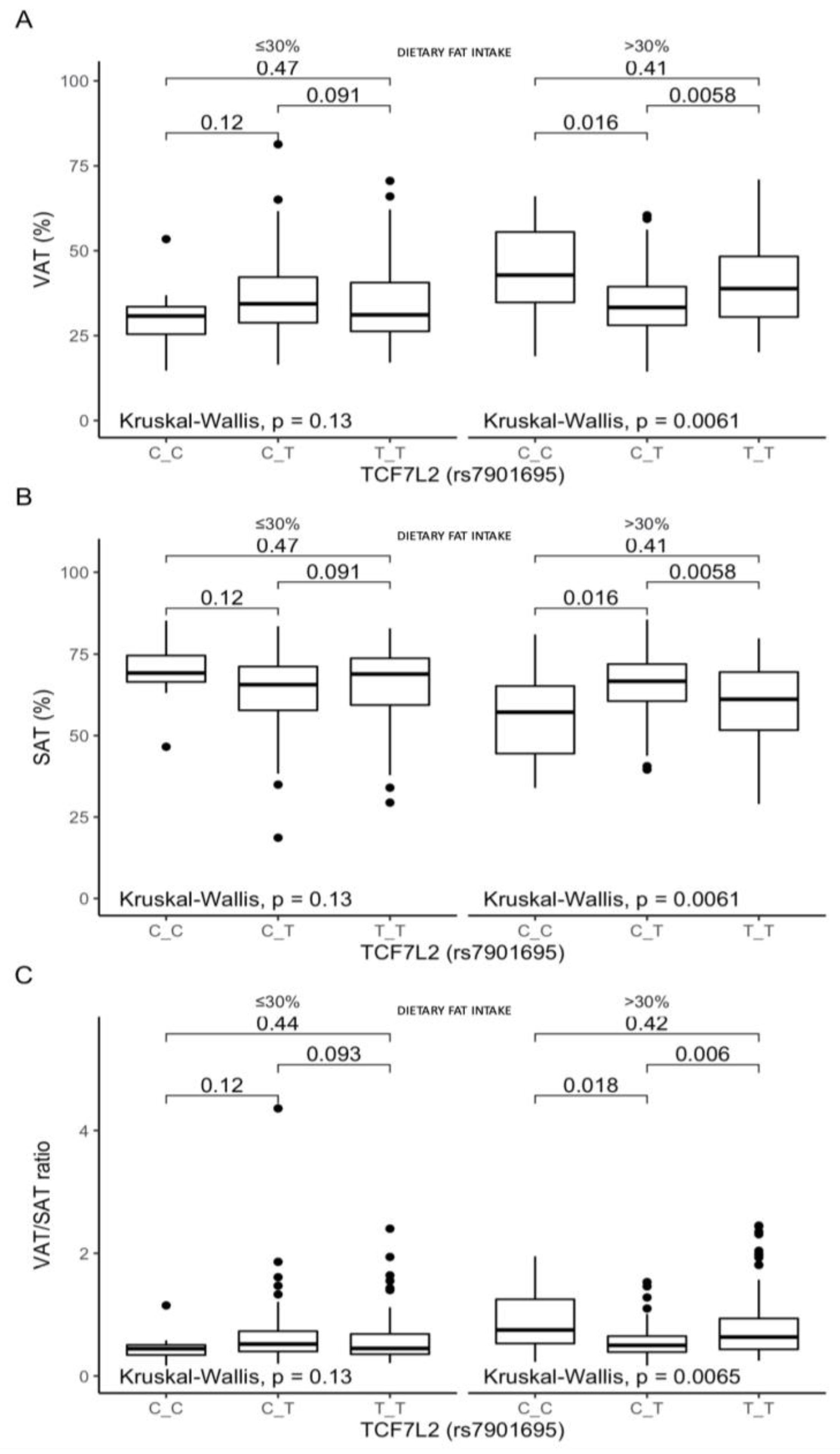
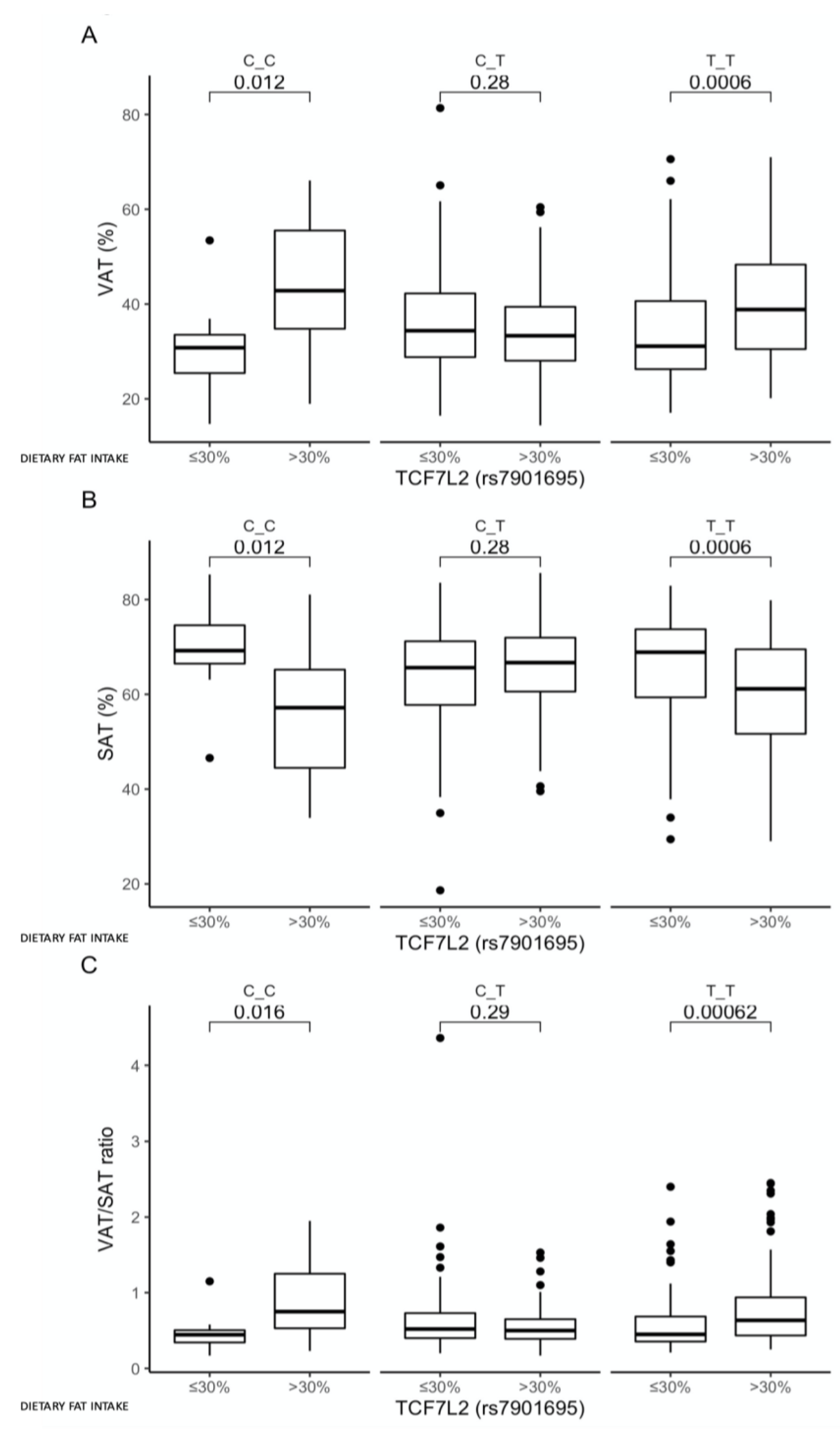
3.4. Associations between the rs7901695 Polymorphism, Glucose Homeostasis, Obesity-Related Parameters, and Dietary Fat Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- González-Sánchez, J.L.; Martínez-Larrad, M.T.; Zabena, C.; Pérez-Barba, M.; Serrano-Ríos, M. Association of variants of the TCF7L2 gene with increases in the risk of type 2 diabetes and the proinsulin: Insulin ratio in the Spanish population. Diabetologia 2008, 51, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, Y.; Nuli, R.; Abudusemaiti, M.; Kadeer, A.; Tian, X.; Xiao, H. Interaction between dietary patterns and TCF7L2 polymorphisms on type 2 diabetes mellitus among Uyghur adults in Xinjiang Province, China. Diabetes Metab. Syndr. Obes. 2019, 12, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Lupi, R.; Marchetti, P.; Del Guerra, S.; Orho-Melander, M.; Almgren, P.; Sjögren, M.; Ling, C.; Eriksson, K.F.; Lethagen, A.L.; et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Investig. 2007, 117, 2155–2163. [Google Scholar] [CrossRef]
- Gloyn, A.L.; Braun, M.; Rorsman, P. Type 2 diabetes susceptibility gene TCF7L2 and its role in beta-cell function. Diabetes 2009, 58, 800–802. [Google Scholar] [CrossRef]
- Tong, Y.; Lin, Y.; Zhang, Y.; Yang, J.; Liu, H.; Zhang, B. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: A large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med. Genet. 2009, 10, 15. [Google Scholar] [CrossRef]
- Yi, F.; Brubaker, P.L.; Jin, T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J. Biol. Chem. 2005, 280, 1457–1464. [Google Scholar] [CrossRef]
- Rulifson, I.C.; Karnik, S.K.; Heiser, P.W.; Ten Berge, D.; Chen, H.; Gu, X.; Taketo, M.M.; Nusse, R.; Hebrok, M.; Kim, S.K. Wnt signaling regulates pancreatic beta cell proliferation. Proc. Natl. Acad. Sci. USA 2007, 104, 6247–6252. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Quiles, L.; Martínez-González, M.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Ortega-Calvo, M.; et al. Polymorphism of the Transcription Factor 7-Like 2 Gene (TCF7L2) Interacts with Obesity on Type-2 Diabetes in the PREDIMED Study Emphasizing the Heterogeneity of Genetic Variants in Type-2 Diabetes Risk Prediction: Time for Obesity-Specific Genetic Risk Scores. Nutrients 2016, 8, 793. [Google Scholar]
- Peng, S.; Zhu, Y.; Lü, B.; Xu, F.; Li, X.; Lai, M. TCF7L2 gene polymorphisms and type 2 diabetes risk: A comprehensive and updated meta-analysis involving 121,174 subjects. Mutagenesis 2013, 28, 25–37. [Google Scholar] [CrossRef]
- Adamska, E.; Kretowski, A.; Goscik, J.; Citko, A.; Bauer, W.; Waszczeniuk, M.; Maliszewska, K.; Paczkowska-Abdulsalam, M.; Niemira, M.; Szczerbinski, L.; et al. The type 2 diabetes susceptibility TCF7L2 gene variants affect postprandial glucose and fat utilization in non-diabetic subjects. Diabetes Metab. 2018, 44, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Dedoussis, G.V.; Kaliora, A.C.; Panagiotakos, D.B. Genes, diet and type 2 diabetes mellitus: A review. Rev. Diabet. Stud. 2007, 4, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Adamska-Patruno, E.; Samczuk, P.; Ciborowski, M.; Godzien, J.; Pietrowska, K.; Bauer, W.; Gorska, M.; Barbas, C.; Kretowski, A. Metabolomics Reveal Altered Postprandial Lipid Metabolism After a High-Carbohydrate Meal in Men at High Genetic Risk of Diabetes. J. Nutr. 2019, 149, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Adamska-Patruno, E.; Goscik, J.; Lipinska, D.; Citko, A.; Krahel, A.; Miniewska, K.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; et al. The Role of Muscle Decline in Type 2 Diabetes Development: A 5-Year Prospective Observational Cohort Study. Nutrients 2019, 11, 834. [Google Scholar] [CrossRef]
- Adamska-Patruno, E.; Goscik, J.; Czajkowski, P.; Maliszewska, K.; Ciborowski, M.; Golonko, A.; Wawrusiewicz-Kurylonek, N.; Citko, A.; Waszczeniuk, M.; Kretowski, A.; et al. The MC4R genetic variants are associated with lower visceral fat accumulation and higher postprandial relative increase in carbohydrate utilization in humans. Eur. J. Nutr. 2019, 58, 2929–2941. [Google Scholar] [CrossRef]
- Nagy, E.; Vicente-Rodriguez, G.; Manios, Y.; Béghin, L.; Iliescu, C.; Censi, L.; Dietrich, S.; Ortega, F.B.; De Vriendt, T.; Plada, M.; et al. Harmonization process and reliability assessment of anthropometric measurements in a multicenter study in adolescents. Int. J. Obes. 2008, 32 (Suppl. S5), S58–S65. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Association, A.D. Abridged for Primary Care Providers. Clin. Diabetes 2020, 38, 10–38. [Google Scholar]
- Damcott, C.M.; Pollin, T.I.; Reinhart, L.J.; Ott, S.H.; Shen, H.; Silver, K.D.; Mitchell, B.D.; Shuldiner, A.R. Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: Replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes 2006, 55, 2654–2659. [Google Scholar] [CrossRef]
- Galderisi, A.; Tricò, D.; Pierpont, B.; Shabanova, V.; Samuels, S.; Dalla Man, C.; Galuppo, B.; Santoro, N.; Caprio, S. A Reduced Incretin Effect Mediated by the rs7903146 Variant in the TCF7L2 Gene Is an Early Marker of β-Cell Dysfunction in Obese Youth. Diabetes Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Florez, J.C.; Jablonski, K.A.; Bayley, N.; Pollin, T.I.; de Bakker, P.I.; Shuldiner, A.R.; Knowler, W.C.; Nathan, D.M.; Altshuler, D.; Diabetes Prevention Program Research Group. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 2006, 355, 241–250. [Google Scholar] [CrossRef]
- Stolerman, E.S.; Manning, A.K.; McAteer, J.B.; Fox, C.S.; Dupuis, J.; Meigs, J.B.; Florez, J.C. TCF7L2 variants are associated with increased proinsulin/insulin ratios but not obesity traits in the Framingham Heart Study. Diabetologia 2009, 52, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qi, L.; Hunter, D.J.; Meigs, J.B.; Manson, J.E.; van Dam, R.M.; Hu, F.B. Variant of Transcription Factor 7-Like 2 (TCF7L2) Gene and the Risk of Type 2 Diabetes in Large Cohorts of U.S. Women and Men. Diabetes 2006, 55, 2645–2648. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Sluiter, W.J.; Erkelens, D.W.; Terpstra, P.; Reitsma, W.D.; Doorenbos, H. Glucose tolerance and insulin release, a mathematical approach. II. Approximation of the peripheral insulin resistance after oral glucose loading. Diabetes 1976, 25, 245–249. [Google Scholar] [CrossRef]
- Fisher, E.; Meidtner, K.; Angquist, L.; Holst, C.; Hansen, R.D.; Halkjær, J.; Masala, G.; Ostergaard, J.N.; Overvad, K.; Palli, D.; et al. Influence of dietary protein intake and glycemic index on the association between TCF7L2 HapA and weight gain. Am. J. Clin. Nutr. 2012, 95, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Mire, E.; Bouchard, C. Abdominal obesity and mortality: The Pennington Center Longitudinal Study. Nutr. Diabetes 2012, 2, e42. [Google Scholar] [CrossRef]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef]
- Maliszewska, K.; Adamska-Patruno, E.; Krętowski, A. The interplay between muscle mass decline, obesity, and type 2 diabetes. Pol. Arch. Intern. Med. 2019, 129, 809–816. [Google Scholar] [CrossRef]
- Hindy, G.; Sonestedt, E.; Ericson, U.; Jing, X.J.; Zhou, Y.; Hansson, O.; Renström, E.; Wirfält, E.; Orho-Melander, M. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 2012, 55, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Qi, L.; Kraft, P.; Hu, F.B. TCF7L2, dietary carbohydrate, and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2009, 89, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Qi, Q.; Hu, F.B.; Sacks, F.M.; Qi, L. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. Am. J. Clin. Nutr. 2012, 96, 1129–1136. [Google Scholar] [CrossRef]
- Grau, K.; Cauchi, S.; Holst, C.; Astrup, A.; Martinez, J.A.; Saris, W.H.; Blaak, E.E.; Oppert, J.M.; Arner, P.; Rössner, S.; et al. TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am. J. Clin. Nutr. 2010, 91, 472–479. [Google Scholar] [CrossRef]
- Phillips, C.M.; Goumidi, L.; Bertrais, S.; Field, M.R.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. Dietary saturated fat, gender and genetic variation at the TCF7L2 locus predict the development of metabolic syndrome. J. Nutr. Biochem. 2012, 23, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, B.L.; Lissner, L. Dietary underreporting by obese individuals—Is it specific or non-specific? BMJ 1995, 311, 986–989. [Google Scholar] [CrossRef]

| rs7901695 | T/T | C/T | C/C | p-Value |
|---|---|---|---|---|
| N | 442 | 317 | 51 | |
| Men/women (%) | 48/52 | 49/51 | 43/57 | |
| Genotype frequency | 54.57% | 39.14% | 6.30% | >0.05 |
| Age (years) | 40.94 | 40.93 | 40.85 | 0.942 |
| BMI (kg/m2) | 28.7 (6.6) | 28.2 (6.8) | 28.3 (6.4) | 0.574 |
| BMI < 25.0 (kg/m2) | 143 (32.6%) | 111 (35.9%) | 16 (32.0%) | 0.856 |
| BMI 25.0–29.9 (kg/m2) | 153 (34.9%) | 105 (34.0%) | 16 (32.0%) | |
| BMI ≥ 30.0 (kg/m2) | 142 (32.4%) | 93 (30.1%) | 18 (36.0%) | |
| Total body fat content (%) | 31.7 (9.8) | 30.8 (9.3) | 32.7 (9.9) | 0.272 |
| WHR | 0.932 (0.086) | 0.925 (0.091) | 0.915 (0.087) | 0.325 |
| Visceral fat content (%) | 37.8 (12.8) | 36.3 (10.8) | 36.7 (13.1) | 0.732 |
| Subcutaneous fat content (%) | 62.1 (13.2) | 63.7 (10.8) | 63.3 (13.1) | 0.738 |
| Visceral/subcutaneous fat ratio | 0.698 (0.465) | 0.633 (0.419 | 0.661 (0.419) | 0.732 |
| Frequency of prediabetes or diabetes | ||||
| Yes | 223 (50.5%) | 159 (50.2%) | 26 (51.0%) | 0.992 |
| No | 219 (49.5%) | 158 (49.8%) | 25 (49.0%) | |
| Fasting blood glucose (mg/dl) | 96.4 (26.4) | 96.1 (19.9) | 104.5 (31.8) | 0.613 |
| Blood glucose at 30′ of OGTT (mg/dl) | 146.9 (34.5) | 145.4 (36.2) | 154.3 (51.7) | 0.811 |
| Blood glucose at 60′ of OGTT (mg/dl) | 131.5 (47.7) | 130.6 (48.8) | 137.1 (63.4) | 0.913 |
| Blood glucose at 120′ of OGTT (mg/dl) | 99.3 (33.0) | 99.5 (36.0) | 101.0 (58.3) | 0.660 |
| Glucose AUC during OGTT | 0.22 (95% CI: 0.21–0.23) | 0.22 (95% CI: 0.21–0.23) | 0.23 (95% CI: 0.19–0.28) | 0.349 |
| HbA1c | 5.6 (1.1) | 5.5 (1.1) | 5.6 (1.1) | 0.427 |
| Fasting insulin (IU/mL) | 12.8 (9.3) | 12.1 (10.4) | 11.1 (7.0) | 0.072 |
| Insulin AUC during OGTT | 0.14 (95% CI: 0.13–0.15) | 0.13 (95% CI: 0.12–0.14) | 0.4 (95% CI: 0.10–0.17) | 0.118 |
| HOMA-IR | 3.1 (2.6) | 2.9 (2.7) | 2.8 (2.2) | 0.084 |
| HOMA-B | 162.5 (218.9) | 157.6 (152.8) | 142.7 (106.4) | 0.287 |
| CIR30 | 0.9 (0.9) | 0.8 (0.7) | 0.8 (0.8) | 0.495 |
| Daily energy intake (kcal) | 1814.7 (702.7) | 1779.3 (708.6) | 1610.1 (538.1) | 0.216 |
| % of daily energy from carbohydrates | 48.0 (8.7) | 47.0 (9.0) | 47.2 (5.4) | 0.329 |
| % of daily energy from protein | 18.8 (4.6) | 19.1 (5.2) | 19.9 (3.6) | 0.334 |
| % of daily energy from fat | 31.1 (7.5) | 31.4 (7.7) | 31.2 (5.2) | 0.154 |
| Protein intake (n in the low/high quantiles) | 140/128 | 92/94 | 10/20 | |
| Carbohydrate intake (n in the low/high quantiles) | 123/145 | 100/86 | 19/11 | |
| Fat intake (n in the low/high quantiles) | 133/135 | 95/91 | 14/16 | |
| Daily physical activity level, n (%) | ||||
| Low | 28 (6.3%) | 28 (8.8%) | 3 (5.9%) | 0.246 |
| Moderate | 89 (20.1%) | 65 (20.5%) | 16 (31.4%) | |
| High | 325 (73.5%) | 224 (70.7%) | 32 (62.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, W.; Adamska-Patruno, E.; Krasowska, U.; Moroz, M.; Fiedorczuk, J.; Czajkowski, P.; Bielska, D.; Gorska, M.; Kretowski, A. Dietary Macronutrient Intake May Influence the Effects of TCF7L2 rs7901695 Genetic Variants on Glucose Homeostasis and Obesity-Related Parameters: A Cross-Sectional Population-Based Study. Nutrients 2021, 13, 1936. https://doi.org/10.3390/nu13061936
Bauer W, Adamska-Patruno E, Krasowska U, Moroz M, Fiedorczuk J, Czajkowski P, Bielska D, Gorska M, Kretowski A. Dietary Macronutrient Intake May Influence the Effects of TCF7L2 rs7901695 Genetic Variants on Glucose Homeostasis and Obesity-Related Parameters: A Cross-Sectional Population-Based Study. Nutrients. 2021; 13(6):1936. https://doi.org/10.3390/nu13061936
Chicago/Turabian StyleBauer, Witold, Edyta Adamska-Patruno, Urszula Krasowska, Monika Moroz, Joanna Fiedorczuk, Przemyslaw Czajkowski, Dorota Bielska, Maria Gorska, and Adam Kretowski. 2021. "Dietary Macronutrient Intake May Influence the Effects of TCF7L2 rs7901695 Genetic Variants on Glucose Homeostasis and Obesity-Related Parameters: A Cross-Sectional Population-Based Study" Nutrients 13, no. 6: 1936. https://doi.org/10.3390/nu13061936
APA StyleBauer, W., Adamska-Patruno, E., Krasowska, U., Moroz, M., Fiedorczuk, J., Czajkowski, P., Bielska, D., Gorska, M., & Kretowski, A. (2021). Dietary Macronutrient Intake May Influence the Effects of TCF7L2 rs7901695 Genetic Variants on Glucose Homeostasis and Obesity-Related Parameters: A Cross-Sectional Population-Based Study. Nutrients, 13(6), 1936. https://doi.org/10.3390/nu13061936







