Associations of Changes in Blood Lipid Concentrations with Changes in Dietary Cholesterol Intake in the Context of a Healthy Low-Carbohydrate Weight Loss Diet: A Secondary Analysis of the DIETFITS Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
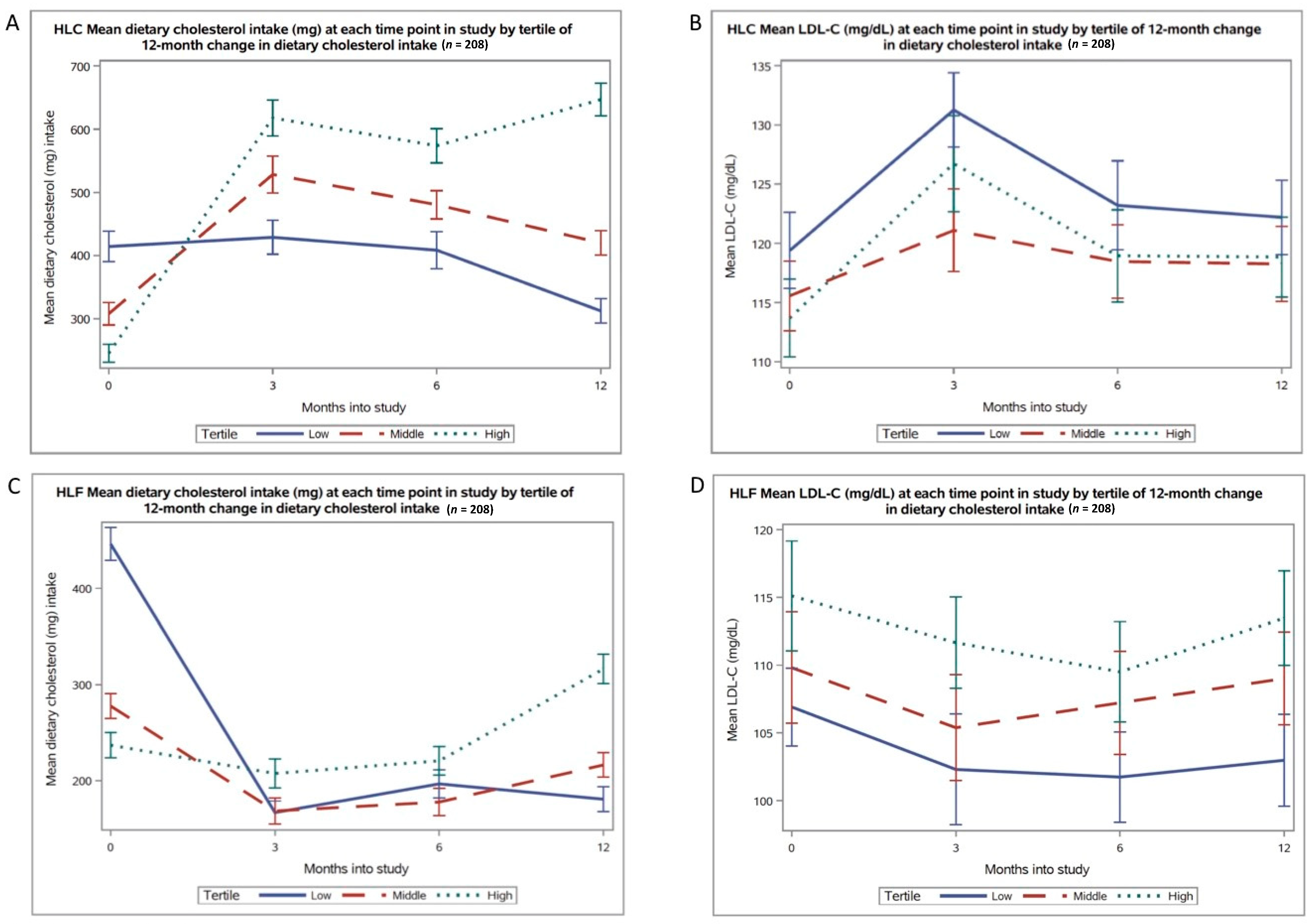
3.2. Changes in Cholesterol Intake
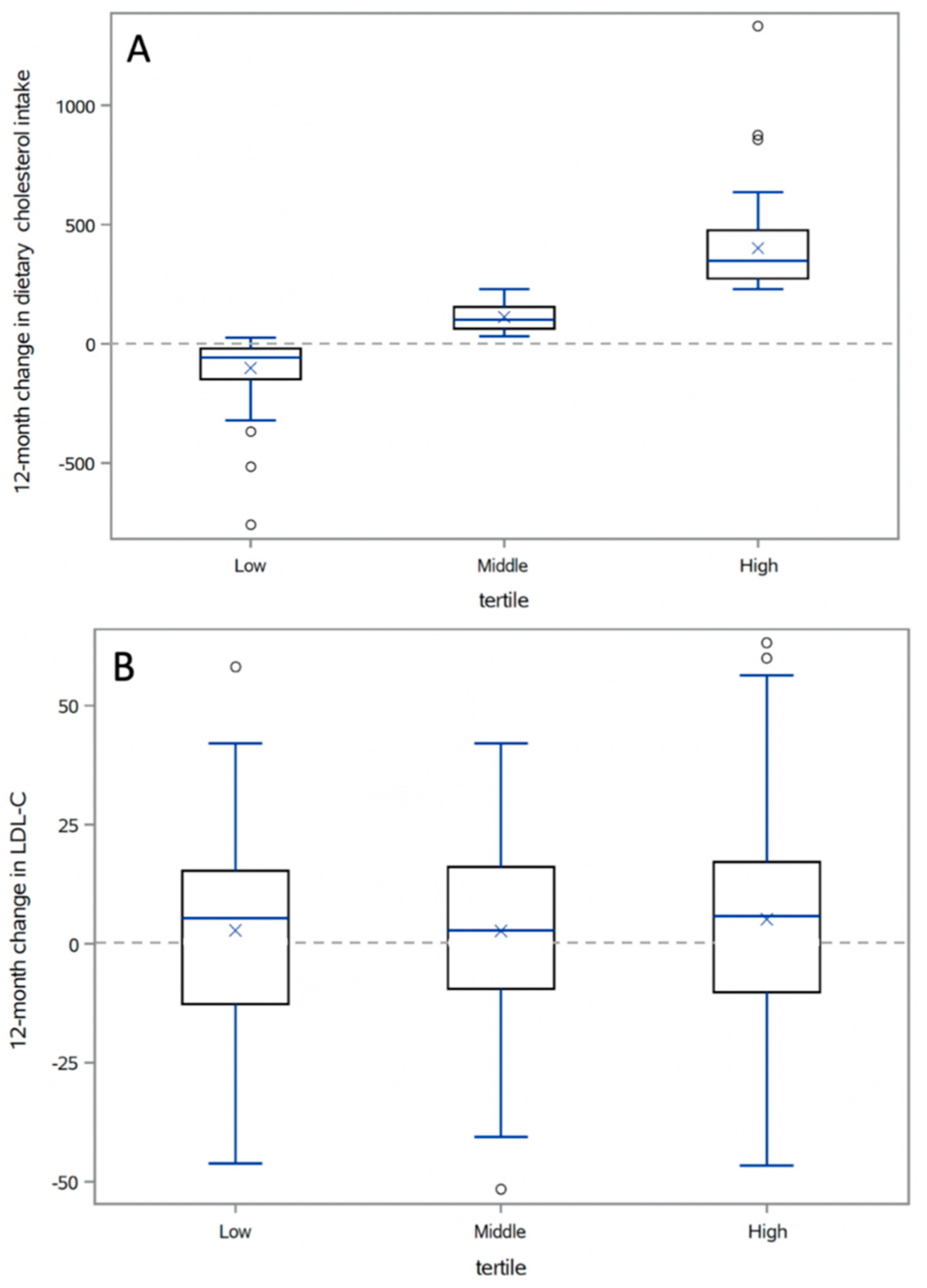
3.3. Correlations between Changes in Cholesterol Intake and Lipid Profile
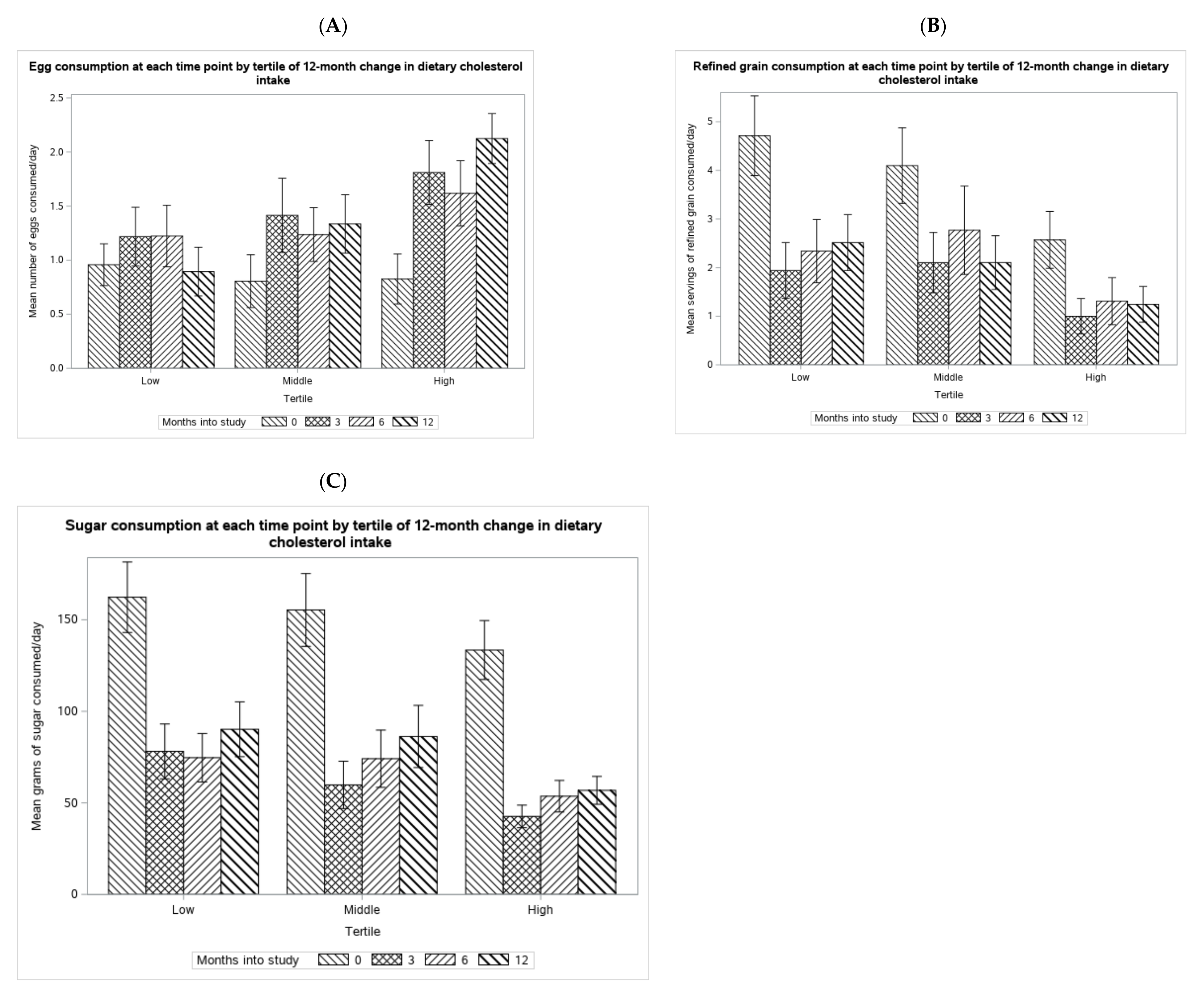
3.4. Qualitative Analysis of Sources of Dietary Cholesterol Increase
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Complete (n = 208) | Missing (n = 96) | p-Value 1 | |
|---|---|---|---|
| Age | 40 ± 6.6 | 38 ± 6.9 | 0.02 |
| Sex, n (%) | 0.5 | ||
| Female | 125 (60) | 54 (56) | |
| Male | 83 (40) | 42 (44) | |
| Race, n (%) 2 | 0.4 | ||
| White | 151 (73) | 62 (68) | |
| Other | 56 (27) | 29 (32) | |
| Education, n (%) | |||
| High School | 7 (3) | 8 (8) | 0.1 |
| College Graduate | 118 (57) | 55 (58) | |
| Postgrad Degree | 83 (40) | 32 (34) | |
| Body Weight (kg) | 95 ± 16 | 99 ± 15 | 0.04 |
| Baseline Diet | |||
| Calories (kcal) | 2198 ± 638 | 2275 ± 686 | 0.3 |
| Carbohydrates (g) | 243 ± 73 | 253 ± 8 | 0.3 |
| Fat (g) | 91 ± 34 | 95 ± 34 | 0.3 |
| Saturated Fat (g) | 30 ± 13 | 32 ± 13 | 0.2 |
| Protein (g) | 92 ± 29 | 95 ± 34 | 0.4 |
| Lipids (mg/dL) | |||
| LDL-C | 116 ± 26 | 109 ± 25 | 0.03 |
| HDL-C | 50 ± 9 | 49 ± 9 | 0.4 |
| Triglycerides | 125 ± 105 | 133 ± 137 | 0.6 |
| HLF-Tertile 2 | |||||
|---|---|---|---|---|---|
| Total (n = 208) | Lowest (n = 69) | Middle (n = 70) | Highest (n = 69) | p-Value 3 | |
| Age | 39 ± 6.6 | 39 ± 7.0 | 39 ± 6.6 | 39 ± 6.1 | 0.86 |
| Gender, n (%) | 0.10 | ||||
| Female | 115 (55) | 32 (54) | 45 (64) | 38 (55) | |
| Male | 93 (45) | 37 (46) | 25 (36) | 31 (45) | |
| Race, n (%) 4 | 0.20 | ||||
| White | 152 (74) | 58 (85) | 46 (66) | 48 (71) | |
| Other | 54 (26) | 10 (25) | 24 (30) | 21 (29) | |
| Education, n (%) 5 | |||||
| High School | 4 (2) | 2 (6) | 1 (1) | 1 (1) | <0.001 6 |
| College Graduate | 104 (50) | 40 (57) | 32 (46) | 32 (47) | |
| Postgrad Degree | 99 (48) | 26 (37) | 37 (53) | 36 (52) | |
| Body Weight (kg) | 97 ± 14 | 99 ± 14 | 95 ± 15 | 96 ± 14 | 0.17 |
| Baseline Diet | |||||
| Calories (kcal) | 2209 ± 665 | 2435 ± 683 a | 2140 ± 590 a | 2051 ± 667 a | 0.002 |
| Carbohydrates (g) | 251 ± 87 | 264 ± 100 | 252 ± 77 | 237 ± 80 | 0.19 |
| Fat (g) | 89 ± 34 | 103 ± 33 a | 84 ± 32 b | 81 ± 34 b | 0.0001 |
| Saturated Fat (g) | 30 ± 13 | 34 ± 11 a | 28 ± 12 b | 27 ± 13 b | 0.0004 |
| Protein (g) | 94 ± 27 | 106 ± 26 a | 89 ± 22 b | 87 ± 28 b | <0.0001 |
| Cholesterol (mg) | 320 ±151 | 446 ± 149 a | 278 ± 109 b | 237 ± 110 c | <0.0001 |
| Lipids (mg/dL) | |||||
| LDL-C | 111 ± 31 | 107 ± 24 | 110 ± 34 | 115 ± 34 | 0.29 |
| HDL-C | 49 ± 9 | 48 ± 11 | 50 ± 8 | 50 ± 9 | 0.52 |
| Triglycerides | 130 ± 75 | 133 ± 68 | 131 ± 88 | 127 ± 67 | 0.89 |
References
- Heart Disease Facts. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 20 April 2021).
- McNamara, D.J. The Fifty Year Rehabilitation of the Egg. Nutrients 2015, 7, 8716–8722. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, U.S. Department of Agriculture Dietary Guidelines for Americans, 1990. 1990. Available online: https://health.gov/dietaryguidelines/1990.asp (accessed on 20 April 2021).
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J. Am. Med. Assoc. 2001, 285, 2486–2497. [CrossRef]
- U.S. Department of Health and Human Services, U.S. Department of Agriculture Dietary Guidelines for Americans, 2010. 2010. Available online: https://health.gov/dietaryguidelines/2010/ (accessed on 20 April 2021).
- Hegsted, D.M.; McGandy, R.B.; Myers, M.L.; Stare, F.J. Quantitative effects of dietary fat on serum cholesterol in man. Am. J. Clin. Nutr. 1965, 17, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Hegsted, D.M.; Ausman, L.M.; Johnson, J.A.; Dallal, G.E. Dietary fat and serum lipids: An evaluation of the experimental data. Am. J. Clin. Nutr. 1993, 57, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Rong, J.; Zhan, Y.; Chen, H.Y.; Engert, J.C.; Sniderman, A.D.; Larson, M.G.; Vasan, R.S.; Thanassoulis, G. Risks of Incident Cardiovascular Disease Associated with Concomitant Elevations in Lipoprotein(a) and Low-Density Lipoprotein Cholesterol-The Framingham Heart Study. J. Am. Heart Assoc. 2020, 9, e014711. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.; Lichtenstein, A. Dietary Cholesterol and Plasma Lipoprotein Profiles: Randomized Controlled Trials. Curr. Nutr. Rep. 2013, 2, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.; Grande, F. Prediction of Serum-Cholesterol Responses of Main to Changes in Fats in the Diet. Lancet 1957, 270, 959–966. [Google Scholar] [CrossRef]
- Health and Human Services, Department of Agriculture, U.S. Department of Dietary Guidelines for Americans 2015–2020; Skyhorse Publishing: La Vergne, TN, USA, 2017.
- Carson, J.A.; Lichtenstein, A.; Anderson, C.; Appel, L.; Kris-Etherton, P.; Meyer, K.; Petersen, K.; Polonsky, T.; Van Horn, L. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.; Lichtenstein, A.; Wu, J.; Appel, L.; Creager, M.; Kris-Etherton, P.; Miller, M.; Rimm, E.; Rudel, L.; Robinson, J.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kühn, T.; Steur, M.; Weiderpass, E.; Wennberg, M.; et al. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease: A Prospective Study of 7198 Incident Cases among 409,885 Participants in the Pan-European EPIC Cohort. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.V.; Robinson, J.L.; Kirkpatrick, S.M.; Farzinkhou, S.; Avery, E.C.; Rigdon, J.; Offringa, L.C.; Trepanowski, J.F.; Hauser, M.E.; Hartle, J.C.; et al. DIETFITS study (diet intervention examining the factors interacting with treatment success)—Study design and methods. Contemp. Clin. Trials 2017, 53, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association with Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2018, 319, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- FoodData Central, Egg, Whole, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171287/nutrients (accessed on 20 April 2021).
- Framingham Heart Study. Available online: https://www.framinghamheartstudy.org/ (accessed on 20 April 2021).
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.N. (University of Utah, Salt Lake City, UT) Effects of dietary cholesterol on serum cholesterol: A meta-analysis and review. Am. J. Clin. Nutr. 1992, 55, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Krauss, R.M. The early years of lipoprotein research: From discovery to clinical application. J. Lipid Res. 2016, 57, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Mickelsen, O.; Adelson, S.F.; Fidanza, F. Diet and serum cholesterol in man; lack of effect of dietary cholesterol. J. Nutr. 1956, 59, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
| HLC-Tertile 2 | |||||
|---|---|---|---|---|---|
| Total (n = 208) | Lowest (n = 69) | Middle (n = 70) | Highest (n = 69) | p-Value 3 | |
| Age | 40 ± 6.6 | 40 ± 6.6 | 40 ± 6.7 | 41 ± 6.3 | 0.45 |
| Gender, n (%) | 0.48 | ||||
| Female | 125 (60) | 45 (65) | 42 (60) | 38 (55) | |
| Male | 83 (40) | 24 (35) | 28 (40) | 31 (45) | |
| Race, n (%) 4 | 0.66 | ||||
| White | 151 (73) | 52 (75) | 48 (70) | 51 (74) | |
| Other | 56 (27) | 17 (25) | 21 (30) | 18 (26) | |
| Education, n (%) | <0.001 5 | ||||
| High School | 7 (3) | 4 (6) | 1 (1) | 2 (3) | |
| College Graduate | 118 (57) | 39 (57) | 39 (56) | 40 (58) | |
| Postgrad Degree | 83 (40) | 26 (37) | 30 (43) | 27 (39) | |
| Body Weight (kg) | 95 ± 16 | 95 ± 15 | 94 ± 16 | 94 ± 17 | 0.94 |
| Baseline Diet | |||||
| Calories (kcal) | 2198 ± 638 | 2338 ± 613 a | 2271 ± 702 a | 1985 ± 537 b | 0.002 |
| Carbohydrates (g) | 243 ± 73 | 252 ± 72 | 246 ± 78 | 231 ± 69 | 0.18 |
| Fat (g) | 91 ± 34 | 100 ± 32 a | 96 ± 36 a | 78 ± 28 b | 0.0002 |
| Saturated Fat (g) | 30 ± 13 | 34 ± 14 a | 32 ± 12 a | 25 ± 10 b | <0.0001 |
| Protein (g) | 92 ± 29 | 101 ± 30 a | 92 ± 28 ab | 85 ± 26 b | 0.003 |
| Cholesterol (mg) | 322 ± 173 | 414 ± 200 a | 307 ± 148 b | 245 ± 120 c | <0.0001 |
| Lipids (mg/dL) | |||||
| LDL-C 6 | 116 ± 26 | 119 ± 27 | 115 ± 24 | 114 ± 27 | 0.43 |
| HDL-C 6 | 50 ± 9 | 51 ± 8 | 51 ± 9 | 49 ± 9 | 0.54 |
| Triglycerides | 125 ± 105 | 128 ± 62 | 119 ± 53 | 131 ± 65 | 0.48 |
| (a) | ||||
|---|---|---|---|---|
| HLC-Tertile | ||||
| Total (n = 208) | Lowest (n = 69) | Middle (n = 70) | Highest (n = 69) | |
| Total dietary cholesterol intake at 12 months (mg) | 460 ± 227 | 312 ± 159 | 420 ± 160 | 647 ± 214 |
| 12-month change in dietary cholesterol intake (mg) | 137 ± 206 | -102 ±131 | 112 ± 56 | 401 ± 185 |
| Range (min, max) | (−760, 1332) | (−760, 26) | (32, 230) | (230, 1332) |
| Calories (kcal) | −507 ± 617 | −664 ± 584 | −578 ± 643 | −277 ± 559 |
| Carbohydrates (g) | −112 ± 76 | −104 ± 68 | −104 ± 83 | −128 ± 75 |
| Saturated Fat (g) | −1.9 ± 14 | −7.7 ± 14 | −3.3 ± 13 | 5.4 ± 11 |
| Body Weight (kg) | −6.3 ± 6.8 | −4.4 ± 6.4 | −6.5 ± 6.5 | −8.0 ± 6.9 |
| LDL-C (mg/dL) | 3.5 ± 20 | 2.8 ± 20 | 2.7 ± 18 | 5.1 ± 23 |
| HDL-C (mg/dL) | 2.8 ± 6.5 | 2.2 ± 6.2 | 2.4 ± 5.8 | 3.9 ± 7.4 |
| Triglycerides (mg/dL) | −25 ± 48 | −17 ± 44 | −24 ± 46 | −35 ± 52 |
| (b) | ||||
| HLF-Tertile | ||||
| Total (n = 208) | Lowest (n = 69) | Middle (n = 70) | Highest (n = 69) | |
| Total dietary cholesterol intake at 12 months (mg) | 238 ± 128 | 181 ± 108 | 216 ± 107 | 316 ± 127 |
| 12-month change in dietary cholesterol intake (mg) | −82 ± 161 | −265 ±103 | −61 ± 39 | 79 ± 74 |
| Range (min, max) | (−655, 365) | (−655, −144) | (−144, −4.4) | (0, 365) |
| Calories (kcal) | −485 ± 627 | −723 ± 700 | −422 ± 593 | −310 ± 509 |
| Carbohydrates (g) | −36 ± 81 | −38 ± 90 | −37 ± 84 | −33 ± 71 |
| Saturated Fat (g) | −11 ± 12 | −19 ± 12 | −8.8 ± 11 | −6.6 ± 10.2 |
| Body Weight (kg) | −5.6 ± 7.3 | −7.0 ± 7.6 | −5.5 ± 8.1 | −4.1 ± 5.7 |
| LDL-C (mg/dL) | −2.1 ± 20 | −3.9 ± 18 | −0.8 ± 24 | −1.6 ± 18 |
| HDL-C (mg/dL) | 0.2 ± 6.0 | −0.5 ± 6.2 | 0 ± 6.0 | 1.0 ± 5.6 |
| Triglycerides (mg/dL) | −11 ± 55 | −14 ± 51 | −13 ± 55 | −6.1 ± 59 |
| (a) | |||
|---|---|---|---|
| Outcome | Model #1 Unadjusted Estimate (95% CI) | Model #2 1 Adjusted Estimate (95% CI) | Model #3 2 Adjusted Estimate (95% CI) |
| LDL-C (mg/dL) | 0.37 (−0.7 to 1.5) | 0.30 (−1.0 to 1.6) | 0.01 (−1.4 to 1.4) |
| HDL-C (mg/dL) | 0.26 (−0.1 to 0.6) | 0.12 (−0.3 to 0.6) | 0.05 (−0.4 to 0.5) |
| Triglycerides (mg/dL) | −2.52 (−5.2 to 0.1) | −0.92 (−4.0 to 2.1) | 0.06 (−3.1 to 3.3) |
| (b) | |||
| Outcome | Model #1 Unadjusted Estimate (95% CI) | Model #2 1 Adjusted Estimate(95% CI) | Model #3 2 Adjusted Estimate (95% CI) |
| LDL-C (mg/dL) | 1.17 (−0.5 to 2.7) | 1.40 (−1.0 to 3.8) | 0.57 (−1.9 to 3.0) |
| HDL-C (mg/dL) | 0.37 (−0.13 to 0.88) | 0.24 (−0.5 to 0.9) | 0.10 (−0.6 to 0.8) |
| Triglycerides (mg/dL) | 0.95 (−3.7 to 5.65) | 0.71 (−5.4 to 6.9) | 0.61 (−5.8 to 7.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara, M.; Hauser, M.E.; Aronica, L.; Rigdon, J.; Fielding-Singh, P.; Shih, C.W.; Gardner, C.D. Associations of Changes in Blood Lipid Concentrations with Changes in Dietary Cholesterol Intake in the Context of a Healthy Low-Carbohydrate Weight Loss Diet: A Secondary Analysis of the DIETFITS Trial. Nutrients 2021, 13, 1935. https://doi.org/10.3390/nu13061935
Vergara M, Hauser ME, Aronica L, Rigdon J, Fielding-Singh P, Shih CW, Gardner CD. Associations of Changes in Blood Lipid Concentrations with Changes in Dietary Cholesterol Intake in the Context of a Healthy Low-Carbohydrate Weight Loss Diet: A Secondary Analysis of the DIETFITS Trial. Nutrients. 2021; 13(6):1935. https://doi.org/10.3390/nu13061935
Chicago/Turabian StyleVergara, Monica, Michelle E. Hauser, Lucia Aronica, Joseph Rigdon, Priya Fielding-Singh, Cynthia W. Shih, and Christopher D. Gardner. 2021. "Associations of Changes in Blood Lipid Concentrations with Changes in Dietary Cholesterol Intake in the Context of a Healthy Low-Carbohydrate Weight Loss Diet: A Secondary Analysis of the DIETFITS Trial" Nutrients 13, no. 6: 1935. https://doi.org/10.3390/nu13061935
APA StyleVergara, M., Hauser, M. E., Aronica, L., Rigdon, J., Fielding-Singh, P., Shih, C. W., & Gardner, C. D. (2021). Associations of Changes in Blood Lipid Concentrations with Changes in Dietary Cholesterol Intake in the Context of a Healthy Low-Carbohydrate Weight Loss Diet: A Secondary Analysis of the DIETFITS Trial. Nutrients, 13(6), 1935. https://doi.org/10.3390/nu13061935







