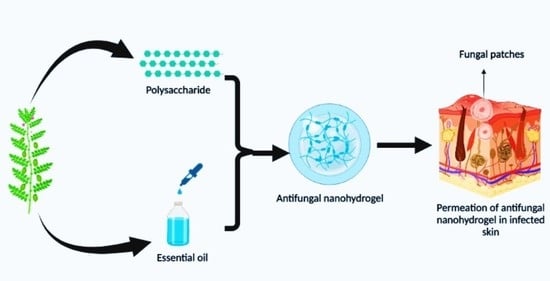
A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels
Abstract
:1. Introduction
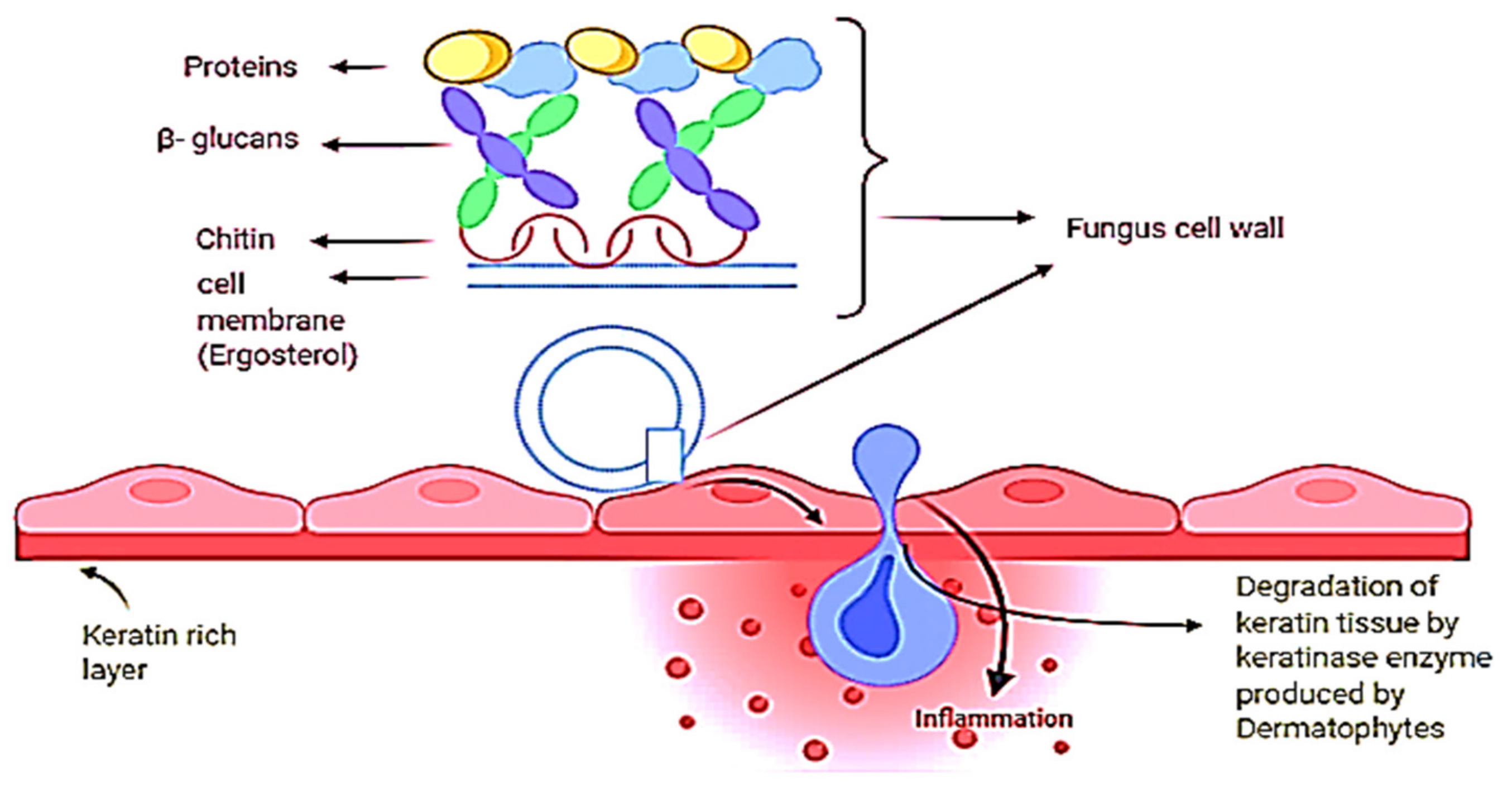
2. Skin Fungal Infections and Causative Agents
3. Fungal Infections of Nails (Onychomycosis)
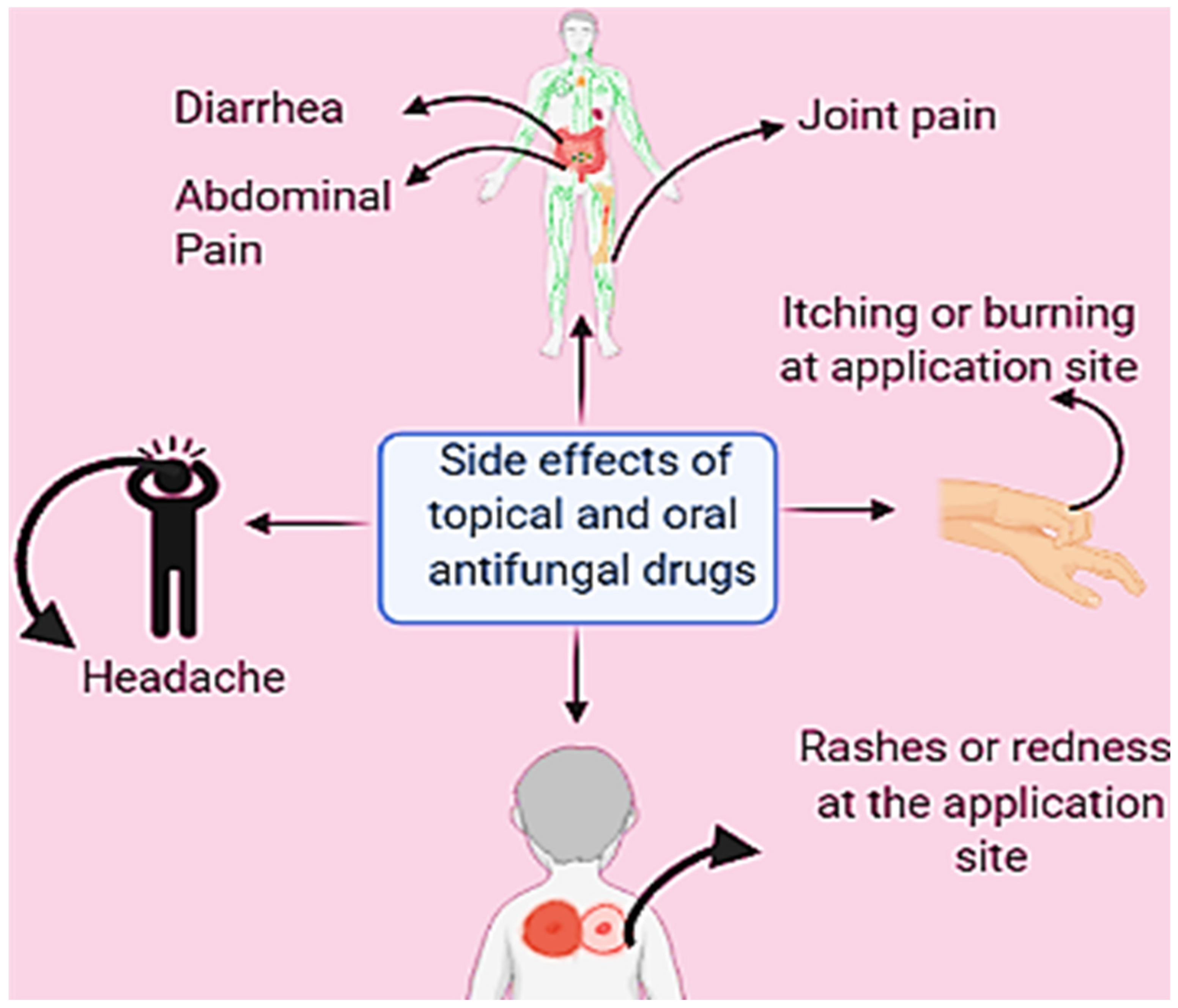
4. Oral and Topical Antifungals and Their Use
5. Candidiasis Treatment by Both Oral and Topical Antifungal Treatments
6. Resistance to Antifungal Drugs
6.1. Mechanism of Drug Resistance
Drug Efflux
6.2. Mutations Affecting Drug Target Genes
6.2.1. Decreased Concentration of Drug within Fungi
6.2.2. Drug Detoxification
6.2.3. Changes in Metabolism to Counteract the Drug’s Effect on De Novo Synthesis of Pyrimidines
6.2.4. Variation in Plasma Membrane Composition
6.2.5. Biofilms
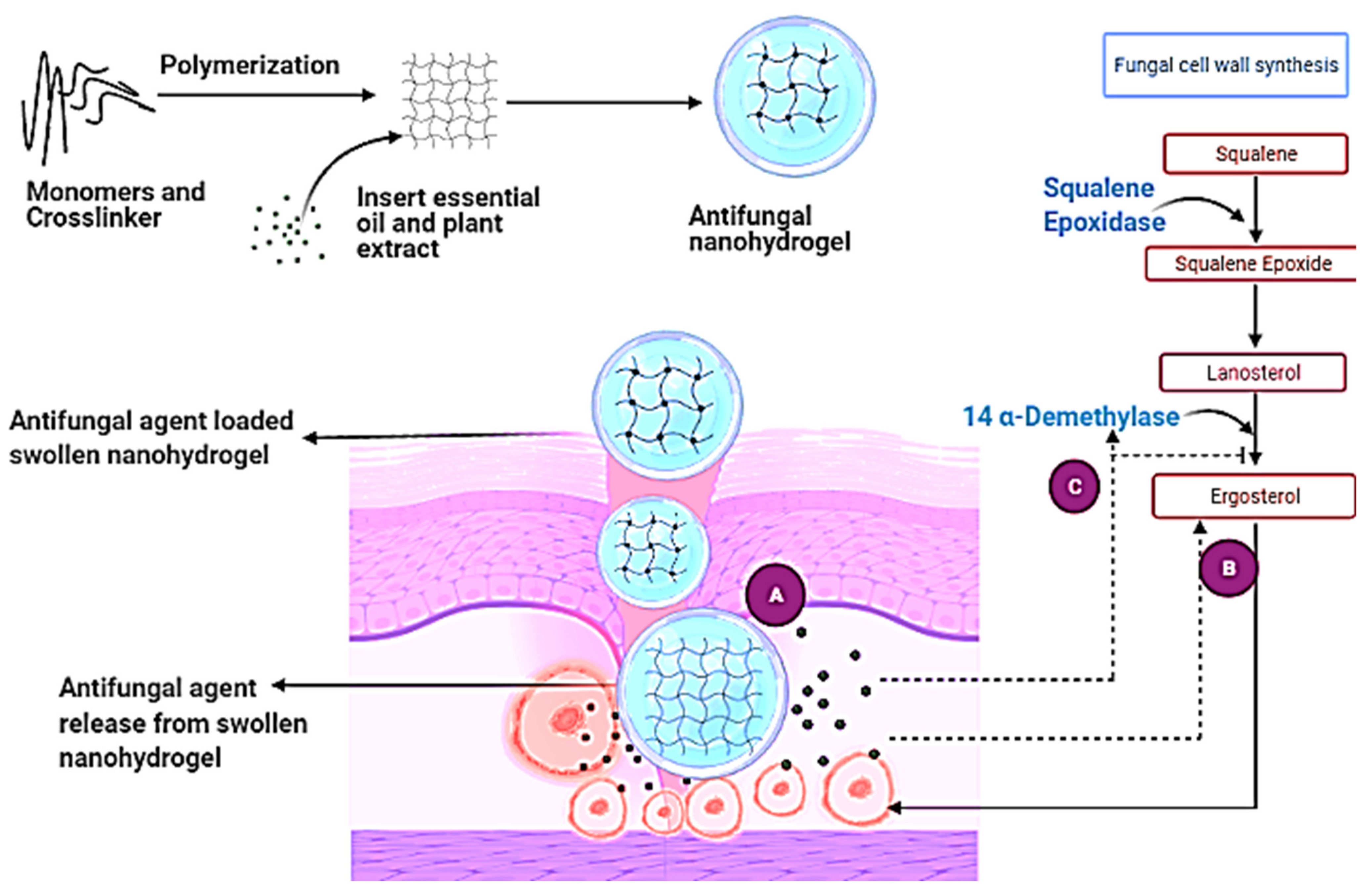
6.2.6. Modifications in the Biosynthesis of Ergosterol
7. Nanohydrogels
7.1. Mechanisms of Different Stimuli-Responsive Hydrogels
- (a)
- Physical stimuli
- TemperatureTemperature changes fluctuate the polymer-polymer and polymer–water interactions that are responsible for swelling and drug release.
- PressureIncreased pressure causes swelling, and vice versa. This is because the lower critical solution temperature (LCST) of hydrogels rises with pressure. The temperature below which negative thermoresponsive hydrogels swell is known as the LCST.
- LightThe hydrogel is reversibly changed from a flowable to a non-flowable state when exposed to light (UV and visible light).
- Electric fieldSwelling–deswelling is caused by changes in the electrical charge distribution within the hydrogel matrix when an electric field is applied, and this is responsible for on-demand drug release.
- Magnetic fieldThe application of a magnetic field causes pores in the gel to expand, resulting in drug release.
- Ultrasound irradiationThe drug is released when the ionic crosslinks in the hydrogels are briefly broken by ultrasound waves, but the crosslinks are repaired when the ultrasound waves are turned off. This allows for on-demand medicine delivery.
- (b)
- Chemical stimuli
- pHThe charge on the polymer chains changes when the pH changes, causing swelling and drug release.
- Ionic strengthChange in ion concentration also causes swelling and drug release
- CO2A pH-sensitive hydrogel disc comes into touch with a bicarbonate solution in CO2 sensors. When exposed to CO2, the pH of the solution changes, causing the hydrogel to swell or de-swell, causing a change in pressure, which is a measure of CO2 partial pressure.
- GlucoseIn reaction to an increase in glucose concentration, hydrogels swell. The combination generated by glucose and phenylboronic acid causes the hydrogels to enlarge, resulting in insulin release.
- RedoxIn a reductive environment (high glutathione concentration = 0.5–10 mM), disulfide links in reduction-sensitive hydrogels cleave in the intracellular matrix, releasing bioactive molecules/drugs.
- (c)
- Biological stimuli
- EnzymesEnzymes are responsible for hydrogel decomposition and, as a result, drug release. This is termed a chemically regulated drug release mechanism.
- AntigenWhen hydrogels detect free antigens, they swell and release the molecule.
- DNAIn the presence of ssDNA, single-stranded (ss) DNA grafted hydrogel probes swell.
7.2. Physical Entrapment Method for Incorporation
7.3. Covalent Conjugation Method for Incorporation
7.4. Controlled Self-Assembly Method for Incorporation
8. Antifungal Mechanism of Essential Oil and Plant Extract-Based Nanohydrogel
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imtiaz, N.; Niazi, M.B.; Fasim, F.; Khan, B.A.; Bano, S.A.; Shah, G.; Badshah, M.; Menaa, F.; Uzair, B. Fabrication of an Original Transparent PVA/Gelatin Hydrogel: In Vitro Antimicrobial Activity against Skin Pathogens. Int. J. Polym. Sci. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shields, B.E.; Rosenbach, M.; Brown-Joel, Z.; Berger, A.P.; Ford, B.A.; Wanat, K.A. Angioinvasive fungal infections impacting the skin: Background, epidemiology, and clinical presentation. JAAD 2019, 80, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Gupta, I.; Anasane, N.; Dolenc-Voljč, M. Nanotechnology for the Treatment of Fungal Infections on Human Skin. Clinical Microbiology Diagnosis, Treatment and prophylaxis of Infections the Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Kon, K., Rai, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 169–184. [Google Scholar]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate preci-sion. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Wijesiri, N.; Yu, Z.; Tang, H.; Zhang, P. Antifungal photodynamic inactivation against dermatophyte Trichophyton rubrum us-ing nanoparticle-based hybrid photosensitizers. Photodiagnosis Photodyn. Ther. 2018, 23, 202–208. [Google Scholar] [CrossRef]
- Gulyuz, U.; Okay, O. Self-healing poly (acrylic acid) hydrogels with shape memory behaviour of high mechanical strength. Macromolecules 2014, 47, 6889–6899. [Google Scholar] [CrossRef]
- Black, A.T. Dermatological Drugs, Topical Agents, and Cosmetics. Side Eff. Drugs Annu. 2015, 37, 175–184. [Google Scholar]
- Baptista, E.B.; Zimmermann-Franco, D.C.; Lataliza, A.A.; Raposo, N.R. Chemical composition and antifungal activity of essential oil from Eucalyptus smithii against dermatophytes. Rev. Soc. Bras. Med. Trop. 2015, 48, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Elaissi, A.; Rouis, Z.; Salem, N.A.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; et al. Chemical composition of 8 eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern Med. 2012, 12, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New antifungal agents and new formulations against dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef]
- Lakshmi, C.V.; Bengalorkar, G.M.; Kumar, V.S. Clinical efficacy of topical terbinafine versus topical luliconazole in treatment of tinea corporis/tinea cruris patients. J. Pharm. Res. Int. 2013, 24, 1001–1014. [Google Scholar] [CrossRef]
- Glynn, M.; Jo, W.; Minowa, K.; Sanada, H.; Nejishima, H.; Matsuuchi, H.; Okamura, H.; Pillai, R.; Mutter, L. Efinaconazole: Developmental and reproductive toxicity potential of a novel antifungal azole. Reprod. Toxicol. 2015, 52, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Abd Elaziz, D.; Abd El-Ghany, M.; Meshaal, S.; El Hawary, R.; Lotfy, S.; Galal, N.; Ouf, S.A.; Elmarsafy, A. Fungal infections in primary immunodeficiency diseases. J. Clin. Immunol. 2020, 219, 108553. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.; Rawat, S. Emerging fungal infections among children: A review on its clinical manifestations, diagnosis, and prevention. J. Pharm. Bioallied. Sci. 2010, 2, 314–320. [Google Scholar] [CrossRef]
- Nami, S.; Aghebati-Maleki, A.; Morovati, H.; Aghebati-Maleki, L. Current antifungal drugs and immunotherapeutic ap-proaches as promising strategies to treatment of fungal diseases. Biomed. Pharmacother. 2019, 110, 857–868. [Google Scholar] [CrossRef]
- Marek, C.L.; Timmons, S.R. Antimicrobials in Pediatric Dentistry. Pediatr. Dent. 2019, 128–141.e1. [Google Scholar] [CrossRef]
- Girois, S.B.; Chapuis, F.; Decullier, E.; Revol, B.G. Adverse effects of antifungal therapies in invasive fungal infections: Review and meta-analysis. Eur. J. Clin. Microbiol. 2006, 25, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Mahlo, S.M.; Chauke, H.R.; McGaw, L.; Eloff, J. Antioxidant and antifungal activity of selected medicinal plant extracts against phytopathogenic fungi. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature influences epimerization and composition of flavanol monomers, di-mers and trimers during cocoa bean roasting. Food Chem. 2013, 141, 3656–3663. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Barreto-Palacios, V.I.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on color and texture of food products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.A.; Corma, A.; Bermejo, M.; Merino, V.; Gon-zalez-Alvarez, M. Ionic hydrogel based on chitosan cross-linked with 6-phosphogluconic trisodium salt as a drug delivery system. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Boddy, L. Interactions with Humans and Other Animals. Fungi 2016, 293–336. [Google Scholar] [CrossRef]
- Natarajan, V.; Nath, A.K.; Thappa, D.M.; Singh, R.; Verma, S.K. Coexistence of onychomycosis in psoriatic nails: A descriptive study. Indian J. Dermatol. 2010, 76, 723. [Google Scholar]
- Ajello, L. A taxonomic review of the dermatophytes and related species. Sabouraudia 1968, 6, 147. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Jacobs, E.; Simpson, S.; Kerdahi, K. Genetic characterization of fungi isolated from the environmental swabs collected from a compounding center known to cause multistate meningitis outbreak in united states using ITS sequencing. Pathogens 2014, 3, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Kalita, J.M.; Sharma, A.; Bhardwaj, A.; Nag, V.L. Dermatophytoses and spectrum of dermatophytes in patients attending a teaching hospital in Western Rajasthan, India. J. Family Med. Prim. Care 2019, 8, 1418. [Google Scholar]
- Sawant, B.; Khan, T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- AbouSamra, M.M.; Basha, M.; Awad, G.E.; Mansy, S.S. A promising nystatin nanocapsular hydrogel as an antifungal polymer-ic carrier for the treatment of topical candidiasis. J. Drug Deliv. Sci. Technol. 2019, 49, 365–374. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabas, P.S. An approach to etiology, diagnosis and management of different types of candidiasis. J. Yeast Fungal Res. 2013, 4, 63–74. [Google Scholar]
- Tovikkai, D.; Maitrisathit, W.; Srisuttiyakorn, C.; Vanichanan, J.; Thammahong, A.; Suankratay, C.S. The case series in Thai-land and literature review in Southeast Asia. Med. Mycol. Case Rep. 2020, 27, 59–63. [Google Scholar] [CrossRef]
- De Lima Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrixschenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, V.K. Sporotrichosis: An Overview and Therapeutic Options. Dermatol. Res. Pract. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chokoeva, A.A.; Zisova, L.; Sotiriou, E.; Miteva-Katrandzhieva, T. Tinea capitis: A retrospective epidemiological comparative study. Wien. Med. Wochenschr. 2017, 167, 51–57. [Google Scholar] [CrossRef]
- Baumgardner, D.J. Fungal infections from human and animal contact. J. Patient Cent. Res. Rev. 2017, 4, 78. [Google Scholar] [CrossRef]
- White, T.C.; Findley, K.; Dawson, T.L.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, 019802. [Google Scholar] [CrossRef] [Green Version]
- Hay, R.J. Tinea capitis: Current status. Mycopathologia 2017, 182, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.M.; Krishnamurthy, K. Histology, Hair and Follicle; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: http://creativecommons.org/licenses/by/4.0/Last (accessed on 10 May 2021).
- Al Aboud, A.M.; Crane, J.S. Tinea Capitis; StatPearls Publishing: Treasure Island, FL, USA, 2020; Bookshelf ID: NBK536909. [Google Scholar]
- Alk, T.M.; Krishnamurthy, A.; Cantrell, W.; Elewski, B. Treatment of tinea capitis. Skin Appendage Disord. 2019, 5, 201–210. [Google Scholar]
- Newland, J.G.; Abdel-Rahman, S.M. Update on terbinafine with a focus on dermatophytoses. Clin. Cosmet. Investing. Dermatol. 2009, 2, 49. [Google Scholar]
- Bennassar, A.; Grimalt, R. Management of tinea capitis in childhood. Clin. Cosmet. Investing. Dermatol. 2010, 3, 89. [Google Scholar]
- Trösken, E.R. Toxicological Evaluation of Azole Fungicides in Agriculture and Food Chemistry. Ph.D. Thesis, Julius-Maximilians-Universität Würzburg, Würzburg, Germany, 2005. [Google Scholar]
- Michaels, B.D.; Del Rosso, J.Q. Tinea capitis in infants: Recognition, evaluation, and management suggestions. J. Clin. Aesthet. Dermatol. 2012, 5, 49. [Google Scholar]
- Basha, B.N.; Prakasam, K.; Goli, D. Formulation and evaluation of gel containing fluconazole-antifungal agent. Int. J. Drug Dev. Res. 2011, 3, 119–127. [Google Scholar]
- Sahoo, A.K.; Mahajan, R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol. Online J. 2016, 7, 77. [Google Scholar]
- Shy, R. Tinea corporis and tinea capitis. Pediatr. Rev. 2007, 28, 164–174. [Google Scholar] [CrossRef]
- Diongue, K.; Ndiaye, M.; Diallo, M.A.; Seck, M.C.; Badiane, A.S.; Diop, A.; Ndiaye, Y.D.; Déme, A.; Ndiaye, T.; Ndir, O.; et al. Fungal interdigital tinea pedis in Dakar (Senegal). J. Mycol. Med. 2016, 26, 312–316. [Google Scholar] [CrossRef]
- Salehi, Z.; Fatahi, N.; Taran, M.; Izadi, A.; Badali, H.; Hashemi, S.J.; Rezaie, S.; Ghazvini, R.D.; Ghaffari, M.; Aala, F.; et al. Com-parison of in vitro antifungal activity of novel triazoles with available antifungal agents against dermatophyte species caused tinea pedis. J. Mycol. Med. 2020, 30, 100935. [Google Scholar] [CrossRef]
- Ilkit, M.; Durdu, M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit. Rev. Microbiol. 2015, 41, 374–388. [Google Scholar] [CrossRef]
- Rotta, I.; Ziegelmann, P.K.; Otuki, M.F.; Riveros, B.S.; Bernardo, N.L.; Correr, C.J. Efficacy of topical antifungals in the treatment of dermatophytosis: A mixed-treatment comparison meta-analysis involving 14 treatments. JAMA Dermatol. 2013, 149, 341–349. [Google Scholar] [CrossRef]
- Khanna, D.; Bharti, S. Luliconazole for the treatment of fungal infections: An evidence-based review. Core Evid. 2014, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Raugi, G.; Nguyen, T.U. Superficial Dermatophyte infections of the skin. In Netter’s Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2012; pp. 102–109. [Google Scholar]
- Banki, A.; Castiglione, F.M. Infections of the Facial Skin and Scalp. In Head, Neck and Orofacial Infections: An Interdisciplinary Approach E-Book; Elsevier: St. Louis, MO, USA, 2015; p. 318. ISBN 978-0-323-3907-2. [Google Scholar]
- Chamorro, M.J.; House, S.A. Tinea Manuum; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 10 August 2020).
- Reddy, K.R. Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm). J. GMC-N. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Noble, S.L.; Forbes, R.C.; Stamm, P.L. Diagnosis and management of common tinea infections. Am. Fam. Physician. 1998, 58, 163. [Google Scholar]
- Ferry, M.; Shedlofsky, L.; Newman, A.; Mengesha, Y.; Blumetti, B. Tinea in Versicolor: A rare distribution of a common erup-tion. Cureus 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin. Microbial. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Sharma, V.; Chauhan, N.S. Promising novel nanopharmaceuticals for improving topical antifungal drug delivery. In Nano-and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 197–228. [Google Scholar]
- Karray, M.; McKinney, W.P. Tinea (Pityriasis) Versicolor; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 10 August 2020).
- Garg, S.; Chandra, A.; Mazumder, A.; Mazumder, R. Green synthesis of silver nanoparticles using Arnebia nobilis root extract and wound healing potential of its hydrogel. Asian J. Pharm. Sci. 2014, 8. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A. Antifungal treatment for pityriasis versicolor. J. Fungi. 2015, 1, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Klafke, G.B.; Silva, R.A.; Pellegrin, K.T.; Xavier, M.O. Analysis of the role of nail polish in the transmission of onychomycosis. Ann. Bras. Dermatol. 2018, 93, 930–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Sharma, S. Non-dermatophytes emerging as predominant cause of onychomycosis in a tertiary care centre in rural part of Punjab, India. JACM 2016, 18, 36. [Google Scholar] [CrossRef]
- Westerberg, D.P.; Voyack, M.J. Onychomycosis: Current trends in diagnosis and treatment. Am. Fam. Phys. 2013, 88, 762–770. [Google Scholar]
- Sahni, K.; Singh, S.; Dogra, S. Newer topical treatments in skin and nail dermatophyte infections. Indian Dermatol. Online J. 2018, 9, 149. [Google Scholar]
- Hay, R. Therapy of Skin, Hair and Nail Fungal Infections. J. Fungi 2018, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- McKeny, P.T.; Nessel, T.A.; Zito, P.M. Antifungal Antibiotics; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 4 May 2021).
- Sahni, T.; Sharma, S.; Arora, G.; Verma, D. Synthesis, Characterization and Antifungal Activity of a Substituted Coumarin and its Derivatives. Pestic. Res. J. 2020, 32, 39–48. [Google Scholar] [CrossRef]
- Danielli, L.J.; Pippi, B.; Duarte, J.A.; Maciel, A.J.; Machado, M.M.; Oliveira, L.F.; Vainstein, M.H.; Teixeira, M.L.; Bordignon, S.A.; Fuentefria, A.M. Antifungal mechanism of action of Schinuslentiscifolius Marchand essential oil and its synergistic effect in vitro with terbinafine and ciclopirox against dermatophytes. J. Pharm. Pharmacol. 2018, 70, 1216–1227. [Google Scholar] [CrossRef]
- Pârvu, M.; Moţ, C.A.; Pârvu, A.E.; Mircea, C.; Stoeber, L.; Roşca-Casian, O.; Ţigu, A.B. Allium sativum extract chemical composition, antioxidant activity and antifungal effect against Meyerozymaguilliermondii and Rhodotorulamucilaginosa causing onychomycosis. Molecules 2019, 21, 3958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.Y.; Camp, W.L.; Elewski, B.E. Advances in topical and systemic antifungals. Clin. Dermatol. 2007, 25, 165–183. [Google Scholar] [CrossRef]
- Jachak, G.R.; Ramesh, R.; Sant, D.G.; Jorwekar, S.U.; Jadhav, M.R.; Tupe, S.G.; Deshpande, M.V.; Reddy, D.S. Silicon incorporated morpholine antifungals: Design, synthesis, and biological evaluation. ACS Med. Chem. Lett. 2015, 6, 1111–1116. [Google Scholar] [CrossRef] [Green Version]
- Tutaj, K.; Szlazak, R.; Szalapata, K.; Starzyk, J.; Luchowski, R.; Grudzinski, W.; Osinska-Jaroszuk, M.; Jarosz-Wilkolazka, A.; Szuster-Ciesielska, A.; Gruszecki, W.I. Amphotericin B-silver hybrid nanoparticles: Synthesis, properties and antifungal activity. Nanomed. Nanotechnol. Biol. 2016, 12, 1095–1103. [Google Scholar] [CrossRef]
- Chowdhry, S.; Gupta, S.; D’souza, P. Topical antifungals used for treatment of seborrheic dermatitis. J. Bacteriol. Mycol. Open Access. 2017, 4, 1–7. [Google Scholar]
- Nankervis, H.; Thomas, K.S.; Delamere, F.M.; Barbarot, S.; Rogers, N.K.; Williams, H.C. Scoping systematic review of treatments for eczema. Program. Grants Appl. Res. 2016, 4, 1–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of action and drug resistance. In Yeast Membrane Transport; Springer: Berlin/Heidelberg, Germany, 2016; pp. 327–349. [Google Scholar]
- Dowd, F.J.; Johnson, B.; Mariotti, A. Pharmacology and Therapeutics for Dentistry-E-Book, 7th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2016; ISBN 978-0-323-39307-2. [Google Scholar]
- Goldstein, A.O.; Goldstein, B.G. Dermatophyte (Tinea) Infections; UpToDate: Walthman, MA, USA, 2017; Available online: https://www.uptodate.com/ (accessed on 27 May 2021).
- Waller, D.G.; Sampson, T. Medical Pharmacology and Therapeutics E-Book, 5th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2017. [Google Scholar]
- Kaul, S.; Yadav, S.; Dogra, S. Treatment of dermatophytosis in elderly, children, and pregnant women. Indian Dermatol. Online J. 2017, 8, 310. [Google Scholar]
- Rengasamy, M.; Chellam, J.; Ganapati, S. Systemic therapy of dermatophytosis: Practical and systematic approach. Clin. Dermatol. Rev. 2017, 1, 19. [Google Scholar] [CrossRef]
- Bondaryk, M.; Kurzątkowski, W.; Staniszewska, M. Antifungal agents commonly used in the superficial and mucosal candidi-asis treatment: Mode of action and resistance development. Postepy Dermatol. Alergol. 2013, 30, 293. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Kofla, G.; Ruhnke, M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of in-vasivecandidosis—Review of the literature. Eur. J. Med. Res. 2011, 16, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marak, M.B.; Dhanashree, B. Antifungal Susceptibility and Biofilm Production of Candida spp. Isolated from Clinical Samples. Int. J. Microbiol. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harriott, M.M.; Lilly, E.A.; Rodriguez, T.E.; Fidel, P.L., Jr.; Noverr, M.C. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 2010, 156, 3635. [Google Scholar] [CrossRef] [Green Version]
- Spampinato, C.; Leonardi, D. CandidaInfections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. BioMed. Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.P.; Chin, V.K.; Wong, W.F.; Madhavan, P.; Yong, V.C.; Looi, C.Y. Transcriptomic and genomic approaches for unrav-elling Candida albicans biofilm formation and drug resistance—An update. Genes 2018, 9, 540. [Google Scholar] [CrossRef] [Green Version]
- Long, N.; Xu, X.; Zeng, Q.; Sang, H.; Lu, L. Erg4A and Erg4B Are Required for Conidiation and Azole Resistance via Regulation of Ergosterol Biosynthesis in Aspergillus fumigatus. Appl. Environ. Microbiol. 2016, 83, e02924-16. [Google Scholar] [CrossRef] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef]
- Hayes, B.M.; Anderson, M.A.; Traven, A.; van der Weerden, N.L.; Bleackley, M.R. Activation of stress signalling pathways en-hances tolerance of fungi to chemical fungicides and antifungal proteins. Cell. Mol. Life Sci. 2014, 71, 2651–2666. [Google Scholar] [CrossRef]
- Pai, V.; Ganavalli, A.; Kikkeri, N.N. Antifungal resistance in dermatology. Indian J. Dermatol. 2018, 63, 361. [Google Scholar] [CrossRef]
- El-Awady, R.; Saleh, E.; Hashim, A.; Soliman, N.; Dallah, A.; Elrasheed, A.; Elakraa, G. The Role of Eukaryotic and Prokaryotic ABC Transporter Family in Failure of Chemotherapy. Front. Pharmacol. 2017, 7, 535. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.J.; Mylonakis, E. Efflux in fungi: La piece de resistance. PLoS Pathog. 2009, 5, e1000486. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Rossi, A. Antifungal Resistance Mechanisms in Dermatophytes. Mycopathology 2008, 166, 369–383. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.; Lang, E.A.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Derma-tophyte resistance to antifungal drugs: Mechanisms and prospectus. Front. Microbiol. 2018, 29, 1108. [Google Scholar] [CrossRef] [Green Version]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef] [Green Version]
- Sagatova, A.; Keniya, M.V.; Wilson, R.K.; Sabherwal, M.; Tyndall, J.D.A.; Monk, B.C. Triazole resistance mediated by mutations of a conserved active site tyrosine in fungal lanosterol 14α-demethylase. Sci. Rep. 2016, 6, 26213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. Rep. 2015, 2, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal Resistance and New Strategies to Control Fungal Infections. Int. J. Microbiol. 2011, 2012, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer hydrogels: A review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Kim, B.; Peppas, N.A. Poly (ethylene glycol)-containing hydrogels for oral protein delivery applications. Biomed. Microdevices 2003, 5, 333–341. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.F.; Adler, H.J. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [Green Version]
- Cretu, A.; Gattin, R.; Brachais, L.; Barbier-Baudry, D. Synthesis and degradation of poly (2-hydroxyethyl methacrylate)-graft-poly (ε-caprolactone) copolymers. Polym. Degrad. Stab. 2004, 83, 399–404. [Google Scholar] [CrossRef]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of sodium alginate-gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jayaramudu, T.; Varaprasad, K.; Reddy, G.S.; Raju, K.M. Antibacterial nanocomposite hydrogels for superi-or biomedical applications: A Facile eco-friendly approach. RSC Adv. 2015, 5, 14351–14358. [Google Scholar] [CrossRef]
- Ajji, Z.; Mirjalili, G.; Alkhatab, A.; Dada, H. Use of electron beam for the production of hydrogel dressings. Radiat. Phys. Chem. 2008, 77, 200–202. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Poly. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Leyden, J.J.; Krochmal, L.; Yaroshinsky, A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. JAAD 2006, 54, 73–81. [Google Scholar] [CrossRef]
- Moftah, N.H.; Ibrahim, S.M.; Wahba, N.H. Intense pulsed light versus photodynamic therapy using liposomal methylene blue gel for the treatment of truncal acne vulgaris: A comparative randomized split body study. Arch. Dermatol. Res. 2016, 308, 263–268. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Staibano, S.; De Rosa, G.; Battimiello, V.; Fardella, N.; Ilardi, G.; La Rotonda, M.I.; Longobardi, A.; Mazzella, M.; Siano, M.; et al. Resveratrol-Containing Gel for the Treatment of Acne Vulgaris. Am. J. Clin. Dermatol. 2011, 12, 133–141. [Google Scholar] [CrossRef]
- Dalwadi, C.; Patel, G. Application of nanohydrogels in drug delivery systems: Recent patents review. Recent Pat. Nanotech. 2015, 9, 17–25. [Google Scholar] [CrossRef]
- Fernández-Ferreiro, A.; Bargiela, N.F.; Varela, M.S.; Martínez, M.G.; Pardo, M.; Ces, A.P.; Méndez, J.B.; Barcia, M.G.; Lamas, M.J.; Otero-Espinar, F.J. Cyclodextrin–polysaccharide-based, in situ-gelled system for ocular antifungal delivery. Beilstein J. Org. Chem. 2014, 10, 2903–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: Design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int. J. Pharm. 2013, 443, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, N.; Ermiş, S.; Özkan, S. Development of topical hydrogels of terbinafine hydrochloride and evaluation of their anti-fungal activity. Drug Dev. Pharm. 2015, 41, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tomé, V.; Luaces-Rodríguez, A.; Silva-Rodríguez, J.; Blanco-Dorado, S.; García-Quintanilla, L.; Llovo-Taboada, J.; Blanco-Méndez, J.; García-Otero, X.; Varela-Fernández, R.; Herranz, M.; et al. Ophthalmic econazole hydrogels for the treatment of fungal keratitis. J. Pharm. Sci. 2018, 107, 1342–1351. [Google Scholar] [CrossRef]
- Harish, N.M.; Prabhu, P.; Charyulu, R.N.; Gulzar, M.A.; Subrahmanyam, E.V. Formulation and evaluation of in situ gels con-taining clotrimazole for oral candidiasis. Indian J. Pharm. Sci. 2009, 71, 421. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of glycerol on the physical and mechanical properties of thin gellan gum films for oral drug delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cyto-toxicity and in vivo evaluation. Artifi. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Serra, E.; Saubade, F.; Ligorio, C.; Whitehead, K.; Sloan, A.; Williams, D.W.; Hidalgo-Bastida, A.; Verran, J.; Malic, S. Methylcellu-lose hydrogel with Melissa officinalis essential oil as a potential treatment for oral candidiasis. Microorganisms 2020, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, A.; Kumari, C.; Kumar, P.A.; Am, H.S.; Divya, K.; Hisana, P.V. Development and Evaluation of Polyherbal GEL FOR Antifungal Activity. Int. J. Curr. Pharm. Res. 2018, 10, 40–43. [Google Scholar]
- Khan, S.; Gowda, B.H.; Deveswaran, R.; Mishra, S.; Sharma, A. Comparison of fluconazole and tea tree oil hydrogels designed for oral candidiasis: An invitro study. In AIP Conference Proceedings 2020; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2274, p. 050004. [Google Scholar]
- Aldawsari, H.M.; Badr-Eldin, S.M.; Labib, G.S.; El-Kamel, A.H. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In Vitro/In Vivo evaluation. Int. J. Nanomed. 2015, 10, 893. [Google Scholar]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Natu, K.N.; Tatke, P.A. Essential oils—Prospective candidates for antifungal treatment? J. Essent. Oil Res. 2019, 31, 347–360. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaci, S.A.; Fogarasi, S.; Jimborean, M.; Pop, C.; Tofană, M.; Rotar, A.; Tibulca, D.; Salagean, D.; Salanta, L. Evaluation of biochemical and microbiological changes occurring in fresh cheese with essential oils during storage time. Stud. Univ. Babeș-Bolyai Chem. 2019, 64, 527–537. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Socaciu, M.-I.; Socaci, S.A.; Mureșan, V.; Fogarasi, M.; Rotar, A.M. Chemometric comparison and Classification of some essential oils extracted from plants belonging to apiaceae and lamiaceae families based on their chemical composition and biological activities. Molecules 2018, 23, 2261. [Google Scholar] [CrossRef] [Green Version]
- Semeniuc, C.A.; Pop, C.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Socaciu, M.-I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Mureşan, V.; Pop, O.L.; Vodnar, D.C. Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil. Polymer 2020, 12, 1748. [Google Scholar] [CrossRef]
- Fajinmi, O.O.; Kulkarni, M.G.; Benická, S.; Zeljković, S.Ć.; Doležal, K.; Tarkowski, P.; Finnie, J.F.; Van Staden, J. Antifungal activity of the volatiles of Agathosmabetulina and Coleonema album commercial essential oil and their effect on the morphology of fungal strains Trichophyton rubrum and T. mentagrophytes. S. Afr. J. Bot. 2019, 122, 492–497. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Koroishi, A.M.; Foss, S.R.; Cortez, D.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. In vitro antifungal activity of extracts and neolignans from Piper regnellii against dermatophytes. J. Ethnopharmacol. 2008, 117, 270–277. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Solujic, S.; Sukdolak, S.; Milosev, M. Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplacacerina. Fitoterapia 2005, 76, 244–246. [Google Scholar] [CrossRef]
- Yemele, B.M.; Krohn, K.; Hussain, H.; Dongo, E.; Schulz, B.; Hu, Q. Tithoniamarin and tithoniamide: A structurally unique iso-coumarin dimer and a new ceramide from Tithonia diversifolia. Nat. Prod. Res. 2006, 20, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Chhillar, A.K.; Yadav, V.; Kamal, P.K.; Gupta, J.; Sharma, G.L. In vitro antifungal activity of 2-(3, 4-dimethyl-2, 5-dihydro-1H-pyrrol-2-yl)-1-methylethyl pentanoate, a dihydropyrrole derivative. J. Med. Microbiol. 2005, 54, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Miran, S.N.; Pirbalouti, A.G.; Mehdizadeh, L.; Ghaderi, Y. Variation in essential oil composition and antioxi-dant activity of cumin (Cuminum cyminum L.) fruits during stages of maturity. Ind. Crops Prod. 2015, 70, 163–169. [Google Scholar] [CrossRef]
- Akram, M.; Hussain, R. Nanohydrogels: History, development, and applications in drug delivery. In Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications; Wiley: Hoboken, NJ, USA, 2017; pp. 297–330. [Google Scholar]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 1–26. [Google Scholar] [CrossRef]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro-and nanogels with labile crosslinks–from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973. [Google Scholar] [CrossRef] [Green Version]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable Hydrogel for Sustained Protein Release by Salt-Induced Association of Hyaluronic Acid Nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. ChemInform Abstract: Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Chemin 2009, 40, 5418. [Google Scholar] [CrossRef]
- Herbal Nanogel Formulation: A Novel Approch. J. Sci. Technol. 2020, 5, 138–146. [CrossRef]
- Missirlis, D.; Kawamura, R.; Tirelli, N.; Hubbell, J.A. Doxorubicin encapsulation and diffusional release from stable, polymer-ic, hydrogel nanoparticles. Eur. J. Pharm. Sci. 2006, 29, 120–129. [Google Scholar] [CrossRef]
- Wang, C.; Mallela, J.; Garapati, U.S.; Ravi, S.; Chinnasamy, V.; Girard, Y.; Howell, M.; Mohapatra, S. A chitosan-modified gra-phene nanogel for noninvasive controlled drug release. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.; Abdel-Mottaleb, M.M.; El-assal, M.; Sammour, O. Novel biocompatible essential oil-based lipid nanocapsules with antifungal properties. J. Drug Deliv. Sci. Technol. 2020, 56, 101605. [Google Scholar] [CrossRef]
- Wang, N.X.; von Recum, H.A. Affinity-based drug delivery. Macromol. Biosci. 2011, 11, 321–332. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002, 54, 135–147. [Google Scholar] [CrossRef] [Green Version]
| Tinea Infection | Affected Locations | References |
|---|---|---|
| Tinea capitis | Scalp | [7] |
| Tinea corporis | Trunk | [8] |
| Tinea faciei | Face | [9] |
| Tinea manuum | Hands | [10] |
| Tinea pedis | Feet | [11] |
| Tinea unguium | Nails | [12] |
| Dermatophytes Based on Their Habitat | Fungal Species Belonging to Different Dermatophyte Group | Infection Site | References |
|---|---|---|---|
| Anthropophillic | Microsporum audouinii | Scalp | [29] |
| Trichophyton concenricum | Body | ||
| Microsporum ferrugineum | Scalp | ||
| Trichophyton interdigitale | Foot, groin, nails | ||
| Trichophyton megninii | Scalp, beard | ||
| Trichophyton rubrum | Foot, nails, body | ||
| Trichophyton schoenleinii | Scalp | ||
| Trichophyton soudanense | Scalp | ||
| Trichophyton tonsurans | Scalp, body | ||
| Trichophyton violaceum | Scalp, body, nails | ||
| Zoophillic | Microsporum canis | Scalp, body | [29,30] |
| Microsporum distortum | Scalp | ||
| Trichophyton equinum | Scalp | ||
| Microsporum nanum | Scalp, body | ||
| Trichophyton verrucosum | Exposed areas | ||
| Geophilic | Microsporum fulvum | Scalp, body | [29] |
| Microsporum gypseum | Scalp, body |
| Onychomycosis Microbiology | Name of Species Cause Onychomycosis |
|---|---|
| Dermatophytes | Epidermophyton floccosum |
| Microsporum species | |
| Trichophyton interdigital | |
| Trichophyton mentagrophytes | |
| Trichophyton rubrum | |
| Trichophyton tonsurans | |
| Nondermatophyte | Acremonium species |
| Alternaria species | |
| Aspergillus species | |
| Cladosporium carrionii | |
| Fusarium species | |
| Geotrichum cadidum | |
| Lasiodiplodia theobromae | |
| Onychocola species | |
| Scopulariopsiss pecies | |
| Scytalidium species | |
| Yeast | Candida albicans |
| Candida parapsilosis |
| Class of Antifungal Agents | Antifungal Agents | Chemical Structure of Different Antifungal Agents | Fungal Infections | Desired Treatment Duration | References |
|---|---|---|---|---|---|
| Imidazoles | Clotrimazole (1%) |  | Tinea corporis Tinea cruris Tinea pedis | 4–6 weeks | [10] |
| Econazole (1%) |  | Tinea corporis Tinea cruris Tinea pedis | 4–6 weeks | [11] | |
| Miconazole (1%) |  | Tinea corporis Tinea cruris Tinea pedis | 4–6 weeks | [73] | |
| Oxiconazole (2%) |  | Tinea corporis Tinea cruris Tinea pedis | 4 weeks | [57] | |
| Sertaconazole (2%) |  | Tinea corporis Tinea cruris Tinea pedis | 4 weeks | [11] | |
| Luliconazole (1%) |  | Tinea corporis Tinea cruris Tinea pedis | 2 weeks | [54] | |
| Eberconazole (1%) |  | Tinea corporis Tinea cruris Tinea pedis | 2–4 weeks | [10] | |
| Triazoles | Efinaconazole (10%) |  | Tinea pedis | 52 weeks | [12] |
| Allylamines | Terbinafine |  | Tinea corporis Tinea cruris Tinea manuum Tinea pedis | 2 weeks 2 weeks 4 weeks 4 weeks | [76] |
| Naftifine (1%) |  | Tinea corporis Tinea cruris Tinea pedis | Used 2 weeks beyond the resolution of symptoms | [77] | |
| Butenafine (1%) |  | Tinea corporis Tinea cruris Tinea pedis | 2–4 weeks | [78] | |
| Others | Amorolfine (0.25%) |  | Tinea corporis | 4 weeks | [79] |
| Amphotericin B (0.1%) |  | Tinea corporis | 4 weeks | [80] |
| Type of Hydrogel | Clinical Study | Agent | Skin Disorder | References |
|---|---|---|---|---|
| Clindamycin/Tretinoin Hydrogel | Clindamycin/Tretinoin Hydrogel | Combination Of Clindamycin (1%) and Tretinoin (0.025%) | Acne vulgaris | [79] |
| Liposomal Methylene Blue Hydrogel | Randomized and comparative study of 35 patients (21 men and 14 women) with varying degrees of acne vulgaris on the back | Methylene Blue | Acne vulgaris (Truncal) | [80] |
| Carboxymethylcellulosebased Hydrogel | Single-blind study on 20 patients (12 men and 8 women) | Resveratrol | Acne vulgaris (Facial) | [122] |
| Hydrogel Patch | Men and women with plaque-type psoriasislesions | Mometasone Furoate | Psoriasis | [123] |
| Hydrogel Micropatch | 100 psoriatic patients (75 men and 25 women) and 100 healthy volunteers | Mometasone Furoate | Psoriasis | [124] |
| Polysaccharide | Active Compounds | References |
|---|---|---|
| Galan gum/cyclodextrin | Fluconazole | [126] |
| Galan gum | Terbinafine HCL | [127] |
| Chitosan/carbopol/natrosol | Terbinafine HCL | [128] |
| Galan gum/carrageenan | Econazole | [129] |
| Galan gum/carbopol934P hydroxyl propyl methyl cellulose E50LV | Clotrimazole | [130] |
| Galan gum/glycerol | Fluconazole | [131] |
| Galan gum | Natamycin | [132] |
| Formulation | Natural Extract | Active Ingredients of the Natural Extract | Effective against | References |
|---|---|---|---|---|
| Methylcellulose hydrogel | Melissa officinalis | citronellal (50%), citronellol (10%), and geraniol (14%) | Candidiasis | [133] |
| Polyherbal gel | Piper betal and Piper nigrum leaf extract | methanolic hydro extracts | Candida albicans | [134] |
| Copper chitosan nanocomposite hydrogel | Thymus vulgaris | p-cymene, thymol, and 1,8-cineole | Aspergillus flavus | [134] |
| Hydroxypropylmethylcellulose hydrogel | Melaleuca alternifolia (Tea tree oil) | terpinene-4-ol | Oral candidiasis | [135] |
| Carbopol hydrogel | Cymbopogon citratus (Lemongrass oil) | geraniol, geranylacetate, and monoterpene olefins | Candida albicans | [136] |
| Antifungal Plant Extracts | Effective against | References |
|---|---|---|
| Leaves of Piperregnellii | Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis | [145] |
| Roots of Rubiatinctorum | Asperegilus niger, Alternaria lternaria, Penicillium verrucosum, Mucor mucedo | [146] |
| Tithoniadiversifolia | Microbotryum violaceum, Chlorella fusca | [147] |
| Daturametel | Candida albicans,Candida tropicalis | [148] |
| Alliumcepa and Alliumsativum | Malassezia furfur, Candida albicans, and other Candida species | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, N.; Bains, A.; Kaushik, R.; Dhull, S.B.; Melinda, F.; Chawla, P. A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels. Nutrients 2021, 13, 2055. https://doi.org/10.3390/nu13062055
Kaur N, Bains A, Kaushik R, Dhull SB, Melinda F, Chawla P. A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels. Nutrients. 2021; 13(6):2055. https://doi.org/10.3390/nu13062055
Chicago/Turabian StyleKaur, Navkiranjeet, Aarti Bains, Ravinder Kaushik, Sanju B. Dhull, Fogarasi Melinda, and Prince Chawla. 2021. "A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels" Nutrients 13, no. 6: 2055. https://doi.org/10.3390/nu13062055
APA StyleKaur, N., Bains, A., Kaushik, R., Dhull, S. B., Melinda, F., & Chawla, P. (2021). A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels. Nutrients, 13(6), 2055. https://doi.org/10.3390/nu13062055