Update on the Role of Allergy in Pediatric Functional Abdominal Pain Disorders: A Clinical Perspective
Abstract
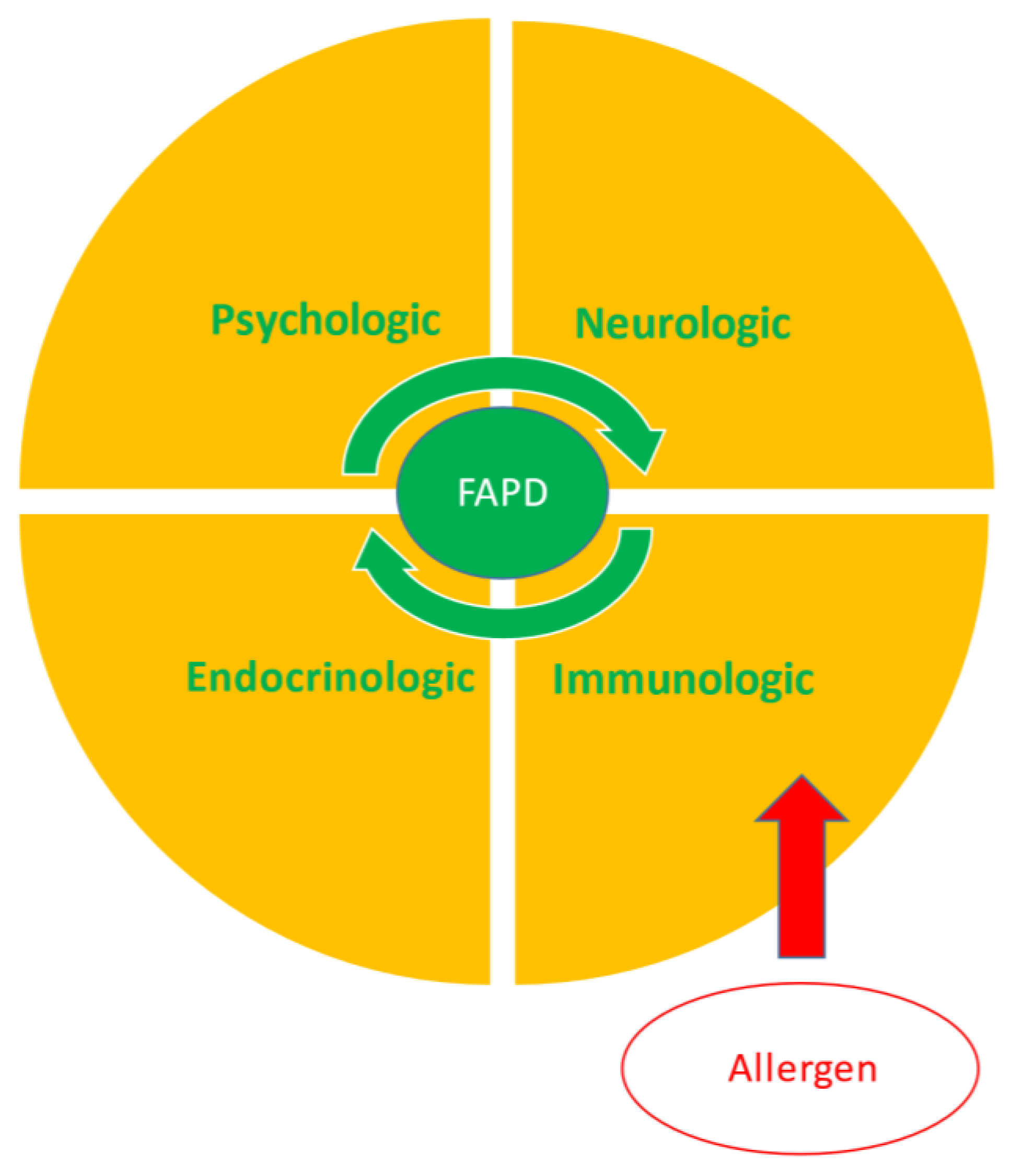
:1. Introduction
2. Inflammation and the Biopsychosocial Model
3. Allergy and Functional Abdominal Pain Disorders
3.1. IgE-Mediated Allergies
3.2. Non-IgE-Mediated Allergies
4. Management
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Korterink, J.J.; Diederen, K.; Benninga, M.A.; Tabbers, M.M. Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS ONE 2015, 10, e0126982. [Google Scholar] [CrossRef] [Green Version]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood gastrointestinal disorders: Child/adolescent. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef]
- Walker, L.S.; Lipani, T.A.; Greene, J.W.; Caines, K.; Stutts, J.; Polk, D.B.; Caplan, A.; Rasquin-Weber, A. Recurrent abdominal pain: Symptom subtypes based on the Rome II criteria for pediatric functional gastrointestinal disorders. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 187–191. [Google Scholar] [CrossRef]
- Schurman, J.V.; Friesen, C.A.; Danda, C.E.; Andre, L.; Welchert, E.; Lavenbarg, T.; Cocjin, J.T.; Hyman, P.E. Diagnosing functional abdominal pain with the Rome II criteria: Parent, child, and clinician agreement. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Weidler, E.M.; Lu, D.Y.; Tsai, C.M.; Shulman, R.J. Self-perceived food intolerances are common and associated with clinical severity in childhood irritable bowel syndrome. J. Acad. Nutr. Diet. 2016, 116, 1458–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, S.E. Food allergy vs food intolerance in patients with irritable bowel syndrome. Gastroenterol. Hepatol. 2019, 15, 38–40. [Google Scholar]
- Pesce, M.; Cargiolli, M.; Cassarano, S.; Polese, B.; De Conno, B.; Aurino, L.; Mancino, N.; Sarnelli, G. Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World J. Gastroenterol. 2020, 26, 456–465. [Google Scholar] [CrossRef]
- De Petrillo, A.; Hughes, L.D.; McGuinness, S.; Roberts, D.; Godfrey, E. A systematic review of psychological, clinical and psychosocial correlates of perceived food intolerance. J. Psychosom. Res. 2021, 141, 110344. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Shulman, R.J. Dietary carbohydrates and childhood functional abdominal pain. Ann. Nutr. Metab. 2016, 68, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Krieger-Grϋbel, C.; Hutter, S.; Hiestand, M.; Brenner, I.; Gϋsewell, S.; Borovicka, J. Treatment efficacy of a low FODMAP diet compared to a low lactose diet in IBS: A randomized, cross-over designed study. Clin. Nutr. ESPEN 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Posovszky, C.; Roesler, V.; Becker, S.; Iven, E.; Hudert, C.; Ebinger, F.; Calvano, C.; Warschburger, P. Roles of lactose and fructose malabsorption and dietary outcomes in children presenting with chronic abdominal pain. Nutrients 2019, 11, 3063. [Google Scholar] [CrossRef] [Green Version]
- Gijsbers, C.F.M.; Kneepkens, C.M.F.; Bϋller, H.A. Lactose and fructose malabsorption in children with recurrent abdominal pain: Results of double-blinded testing. Acta Paediatr. 2012, 101, e411–e415. [Google Scholar] [CrossRef]
- Yang, J.; Fox, M.; Cong, Y.; Chu, H.; Zheng, X.; Long, Y.; Fried, M.; Dai, N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: The roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment. Pharmacol. Ther. 2014, 39, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Kamphuis, J.B.J.; Guiard, B.; Leveque, M.; Olier, M.; Jouanin, I.; Yvon, S.; Tondereau, V.; Rivière, P.; Guéraud, F.; Chevolleau, S.; et al. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology 2020, 158, 652–663. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.; Friesen, C.A. Colonic mucosal inflammatory cells in children and adolescents with lactase deficiency. Pathol. Res. Pract. 2020, 216, 152971. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, J.; Parashette, K.; Zhang, J.; Fan, R. Association of lymphocytic colitis and lactase deficiency in pediatric population. Pathol. Res. Pract. 2015, 211, 138–144. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in irritable bowel syndrome (IBS): Interaction with gut microbiota and gut hormones. Nutrients 2019, 11, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.-R.; Du, L.-J.; He, H.-Q.; Kim, J.J.; Zhao, Y.; Zhang, Y.-W.; Luo, L.; Dai, N. Fructo-oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress-induced irritable bowel syndrome mouse model. World J. Gastroenterol. 2017, 23, 8321–8333. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the molecular mechanisms underlying protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tan, H.Y.; Kaiko, G.E. Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Uranga, J.A.; Martinez, V.; Abalo, R. Mast cell regulation and irritable bowel syndrome: Effects of food components with potential nutraceutical use. Molecules 2020, 25, 4314. [Google Scholar] [CrossRef] [PubMed]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 474–479. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkerts, J.; Stadhouders, R.; Redegeld, F.A.; Tam, S.Y.; Hendriks, R.W.; Galli, S.J.; Maurer, M. Effect of dietary fiber and metabolites on mast cell activation and mast cell-associated diseases. Front. Immunol. 2018, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Onyimba, F.; Crowe, S.E.; Johnson, S.; Leung, J. Food allergies and intolerances: A clinical approach to the diagnosis and management of adverse reactions to foods. Clin. Gastroenterol. Hepatol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Aversa, T.; Caminiti, L.; Lombardo, F.; Wasniewska, M.; Battista Pajno, G. Nutrition and avoidance diets in children with food allergy. Front. Pediatr. 2020, 8, 518. [Google Scholar] [CrossRef]
- Vickery, B.P.; Scurlock, A.M.; Jones, S.M.; Burks, A.W. Mechanisms of immune tolerance relevant to food allergy. J. Allergy Clin. Immunol. 2011, 127, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Schmiechen, Z.C.; Weissler, K.A.; Frischmeyer-Guerrerio, P.A. Recent developments in understanding the mechanisms of food allergy. Curr. Opin. Pediatr. 2019, 31, 807–814. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Youssef, N.N.; Sigurdsson, L.; Scharff, L.; Griffiths, J.; Wald, A. Visceral hyperalgesia in children with functional abdominal pain. J. Pediatr. 2001, 139, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, R.; Voskuijl, W.P.; Benninga, M.A.; Taminiau, J.A.; Boeckxstaens, G.E. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology 2001, 120, 31–38. [Google Scholar] [CrossRef]
- Halac, U.; Noble, A.; Faure, C. Rectal sensory threshold for pain is a diagnostic marker of irritable bowel syndrome and functional abdominal pain in children. J. Pediatr. 2010, 156, 60–65.e1. [Google Scholar] [CrossRef]
- Traina, G. The role of mast cells in the gut and brain. J. Integr. Neurosci. 2021, 20, 185–196. [Google Scholar] [CrossRef]
- Krammer, L.; Sergeevna Sowa, A.; Lorentz, A. Mast cells in irritable bowel syndrome: A systematic review. J. Gastrointest. Liver Dis. 2019, 28, 463–472. [Google Scholar] [CrossRef]
- Du, L.; Chen, B.; Kim, J.J.; Chen, X.; Dai, N. Micro-inflammation in functional dyspepsia: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2018, 30, e13304. [Google Scholar] [CrossRef]
- Friesen, C.A.; Schurman, J.V.; Colombo, J.M.; Abdel-Rahman, S.M. Eosinophils and mast cells as therapeutic targets in pediatric functional dyspepsia. World J. Gastrointest. Pharmacol. Ther. 2013, 4, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Moossavi, S.; Cremon, C.; Barbaro, M.R.; Moraveji, S.; Talmon, G.; Rezaei, N.; Hughes, P.A.; Bian, Z.X.; Choi, C.H.; et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2018, 30, e13192. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.; Carroll, G.; Mathe, A.; Horvat, J.; Foster, P.; Walker, M.M.; Talley, N.J.; Keely, S. Evidence for local and systemic immune activation in functional dyspepsia and irritable bowel syndrome: A systematic review. Am. J. Gastroenterol. 2019, 114, 429–436. [Google Scholar] [CrossRef]
- Robles, A.; Perez Ingles, D.; Myneedu, K.; Deoker, A.; Sarosiek, I.; Zuckerman, M.J.; Schmulson, M.J.; Bashashati, M. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2019, 31, e13718. [Google Scholar]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Singh, V.; Schurman, J.V.; Colombo, J.M.; Friesen, C.A. The relationship between mucosal inflammatory cells, specific symptoms, and psychological functioning in youth with irritable bowel syndrome. Sci. Rep. 2020, 10, 11988. [Google Scholar] [CrossRef]
- Wauters, L.; Nightingale, S.; Talley, N.J.; Sulaiman, B.; Walker, M.M. Functional dyspepsia is associated with duodenal eosinophilia in an Australian paediatric cohort. Aliment. Pharmacol. Ther. 2017, 45, 1358–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Li, Z.; Li, X.; Li, X.; Yuan, H.; Wang, X. Association between food allergy and duodenal mast cells in patients with functional dyspepsia. Biomed. Res. 2017, 28, 7274–7280. [Google Scholar]
- Friesen, C.A.; Andre, L.; Garola, R.; Hodge, C.; Roberts, C. Activated duodenal mucosal eosinophils in children with dyspepsia: A pilot transmission electron microscopic study. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 329–333. [Google Scholar] [CrossRef]
- Hou, X.H.; Zhu, L.R.; Li, Q.X.; Chen, J.D.Z. Alterations in mast cells and 5-HT positive cells in gastric mucosa in functional dyspepsia patients with hypersensitivity. Neurogastroenterol. Motil. 2001, 13, 398–399. [Google Scholar]
- Walker, M.M.; Salehian, S.S.; Murray, C.E.; Rajendran, A.; Hoare, J.M.; Negus, R.; Powell, N.; Talley, N.J. Implications of eosinophilia in the normal duodenal biopsy- an association with allergy and functional dyspepsia. Aliment. Pharmacol. Ther. 2010, 31, 1229–1236. [Google Scholar] [CrossRef]
- Schäppi, M.G.; Borrelli, O.; Knafelz, D.; Williams, S.; Smith, V.V.; Milla, P.J.; Lindley, K.J. Mast cell-nerve interactions in children with functional dyspepsia. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Hollier, J.M.; van Tilburg, M.A.L.; Liu, Y.; Czyzewski, D.I.; Self, M.M.; Weidler, E.M.; Heitkemper, M.; Shulman, R.J. Multiple psychological factors predict abdominal pain severity in children with irritable bowel syndrome. Neurogastroenterol. Motil. 2019, 31, e13509. [Google Scholar] [CrossRef] [PubMed]
- Newton, E.; Schosheim, A.; Patel, S.; Chitkara, D.K.; van Tilburg, M.A.L. The role of psycohological factors in pediatric functional abdominal pain disorders. Neurogastroenterol. Motil. 2019, 31, e13538. [Google Scholar] [CrossRef] [PubMed]
- Deacy, A.D.; Friesen, C.A.; Staggs, V.S.; Schurman, J.V. Evaluation of clinical outcomes in an interdisciplinary abdominal pain clinic: A retrospective, exploratory review. World J. Gastroenterol. 2019, 25, 3079–3090. [Google Scholar] [CrossRef]
- Liu, L.Y.; Coe, C.L.; Swenson, C.A.; Kelly, E.A.; Kita, H.; Busse, W.W. School examinations enhance airway inflammation to antigen challenge. Am. J. Respir. Crit. Care Med. 2002, 165, 1062–1067. [Google Scholar] [CrossRef]
- Oland, A.A.; Booster, G.D.; Bender, B.G. Integrated behavioral health care for management of stress in allergic diseases. Ann. Allergy Asthma Immunol. 2018, 121, 31–36. [Google Scholar] [CrossRef]
- Schurman, J.V.; Singh, M.; Singh, V.; Neilan, N.; Friesen, C.A. Symptoms and subtypes in pediatric functional dyspepsia: Relation to mucosal inflammation and psychological functioning. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 298–303. [Google Scholar] [CrossRef]
- Yuan, H.-P.; Li, Z.; Zhang, Y.; Li, X.-P.; Li, F.-K.; Li, Y.-Q. Anxiety and depression are associated with increased counts and degranulation of duodenal mast cells in functional dyspepsia. Int. J. Clin. Exp. Med. 2015, 8, 8010–8014. [Google Scholar]
- Piche, T.; Saint-Paul, M.C.; Dainese, R.; Marine-Barjoan, E.; Iannelli, A.; Montoya, M.L.; Peyron, J.F.; Czerucka, D.; Cherikh, F.; Filippi, J.; et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut 2008, 57, 468–473. [Google Scholar] [CrossRef]
- He, S.-H. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Saperas, E.; Nogueiras, C.; Mourelle, M.; Antolín, M.; Cadahia, A.; Malagelada, J.R. release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 1998, 114, 640–648. [Google Scholar] [CrossRef]
- Loo, E.X.L.; Wang, D.Y.; Siah, K.T.H. Association between irritable bowel syndrome and allergic diseases: To make a case for aeroallergen. Int. Arch. Allergy Immunol. 2020, 181, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.V.; Devanarayana, N.M.; Amarasiri, L.; Rajindrajith, S. Association between functional abdominal pain disorders and asthma in adolescents: A cross-sectional study. World J. Clin. Cases 2018, 6, 944–951. [Google Scholar] [CrossRef]
- Koloski, N.; Jones, M.; Walker, M.M.; Veysey, M.; Zala, A.; Keely, S.; Holtmann, G.; Talley, N.J. Population based study: Atopy and autoimmune diseases are associated with functional dyspepsia and irritable bowel syndrome, independent of psychological distress. Aliment. Pharmacol. Ther. 2019, 49, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Vila-Nadal, G.; Shah, J.; Shamji, M.; Swan, L.; Durham, S.R.; Patel, K.; Skypala, I.J. Is pollen-food syndrome a frequent comorbidity in adults with irritable bowel syndrome? Allergy 2020, 75, 1780–1783. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.C.; Moparty, B.; Farhadi, A.; DeMeo, M.T.; Bansal, P.J.; Keshavarzian, A. Atopic irritable bowel syndrome: A novel subgroup of irritable bowel syndrome with allergic manifestations. Ann. Allergy Asthma Immunol. 2008, 100, 49–53. [Google Scholar] [CrossRef]
- Jones, M.P.; Walker, M.M.; Ford, A.C.; Talley, N.J. The overlap of atopy and functional gastrointestinal disorders among 23,471 patients in primary care. Aliment. Pharmacol. Ther. 2014, 40, 382–391. [Google Scholar] [CrossRef]
- Deshmukh, F.; Vasudevan, A.; Mengalie, E. Association between irritable bowel syndrome and asthma: A meta-analysis and systematic review. Ann. Gastroenterol. 2019, 32, 570–577. [Google Scholar] [CrossRef]
- Tan, T.-K.; Chen, A.-C.; Lin, C.-L.; Shen, T.-C.; Li, T.-C.; Wei, C.-C. Preschoolers with allergic diseases have an increased risk of irritable bowel syndrome when reaching school age. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-D.; Wang, I.-C.; Shen, T.-C.; Lin, C.-L.; Wei, C.-C. A 8-year population-based cohort study of irritable bowel syndrome in childhood with a history of atopic dermatitis. J. Investig. Med. 2018, 66, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Cremon, C.; Frediani, S.; Lucarelli, S.; Pia Villa, M.; Stanghellini, V.; La Torre, G.; Martemucci, L.; Barbara, G. Allergic proctocolitis is a risk factor for functional gastrointestinal disorders in children. J. Pediatr. 2018, 195, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bificobacterium mixture (B longum BB536, B infantis M-63, M breve M-16v) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef] [Green Version]
- Van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.-M.; De Meester, I.; De Man, J.G.; De Winter, B.Y. Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106–2123. [Google Scholar] [CrossRef]
- Mansueto, P.; D’Alcamo, A.; Seidita, A.; Carroccio, A. Food allergy in irritable bowel syndrome: The case of non-celiac wheat sensitivity. World J. Gastroenterol. 2015, 21, 7089–7109. [Google Scholar] [CrossRef]
- Choung, R.S.; Murray, J.A. The role for food allergies in the pathogenesis of irritable bowel syndrome: Understanding mechanisms of intestinal mucosal responses against food antigens. Gastroenterology 2019, 157, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Hammond, C.; Lieberman, J.A. Unproven diagnostic tests for food allergy. Immunol. Allergy Clin. N. Am. 2018, 38, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.W.; Abid, S.; Awan, S.; Lubna, F. Frequency of food hypersensitivity in patients with functional gastrointestinal disorders. Acta Gastroenterol. Belg. 2018, 81, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.L.; Li, Y.Q.; Li, W.J.; Guo, Y.T.; Lu, X.F.; Li, J.M.; Desmond, P.V. Alterations of food antigen-spedific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin. Exp. Allergy 2007, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Neilan, N.A.; Dowling, P.J.; Taylor, D.L.; Ryan, P.; Schurman, J.V.; Friesen, C.A. Useful biomarkers in pediatric eosinophilic duodenitis and their existence: A case-control, single-blind, observational pilot study. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 377–384. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Mayer, J.; Meier, P.N.; Zeck-Kapp, G.; Manns, M.P. Clinical significance of the colonoscopic allergen provocation test. Int. Arch. Allergy Immunol. 1997, 113, 348–351. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021, 590, 151–156. [Google Scholar] [CrossRef]
- Jones, A.L.; Curran-Everett, D.; Leung, D.Y.M. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1247–1248. [Google Scholar] [CrossRef]
- Vivinus-Nébot, M.; Dainese, R.; Anty, R.; Saint-Paul, M.C.; Nano, J.L.; Gonthier, N.; Marjoux, S.; Frin-Mathy, G.; Bernard, G.; Hébuterne, X.; et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: Role of barrier defects and mast cells. Am. J. Gastroenterol. 2012, 107, 75–81. [Google Scholar] [CrossRef]
- Schurman, J.V.; Friesen, C.A. Identifying potential pediatric chronic abdominal pain triggers using ecological momentary assessment. Clin. Pract. Pediatr. Psychol. 2015, 3, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, J.; Lin, X.P.; Dahlman-Höglund, A.; Hanson, L.A.; Esbjörn, T.; Magnusson, O.; Bengtsson, U.; Ahlstedt, S. Seasonal intestinal inflammation in patients with birch pollen allergy. J. Allergy Clin. Immunol. 2003, 112, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Rentzos, G.; Lundberg, V.; Stotzer, P.O.; Pullerits, T.; Telemo, E. Intestinal allergic inflammation in birch pollen allergic patients n relation to pollen season, IgE sensitization profile and gastrointestinal symptoms. Clin. Transl. Allergy 2014, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickert, C.N.; Lorentz, A.; Manns, M.P.; Bischoff, S.C. Colonoscopic allergen provocation test with rBet v 1 in patients with pollen-associated allergy. Allergy 2012, 67, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C. Advances in understanding immune mechanisms of food protein-induced enterocolitis syndrome. Ann. Allergy Asthma Immunol. 2021, 126, 478–481. [Google Scholar] [CrossRef]
- Mehr, S.; Brown-Whitehorn, T. What do allergists in practice need to know about non-IgE-mediated food allergies. Ann. Allergy Asthma Immunol. 2019, 122, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, N.V.; Ahmed, A.; Fortunato, J.E. Food protein-induced enterocolitis syndrome. Dynamic relationship among gastrointestinal symptoms, immune response, and the autonomic nervous system. Ann. Allergy Asthma Immunol. 2021, 126, 498–505. [Google Scholar]
- Caubet, J.C.; Ford, L.S.; Sickles, L.; Järvinen, K.M.; Sicherer, S.H.; Sampson, H.A.; Nowak-Węgrzyn, A. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J. Allergy Clin. Immunol. 2014, 134, 382–389. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Berin, M.C.; Mehr, S. Food protein-induced enterocolitis syndrome. J. Allergy Clin. Immunol. Pract. 2020, 8, 24–35. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, P.; Caparrós, E.; Moreno, V.; Cueva, B.; Fernández, J. Food protein-induced enterocolitis-like syndrome in a population of adolescents and adults caused by seafood. J. Allergy Clin. Immunol. Pract. 2019, 7, 670–672. [Google Scholar] [CrossRef]
- Bauché, D.; Marie, J.C. Transforming growth factor β: A master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef]
- Luo, A.; Leach, S.T.; Barres, R.; Hesson, L.B.; Grimm, M.C.; Simar, D. The microbiota and epigenetic regulation of T helper 17/regulatory T cells: In search of a balanced immune system. Front. Immunol. 2017, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, R.; Lee, E.-J.; Jang, M.S.; Jeun, E.-J.; Hong, C.-P.; Kim, J.-H.; Park, A.; Yun, C.H.; Hong, S.-W.; Kim, Y.-M.; et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J. Exp. Med. 2016, 213, 555–567. [Google Scholar] [CrossRef]
- Bin Dhuban, K.; d’Hennezel, E.; Ben-Shoshan, M.; McCusker, C.; Clarke, A.; Fiset, P.; Mazer, B.; Piccirillo, C.A. Altered T helper 17 responses in children with food allergy. Int. Arch. Allergy Immunol. 2013, 162, 318–322. [Google Scholar] [CrossRef]
- Wakashin, H.; Hirose, K.; Maezawa, Y.; Kagami, S.; Suto, A.; Watanabe, N.; Saito, Y.; Hatano, M.; Tokuhisa, T.; Iwakura, Y.; et al. IL-23 and TH17 cells enhance Th2-cell-medicated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008, 178, 1023–1032. [Google Scholar] [CrossRef]
- Dias, P.M.; Banerjee, G. The role of Th17/IL-17 on eosinophilic inflammation. J. Autoimmun. 2013, 40, 9–20. [Google Scholar] [CrossRef]
- Cheung, P.F.Y.; Wong, C.K.; Lam, C.W.K. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: Implications for Th17 lymphocytes-mediated allergic inflammation. J. Immunol. 2008, 180, 5625–5635. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.-A.; Park, M.; Kim, Y.-H.; Woo, S.-Y. Th17 cell-mediated immune responses promote mast cell proliferation by triggering stem cell factor in keratinocytes. Biochem. Biophys. Res. Commun. 2017, 487, 856–861. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.; Schurman, J.V.; Friesen, C.A. Mucosal Th17 cells are increased in pediatric functional dyspepsia associated with chronic gastritis. Dig. Dis. Sci. 2020, 65, 3184–3190. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Pflaum, t.; Mösinger, M.; Ruchay, Z.; Röchen, C.; Milla, P.J.; Das, M.; Böttner, M.; Wedel, T.; Schuppan, d. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019, 157, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Lee, K.J. Alterations of food-specific serum IgG4 titers to common food antigens in patients with irritable bowel syndrome. J. Neurogastroenterol. Motil. 2017, 23, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Kanagaratham, C.; El Ansari, Y.S.; Lewis, O.L.; Oettgen, H.C. IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Front. Immunol. 2020, 11, 603050. [Google Scholar] [CrossRef]
- Karakula-Juchnowicz, H.; Gałęcka, M.; Rog, J.; Bartnicka, A.; Łukaszewicz, Z.; Krukow, P.; Morylowska-Topolska, J.; Skonieczna-Zydecka, K.; Krajka, T.; Jonak, K.; et al. The food-specific serum IgG reactivity in major depressive disorder patients, irritable bowel syndrome patients and healthy controls. Nutrients 2018, 10, 548. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhou, G.; Xu, Y.; He, B.; Wang, Y.; Ma, R.; Chang, Y.; He, D.; Xu, C.; Xiao, Z. Effects of diet based on IgG elimination combined with probiotics on migraine plus irritable bowel syndrome. Pain Res. Manag. 2019, 2019, 7890461. [Google Scholar] [CrossRef] [Green Version]
- Ostrowska, L.; Wasiluk, D.; Lieners, C.F.J.; Gałęcka, M.; Bartnicka, A.; Tveiten, D. IgG food antibody guided elimination-rotation diet was more effective than FODMAP diet and control diet in the treatment of women with mixed IBS-Results from an open label study. Res. Sq. 2020, in press. [Google Scholar] [CrossRef]
- Cappelletti, M.; Tognon, E.; Vona, L.; Basello, K.; Costanzi, A.; Speciani, M.C.; Speciani, A.F. Food-specific serum IgG and sumptom reduction with a personalized, unrestricted-calorie diet of six weeks in irritable bowel syndrome (IBS). Nutr. Metab. 2020, 17, 101. [Google Scholar] [CrossRef]
- Atkinson, W.; Sheldon, T.A.; Shaath, N.; Whorwell, P.J. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomized controlled trial. Gut 2004, 53, 1459–1464. [Google Scholar] [CrossRef]
- Zar, S.; Mincher, L.; Benson, M.J.; Kumar, D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand. J. Gastroenterol. 2005, 40, 800–807. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, T.; Wang, J.; Chang, Y.; Guo, H.; Zhang, W. The value of eliminating foods according to food-specific immunoglobin G antibodies in irritable bowel syndrome with diarrhoea. J. Int. Med. Res. 2012, 40, 204–210. [Google Scholar] [CrossRef]
- Stierstorfer, M.B.; Sha, C.T.; Sasson, M. Food patch testing for irritable bowel syndrome. J. Am. Acad. Dermatol. 2013, 68, 377–384. [Google Scholar] [CrossRef]
- Shin, G.H.; Smith, M.S.; Toro, B.; Ehrlich, A.C.; Luther, S.; Midani, D.; Hong, I.; Stierstorfer, M. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin 2018, 2, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Lucendo, A.J.; Serrano-Montalbán, B.; Arias, Á.; Redondo, O.; Tenias, J.M. Efficacy of dietary treatment for inducing disease remission in eosinophilic gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 56–64. [Google Scholar] [CrossRef]
- Gutiérrez-Junquera, C.; Zevit, N. Dietary treatment of eosinophilic gastrointestinal disorders in children. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 210–216. [Google Scholar] [CrossRef]
- Kalmpourtzidou, A.; Xinias, I.; Agakidis, C.; Mavroudi, A.; Mouselimis, D.; Tsarouchas, A.; Agakidou, E.; Karagiozoglou-Lampoudi, T. Diet quality: A neglected parameter in children with food allergies; A cross-sectional study. Front. Pediatr. 2021, 9, 658778. [Google Scholar] [CrossRef]
- Schurman, J.V.; Wu, Y.P.; Grayson, P.; Friesen, C.A. A pilot study to assess the efficacy of biofeedback-assisted relaxation training as an adjunct treatment for pediatric functional dyspepsia associated with duodenal eosinophilia. J. Pediatr. Psychol. 2010, 35, 837–847. [Google Scholar] [CrossRef]
- Pesek, R.D.; Gupta, S.K. Future therapies for eosinophilic gastrointestinal disorders. Ann. Allergy Asthma Immunol. 2020, 124, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, J.B.; Hirano, I. Biological therapies for eosinophilic gastrointestinal diseases. J. Allergy Clin. Immunol. 2018, 142, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Navinés-Ferrer, A.; Serrano-Candelas, E.; Molina-Molina, G.J.; Martin, M. EgE-related diseases and anti-IgE-based treatments. J. Immunol. Res. 2016, 2016, 8163803. [Google Scholar] [CrossRef]
- Foroughi, S.; Foster, B.; Kim, N.; Bernadino, L.B.; Scott, L.M.; Hamilton, R.G.; Metcalfe, D.D.; Mannon, P.J.; Prussin, C. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J. Allergy Clin. Immunol. 2007, 120, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Zhou, H.; Gu, W.; Wang, X.; Yang, J. Clinical efficacy and safety of ketotifen in treating irritable bowel syndrome with diarrhea. Eur. J. Gastroenterol. Hepatol. 2020, 32, 706–712. [Google Scholar] [CrossRef]
- Klooker, T.K.; Braak, B.; Koopman, K.E.; Welting, O.; Wouters, M.M.; van der Heide, S.; Schemann, M.; Bischoff, S.C.; van den Wijngaard, R.M.; Boeckxstaens, G.E. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010, 59, 1213–1221. [Google Scholar] [CrossRef]
- Grazioli, I.; Melzi, G.; Balsamo, V.; Castellucci, G.; Castro, M.; Catassi, C.; Rätsch, J.M.; Scotta, S. Food intolerance and irritable bowel syndrome of childhood: Clinical efficacy of oral sodium cromoglycate and elimination diet. Minerva Pediatr. 1993, 45, 253–258. [Google Scholar]
- Burks, A.W.; Sampson, H.A. Double-blind placebo-controlled trial of oral cromolyn in children with atopic dermatitis and documented food hypersensitivity. J. Allergy Clin. Immunol. 1988, 81, 417–423. [Google Scholar] [CrossRef]
- Lunardi, C.; Bambara, L.M.; Biasi, D.; Cortina, P.; Peroli, P.; Nicolis, F.; Favari, F.; Pacor, M.L. Double-blind cross-over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin. Exp. Allergy 1991, 21, 569–572. [Google Scholar] [CrossRef]
- Stefanini, G.F.; Saggioro, A.; Alvisi, V.; Angelini, G.; Capurso, L.; di Lorenzo, G.; Dobrilla, G.; Dodero, M.; Galimberti, M.; Gasbarrini, M.; et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand. J. Gastroenterol. 1995, 30, 535–541. [Google Scholar] [CrossRef]
- Lobo, B.; Ramos, L.; Martínez, C.; Guilarte, M.; González-Castro, A.M.; Alonso-Cotoner, C.; Pigrau, M.; de Torres, I.; Rodiño-Janeiro, B.K.; Salvo-Romero, E.; et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United Eur. Gastroenterol. J. 2017, 5, 887–897. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-siglec-8 antibody for eosinophilic gastritis and duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016, 150, 875–887.e9. [Google Scholar] [CrossRef] [Green Version]
- Matter, S.E.; Bhatia, P.S.; Miner, P.B., Jr. Evaluation of antral mast cells in nonulcer dyspepsia. Dig. Dis. Sci. 1990, 35, 1358–1363. [Google Scholar] [CrossRef]
- Friesen, C.A.; Sandridge, L.; Andre, L.; Roberts, C.C.; Abdel-Rahman, S.M. Mucosal eosinophilia and response to H1/H2 antagonist and cromolyn therapy in pediatric dyspepsia. Clin. Pediatr. 2006, 45, 143–147. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Goodsall, T.M.; Walker, M.M.; Talley, N.J. Dual histamine blockade for the treatment of adult functional dyspepsia: A single centre experience. Gut 2020, 69, 966. [Google Scholar] [CrossRef]
- Friesen, C.A.; Kearns, G.L.; Andre, L.; Neustrom, M.; Roberts, C.C.; Abdel-Rahman, S.M. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 343–351. [Google Scholar] [CrossRef]
- Friesen, C.A.; Neilan, N.A.; Schurman, J.V.; Taylor, D.L.; Kearns, G.L.; Abdel-Rahman, S.M. Montelukast in the treatment of duodenal eosinophilia in children with dyspepsia: Effect on eosinophil density and activation in relation to pharmacokinetics. BMC Gastroenterol. 2009, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Junquera, C.; Fernández-Fernández, S.; Cilleruelo, M.L.; Rayo, A.; Echeverría, L.; Borrell, B.; Román, E. Long-term treatment with proton pump inhibitors is effective in children with eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 210–216. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Wodk, N.K.; Walker, M.M.; Jones, M.P.; Talley, N.J. Proton pump inhibitors and suppression of duodenal eosinophilia in functional dyspepsia. Gut 2019, 68, 1339–1340. [Google Scholar] [CrossRef]
- Wauters, L.; Ceulemans, M.; Frings, D.; Lambaerts, M.; Accarie, A.; Toth, J.; Mols, R.; Augustijns, P.; De Hertogh, G.; Van Oudenhove, L.; et al. Proton pump inhibitors reduce duodenal eosinophilia, mast cells, and permeability in patients with functional dyspepsia. Gastroenterology 2021, 160, 1521–1531. [Google Scholar] [CrossRef]
- Talley, N.J.; Walker, M.M.; Jones, M.; Keely, S.; Koloski, N.; Cameron, R.; Fairlie, T.; Burns, G.; Shah, A.; Hansen, T.; et al. Letter: Budesonide for functional dyspepsia with duodenal eosinophilia- randomised, double-blind, placebo-controlled parallel-group trial. Aliment. Pharmacol. Ther. 2021, 53, 1332–1333. [Google Scholar] [CrossRef]

| Medication | Mode of Action | Population | Study Type | Result |
|---|---|---|---|---|
| Diphenhydramine [129] | H1 antagonist | Adults with FD and mucosal mast cell density elevation | Open-label trial | Symptomatic improvement in 79% |
| Ebastine [128] | H1 antagonist | Adults with IBS | Randomized, double-blind placebo-controlled trial | Symptomatic improvement and reduced visceral sensitivity |
| Hydroxyzine/Ranitidine [130] | H1/H2 antagonists | Children with FD and mucosal eosinophilia | Retrospective case series | Symptomatic improvement in 50% |
| Loratidine/Ranitidine [131] | H1/H2 antagonists | Adults with FD | Retrospective case series | Symptomatic improvement in 71% |
| Montelukast [132] | Cys-Leukotriene antagonist | Children with FD and mucosal eosinophilia | Randomized, double-blind placebo-controlled cross-over trial | Superior to placebo in pain relief |
| Montelukast [133] | Cys-Leukotriene antagonist | Children with FD and mucosal eosinophilia | Open-label trial | Symptomatic improvement unrelated to changes in mucosal eosinophilia or mast cell density |
| Budesonide [137] | Steroid | Adults with FD and mucosal eosinophilia | Randomized, double-blind placebo-controlled trial | Symptomatic response not different from placebo |
| Unspecified PPI [135] | Proton pump inhibitor | Adults with FD and mucosal eosinophilia | Case-control study | Lower eosinophil density without symptomatic improvement |
| Pantoprazole [136] | Proton pump inhibitor | Adults with FD | Open-label trial | Symptomatic improvement and decreased mucosal eosinophil and mast cell densities |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friesen, C.; Colombo, J.; Schurman, J. Update on the Role of Allergy in Pediatric Functional Abdominal Pain Disorders: A Clinical Perspective. Nutrients 2021, 13, 2056. https://doi.org/10.3390/nu13062056
Friesen C, Colombo J, Schurman J. Update on the Role of Allergy in Pediatric Functional Abdominal Pain Disorders: A Clinical Perspective. Nutrients. 2021; 13(6):2056. https://doi.org/10.3390/nu13062056
Chicago/Turabian StyleFriesen, Craig, Jennifer Colombo, and Jennifer Schurman. 2021. "Update on the Role of Allergy in Pediatric Functional Abdominal Pain Disorders: A Clinical Perspective" Nutrients 13, no. 6: 2056. https://doi.org/10.3390/nu13062056







