Cardiometabolic Associations between Physical Activity, Adiposity, and Lipoprotein Subclasses in Prepubertal Norwegian Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study and Participants
2.2. Lipoprotein Subclasses
2.3. Physical Activity
2.4. Adiposity
2.5. Data Analysis
3. Results
3.1. Descriptive Statistics for the Variables
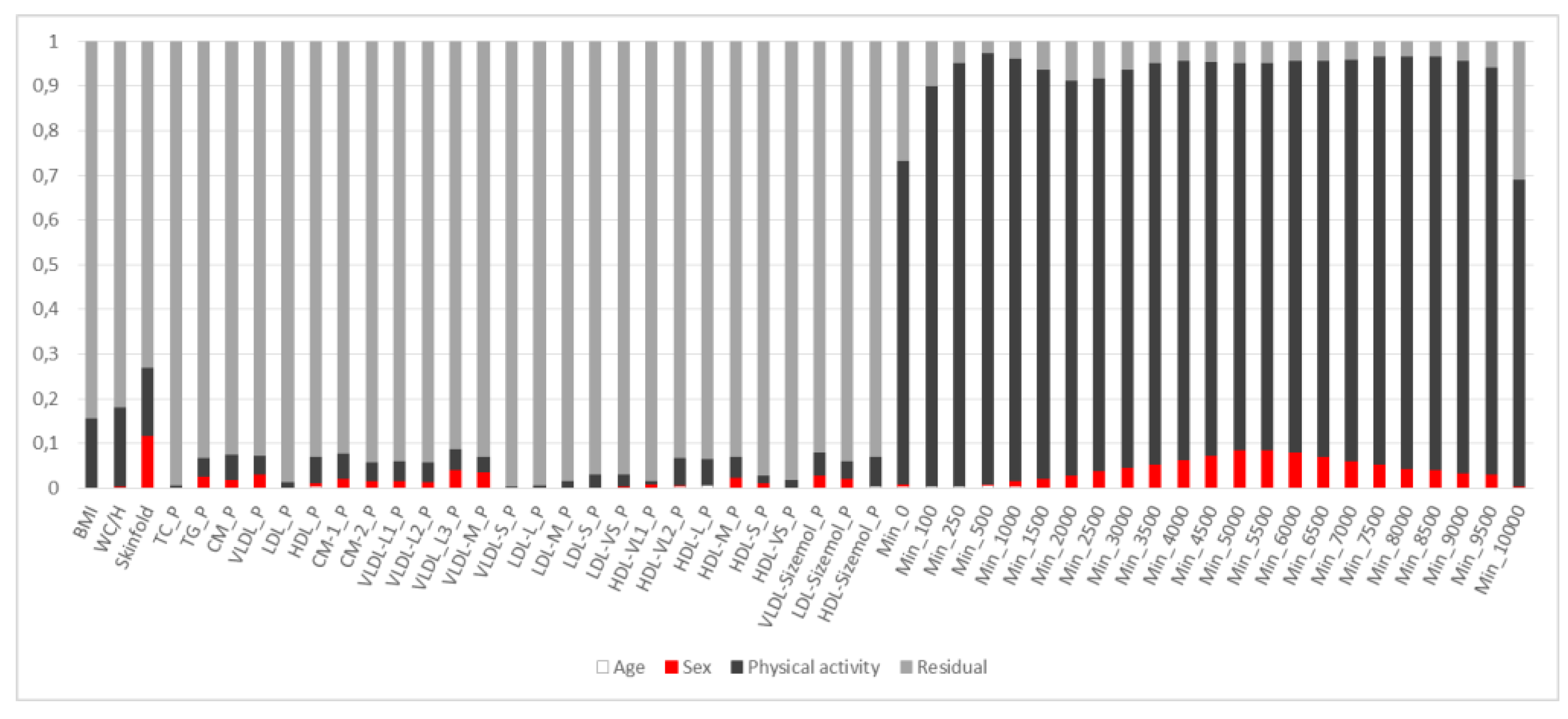
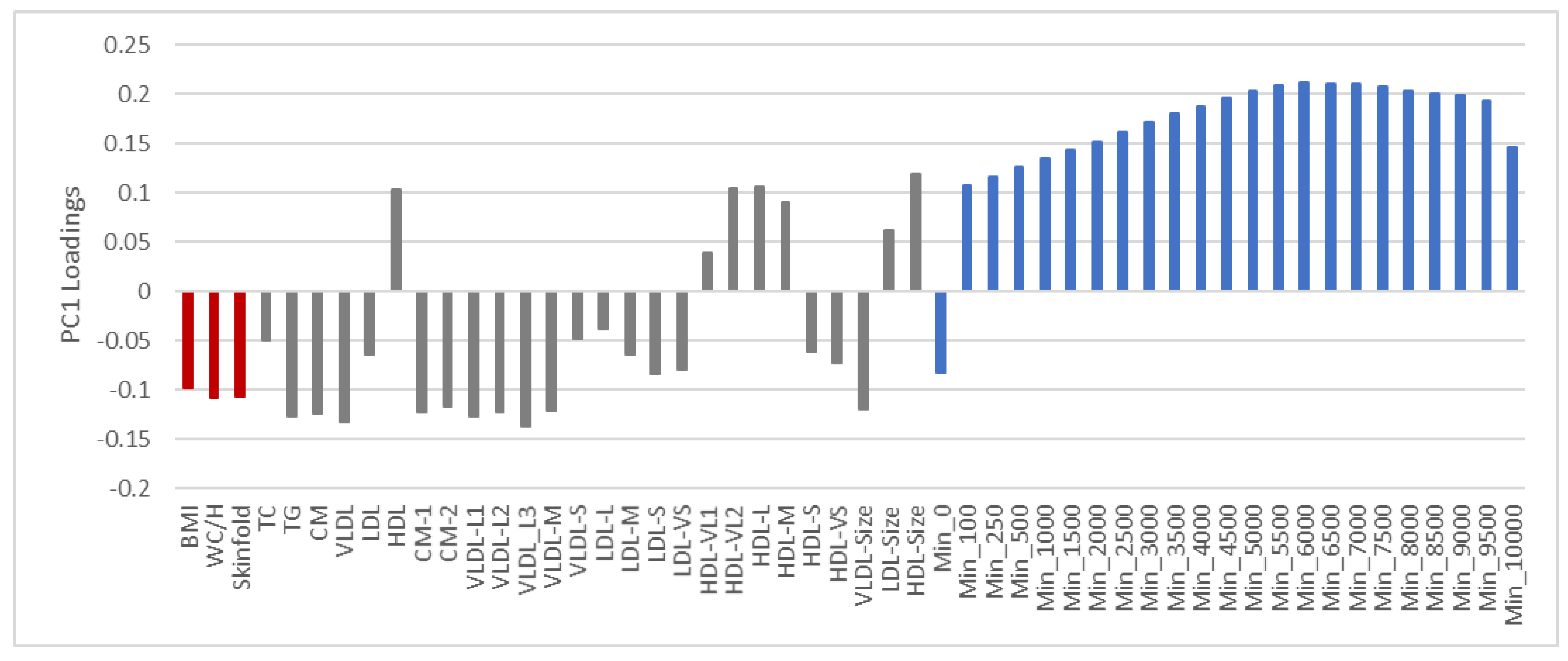
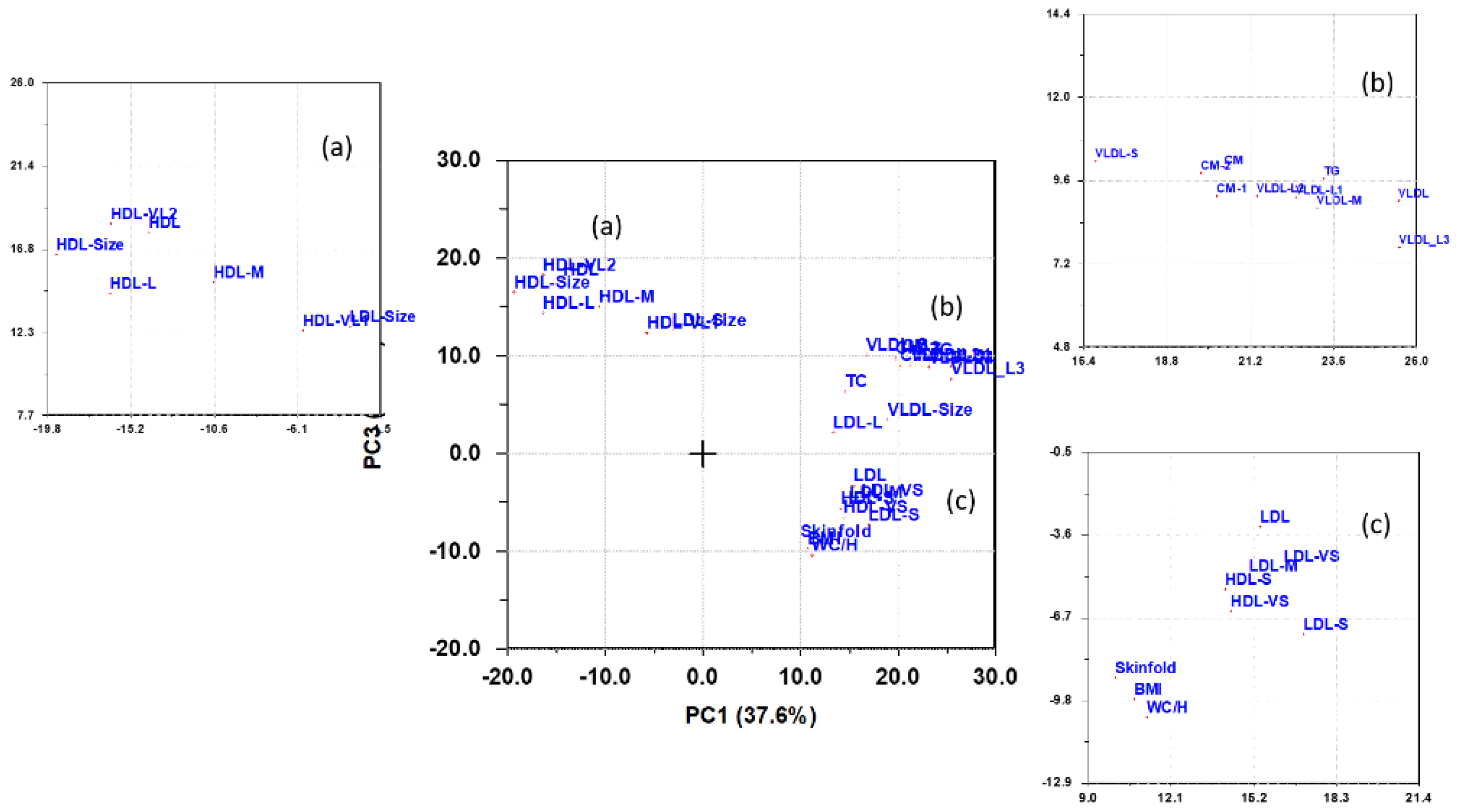
3.2. PCA of Lipoproteins Adjusted for Age and Sex
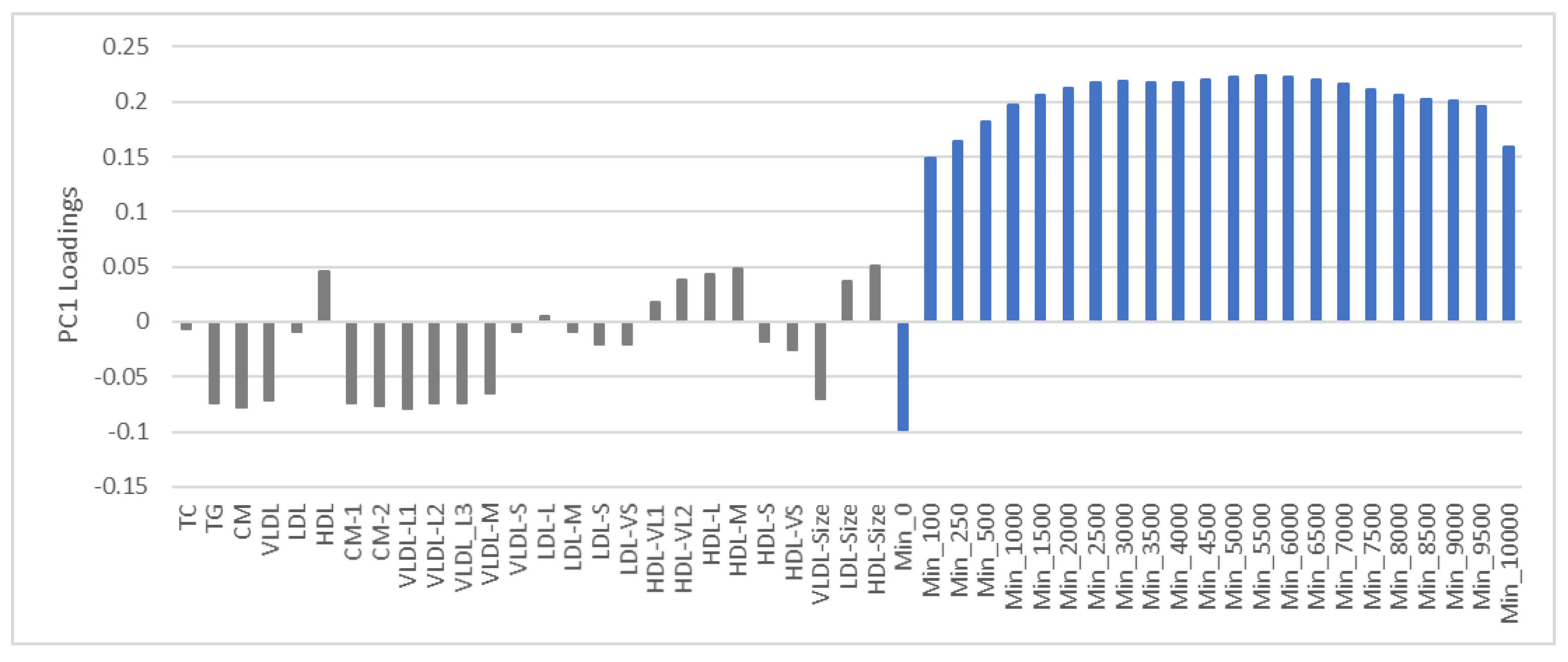
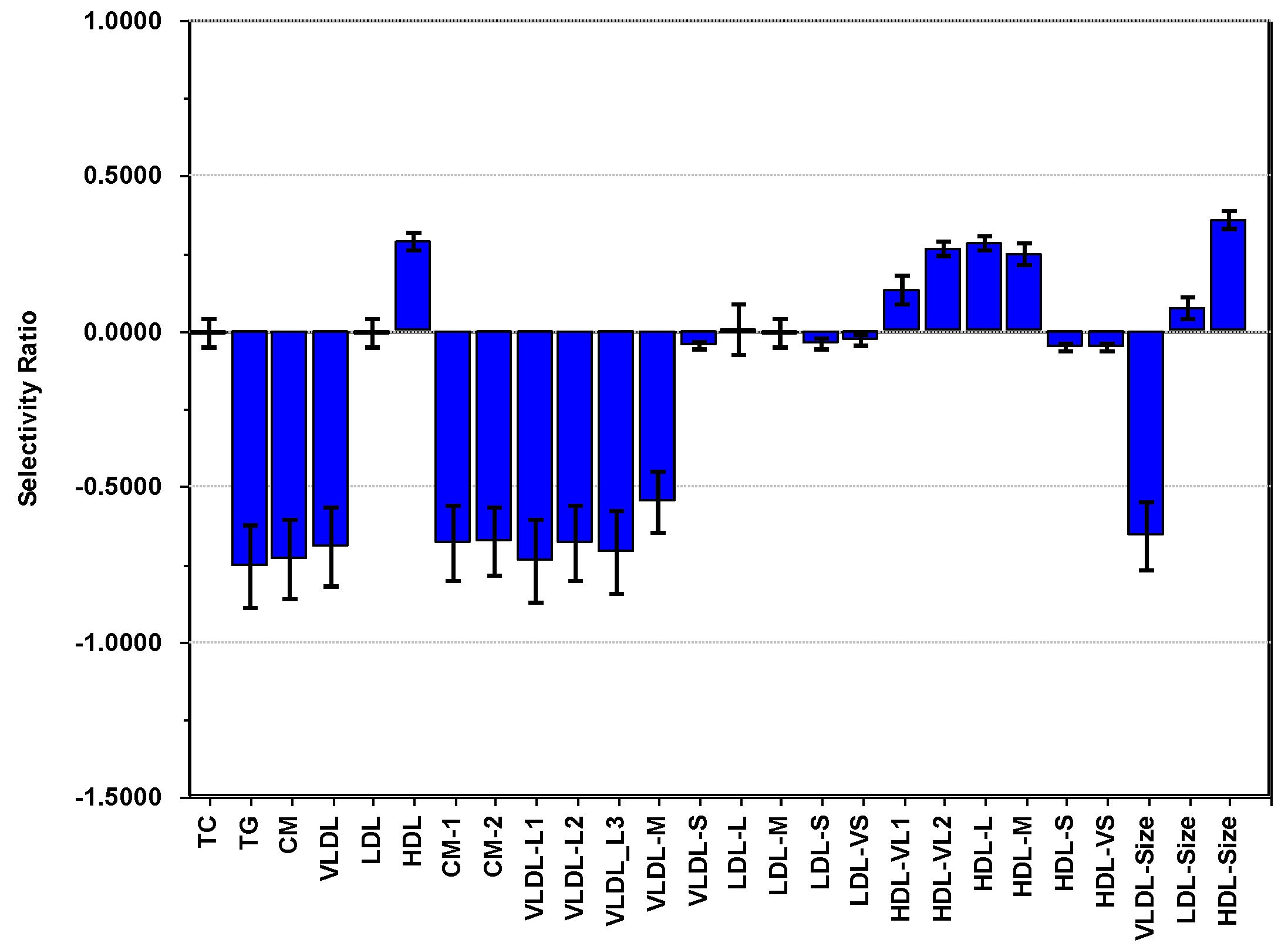
3.3. PCA of Lipoproteins and PA Adjusted for Age, Sex and Adiposity
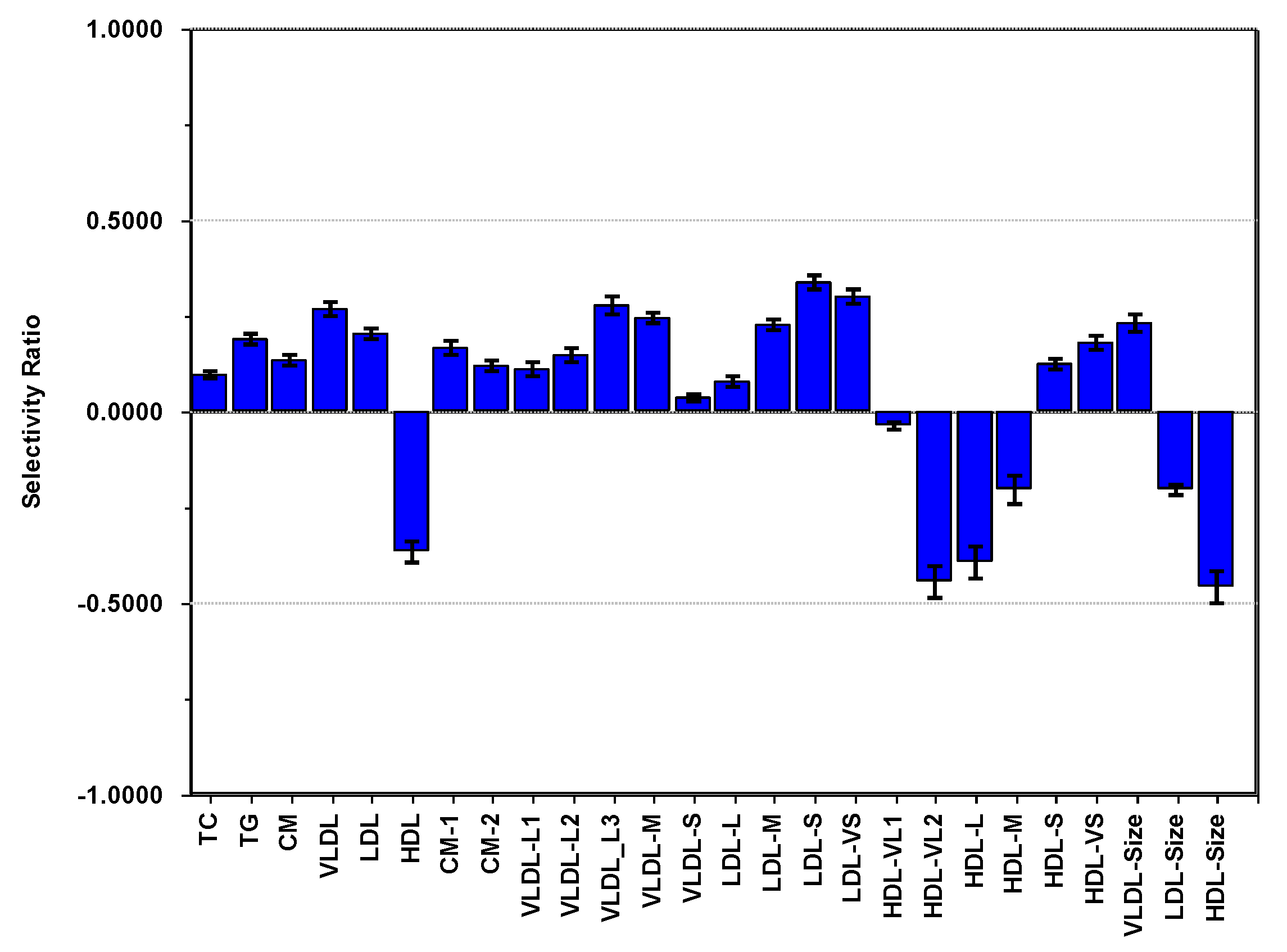
3.4. PCA of Lipoproteins and Adiposity Adjusted for Age, Sex and PA
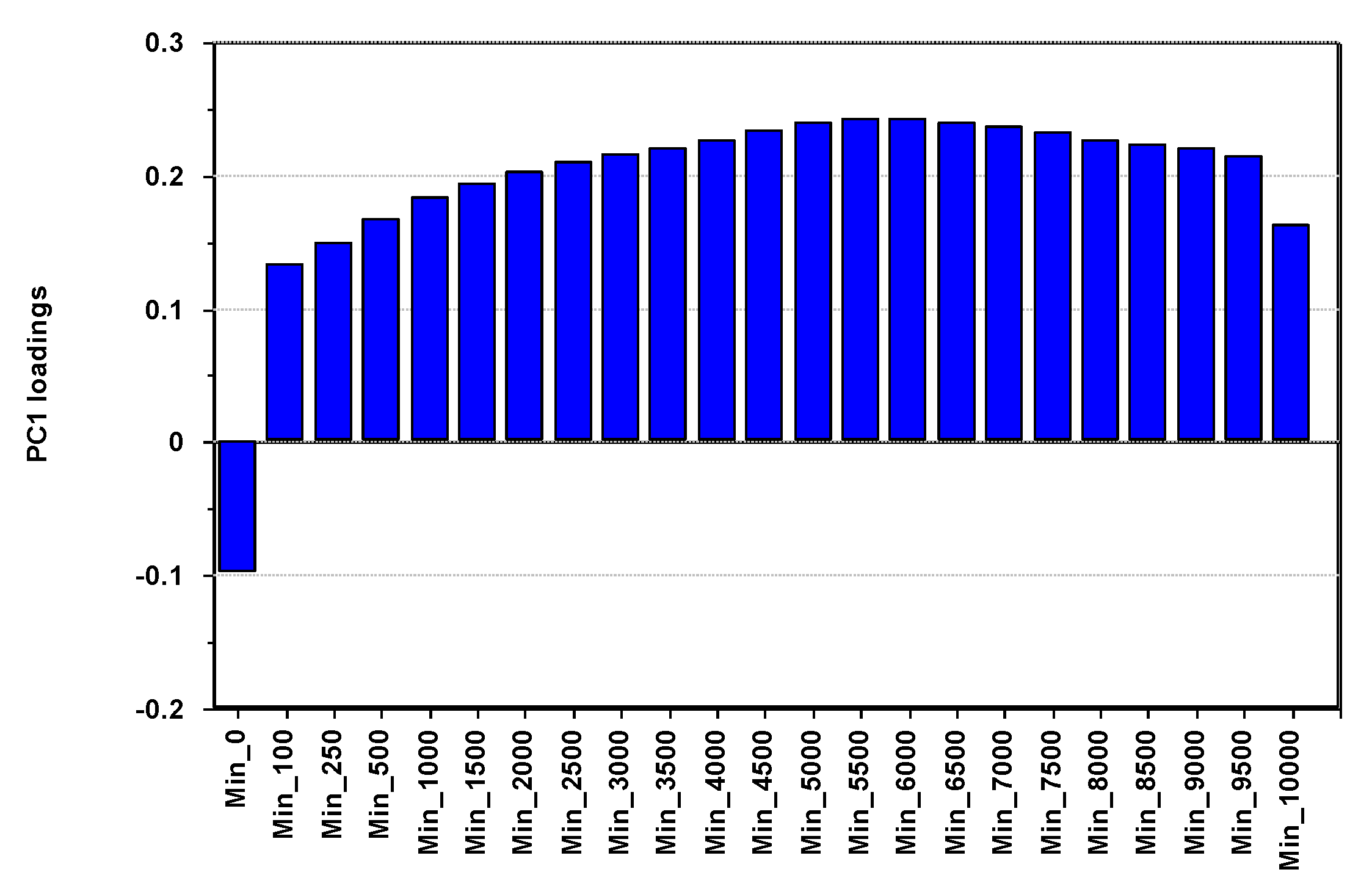
3.5. Multivariate Pattern Analysis of Lipoproteins
3.6. Summary of Findings
4. Discussion
Strengths and Limitations of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Clinical Trial Registration
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
References
- Okazaki, M.; Usui, S.; Ishigami, M.; Sakai, N.; Nakamura, T.; Matsuzawa, Y.; Yamashita, S. Identification of Unique Lipoprotein Subclasses for Visceral Obesity by Component Analysis of Cholesterol Profile in High-Performance Liquid Chromatography. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Lamarche, B.; Tchernof, A.; Moorjani, S.; Cantin, B.; Dagenais, G.R.; Lupien, P.J.; Despre’s, J.-P. Small, Dense Low-Density Lipoprotein Particles as a Predictor of the Risk of Ischemic Heart Disease in Men. Circulation 1997, 95, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Freedman, D.S.; Otvos, J.D.; Jeyarajah, E.J.; Barboriak, J.J.; Anderson, A.J.; Walker, J.A. Relation of Lipoprotein Subclasses as Measured by Proton Nuclear Magnetic Resonance Spectroscopy to Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1046–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otvos, J.D.; Collins, D.; Freedman, D.S.; Shalaurova, I.; Schaefer, E.J.; McNamara, J.R.; Bloomfield, H.; Robins, S.J. Low-Density Lipoprotein and High-Density Lipoprotein Particle Subclasses Predict Coronary Events and Are Favorably Changed by Gemfibrozil Therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006, 113, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Slyper, A.H.; Rosenberg, H.; Kabra, A.; Weiss, M.J.; Blech, B.; Gensler, S.; Matsumura, M. Early atherogenesis and visceral fat in obese adolescents. Int. J. Obes. 2014, 38, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Miida, T. Small dense LDL: An emerging risk factor for cardiovascular disease. Clin. Chim. Acta 2012, 414, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Halverstadt, A.; Pharesa, D.A.; Wilund, K.R.; Goldberg, A.P.; Hagberg, J.M. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metab. Clin. Exp. 2007, 56, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Mäkinen, V.-P.; Heinonen, I.; Soininen, P.; Kangas, A.J.; Leskinen, T.; Rahkila, P.; Würtz, P.; Kovanen, V.; Cheng, S.; et al. Long-term Leisure-time Physical Activity and Serum Metabolome. Circulation 2013, 127, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Després, J.-P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R.; et al. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. BBA Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef] [PubMed]
- James, R.W.; Brulhart-Meynet, M.C.; Lehmann, T.; Golay, A. Lipoprotein distribution and composition in obesity: Their association with central adiposity. Int. J. Obes. 1997, 21, 1115–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainwater, D.L.; Mitchell, B.D.; Comuzzie, A.G.; Haffner, S.M. Relationship of low-density lipoprotein particle size and measures of adiposity. Int. J. Obes. 1999, 23, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.-S.; Gutin, B.; Barbeau, P.; Litaker, M.S.; Allison, J.; Le, N.A. Low-density lipoprotein particle size; central obesity, cardiovascular fitness, and insulin resistance syndrome markers in obese youths. Int. J. Obes. 2002, 26, 1030–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, D.C.; D’Agostino, R.B., Jr.; Haffner, S.M.; Otvos, J.D. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metab. Clin. Exp. 2005, 54, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Mohammed, B.S.; Mittendorfer, B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int. J. Obes. 2008, 32, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- Aadland, E.; Kvalheim, O.M.; Anderssen, S.A.; Resaland, G.K.; Andersen, L.B. The multivariate physical activity signature associated with metabolic health in children. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 77. [Google Scholar] [CrossRef]
- Aadland, E.; Kvalheim, O.M.; Anderssen, S.A.; Resaland, G.K.; Andersen, L.B. Multicollinear physical activity accelerometry data and associations to cardiometabolic health: Challenges; pitfalls.; and potential solutions. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 74. [Google Scholar] [CrossRef]
- Kvalheim, O.M. Latent-structure decompositions (projections) of multivariate data. Chemom. Int. Lab. Syst. 1987, 2, 283–290. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Juonala, M.; Kahonen, M.; Taittonen, L.; Laitinen, T.; Maki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef]
- Eisenmann, J.C.; Welk, G.J.; Wickel, E.E.; Blair, S.N. Aerobics Center Longitudinal Study. Stability of variables associated with the metabolic syndrome from adolescence to adulthood: The Aerobics Center Longitudinal Study. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2004, 16, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Hamer, M.; Richmond, R.C.; Timpson, N.J.; Carslake, D.; Smith, G.D. Associations of device-measured physical activity across adolescence with metabolic traits: Prospective cohort study. PLoS Med. 2018, 15, e1002649. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.R.; Rajalahti, T.; Resaland, G.K.; Aadland, E.; Steene-Johannessen, J.; Anderssen, S.A.; Bathen, T.F.; Andreassen, T.; Kvalheim, O.M.; Ekelund, U. Associations of physical activity and sedentary time with lipoprotein subclasses in Norwegian schoolchildren: The Active Smarter Kids (ASK) study. Atherosclerosis 2019, 288, 186–193. [Google Scholar] [CrossRef]
- Okuma, H.; Okada, T.; Abe, Y.; Saito, E.; Iwata, F.; Hara, M.; Ayusawa, M.; Mugishima, H.; Takahashi, S. Abdominal adiposity is associated with high-density lipoprotein subclasses in Japanese schoolchildren. Clin. Chim. Acta 2013, 425, 80–84. [Google Scholar] [CrossRef]
- Resaland, G.K.; Rajalahti, T.; Aadland, E.; Kvalheim, O.M. Strong association between cardiorespiratory fitness and lipoprotein subclass pattern in prepubertal healthy children. Scand. J. Med. Sci. Sports 2018, 28, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Resaland, G.K.; Moe, V.F.; Aadland, E.; Steene-Johannessen, J.; Glosvik, Ø.; Andersen, J.R.; Kvalheim, O.M.; McKay, H.A.; Anderssen, S.A.; on behalf of the ASK Study Group. Active Smarter Kids (ASK): Rationale and design of a cluster-randomized controlled trial investigating the effects of daily physical activity on children’s academic performance and risk factors for non-communicable diseases. BMC Public Health 2015, 15, 709. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Rajalahti, T.; Mjøs, S.A.; Kvalheim, O.M. Predictive associations between serum fatty acid and lipoproteins in healthy non-obese Norwegians—Implications for cardiovascular health. Metabolomics 2016, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W.J., III. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Kvalheim, O.M.; Arneberg, R.; Grung, B.; Rajalahti, T. Determination of optimum number of components in partial least squares regression from distributions of the root-mean-squared error obtained by Monte Carlo resampling. J. Chemom. 2018, 32, e2993. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.; Holmes, E.; Lindon, J.; Nicholson, J.K. Precision High-Throughput Proton NMR Spectroscopy of Human Urine, Serum, and Plasma for Large-Scale Metabolic Phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- John, D.; Freedson, P. Actigraph and Actical physical activity monitors: A peek under the hood. Med. Sci. Sports Exerc. 2012, 44, S86–S89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2851. [Google Scholar] [CrossRef] [Green Version]
- Kvalheim, O.M.; Karstang, T.V. Interpretation of latent-variable regression models. Chemom. Int. Lab. Syst. 1989, 7, 39–51. [Google Scholar] [CrossRef]
- Rajalahti, T.; Kvalheim, O.M. Multivariate data analysis in pharmaceutics: A tutorial review. Intern. J. Pharm. 2011, 417, 280–290. [Google Scholar] [CrossRef]
- Rajalahti, T.; Arneberg, R.; Berven, F.S.; Myhr, K.-M.; Ulvik, R.J.; Kvalheim, O.M. Biomarker discovery in mass spectral profiles by means of selectivity ratio plot. Chemom. Intell. Lab. Syst. 2009, 95, 35–48. [Google Scholar] [CrossRef]
- Aadland, E.; Andersen, J.R.; Anderssen, S.A.; Kvalheim, O.M. Physical Activity versus Sedentary Behavior: Associations with Lipoprotein Particle Subclass Concentrations in Healthy Adults. PLoS ONE 2013, 8, e85223. [Google Scholar] [CrossRef]
- Blackett, P.R.; Blevins, K.S.; Stoddart, M.; Wang, W.; Quintana, E.; Alaupovic, P.; Lee, E.T. Body Mass Index and High-Density Lipoproteins in Cherokee Indian Children and Adolescents. Pediatr. Res. 2005, 58, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Spinneker, A.; Egert, S.; Gonzalez-Gross, M.; Breidenassel, C.; Albers, U.; Stoffel-Wagner, B.; Huybrechts, I.; Manios, Y.; Venneria, E.; Molna, D.; et al. Lipid, lipoprotein and apolipoprotein profiles in European adolescents and its associations with gender, biological maturity and body fat—The HELENA Study. Eur. J. Clin. Nutr. 2012, 66, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Kaitosaari, T.; Rönnemaa, T.; Viikari, J.; Leino, A.; Jokinen, E.; Simell, O. Low-density lipoprotein (LDL) particle size in healthy prepubertal children: The STRIP study. Acta Paediatr. 2006, 95, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajalahti, T.; Aadland, E.; Resaland, G.K.; Anderssen, S.A.; Kvalheim, O.M. Cardiometabolic Associations between Physical Activity, Adiposity, and Lipoprotein Subclasses in Prepubertal Norwegian Children. Nutrients 2021, 13, 2095. https://doi.org/10.3390/nu13062095
Rajalahti T, Aadland E, Resaland GK, Anderssen SA, Kvalheim OM. Cardiometabolic Associations between Physical Activity, Adiposity, and Lipoprotein Subclasses in Prepubertal Norwegian Children. Nutrients. 2021; 13(6):2095. https://doi.org/10.3390/nu13062095
Chicago/Turabian StyleRajalahti, Tarja, Eivind Aadland, Geir Kåre Resaland, Sigmund Alfred Anderssen, and Olav Martin Kvalheim. 2021. "Cardiometabolic Associations between Physical Activity, Adiposity, and Lipoprotein Subclasses in Prepubertal Norwegian Children" Nutrients 13, no. 6: 2095. https://doi.org/10.3390/nu13062095





