The Functional DNA Methylation Signatures Relevant to Altered Immune Response of Neonatal T Cells with l-Arginine Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Enrollment
2.2. Cell Enrichment, Cell Culture and l-Arginine Treatment
2.3. DNA Methylation Assay
2.4. Reverse-Transcription qPCR and ELISAs
2.5. Statistics Analysis
3. Results
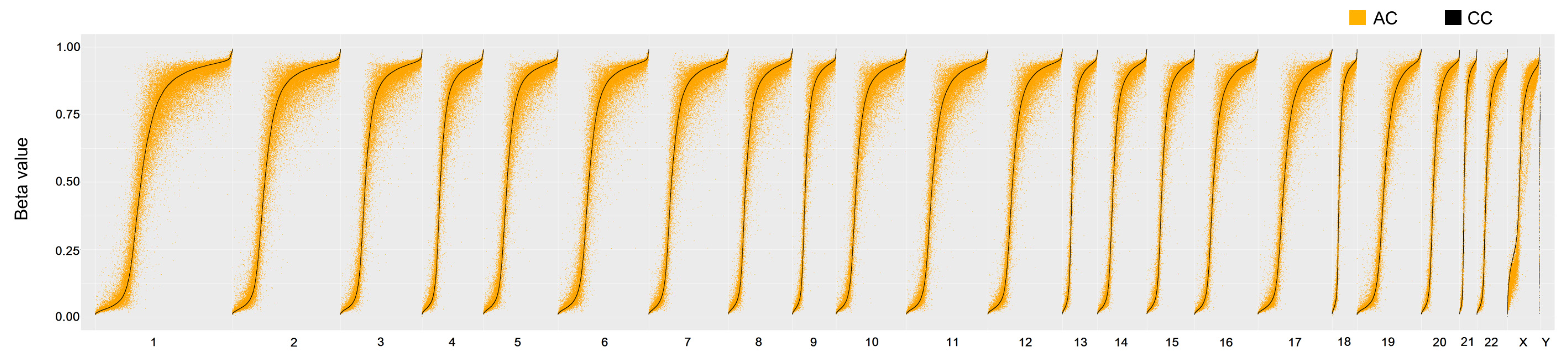
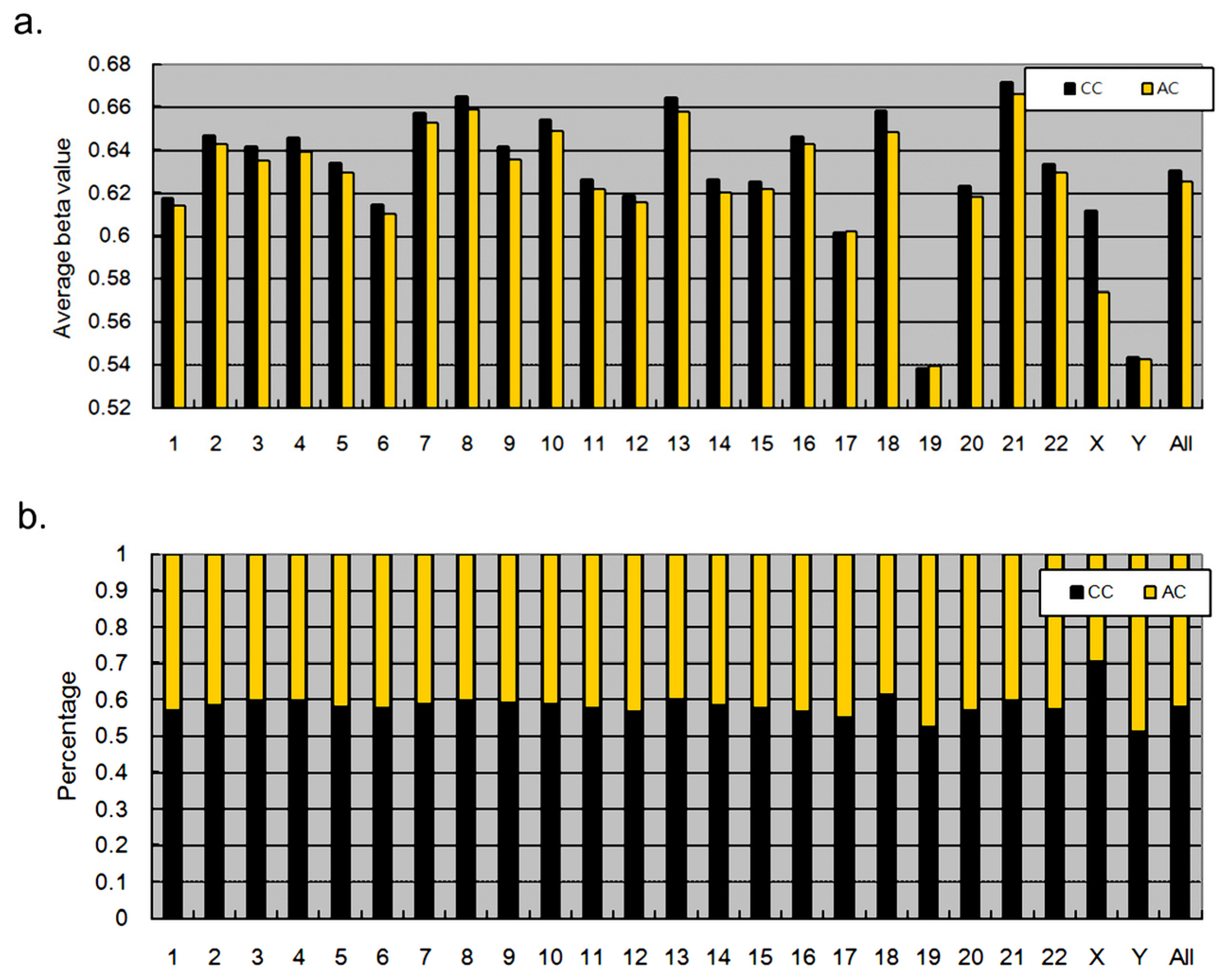
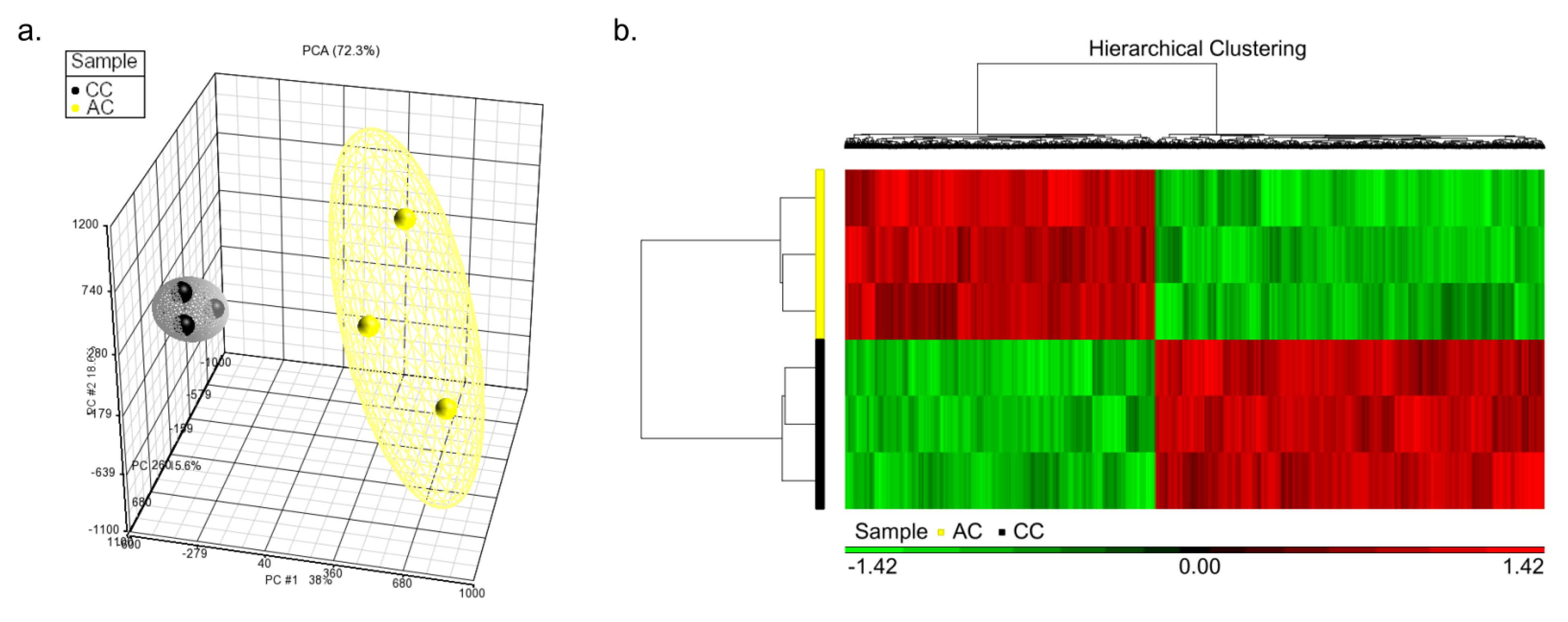
3.1. Correlation of Overall DNA Methylation Patterns with CC and AC Treatments
3.2. Methylation Proportions of CpG Dinucleotides in CC and AC
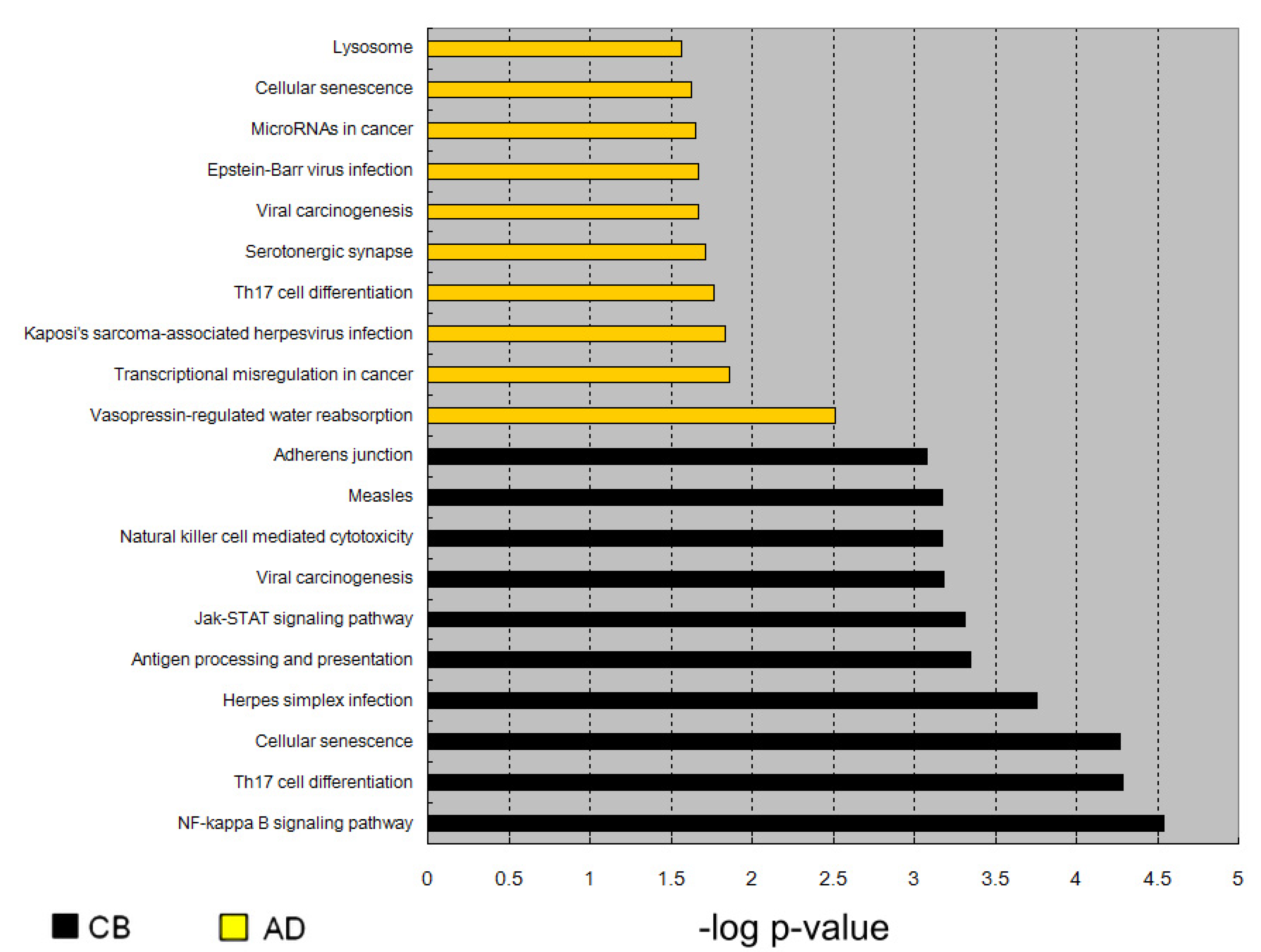
3.3. Genes with Significantly Different CpG Dinucleotides between CC and AC Samples Were Mainly Involved in Cell Adhesion and Metabolism
3.4. Expression Intensity and DNA Methylation of T Cell-Specific Genes
3.5. Enrichment of Genes Activated by l-Arginine Treatment
3.6. IL-13 Was Activated by DNA Hypomethylation in Neonatal CD4+ Cells Due to the l-Arginine Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, H.R.; Huang, Y.C.; Yang, K.D. Neonatal varicella frequently associated with visceral complications: A retrospective analysis. Acta Paediatr. Taiwanica Taiwan Er Ke Yi Xue Hui Za Zhi 2003, 44, 25–28. [Google Scholar]
- Yang, K.D.; Hill, H.R. Immune responses to infectious diseases: An evolutionary perspective. Pediatr. Infect. Dis. J. 1996, 15, 355–364. [Google Scholar] [CrossRef]
- Yu, H.R.; Chang, J.C.; Chen, R.F.; Chuang, H.; Hong, K.C.; Wang, L.; Yang, K.D. Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J. Leukoc. Biol. 2003, 74, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, D.A. Prevention and management of neonatal infections. Infect. Dis. Clin. N. Am. 1989, 3, 779–813. [Google Scholar] [CrossRef]
- Yu, H.R.; Kuo, H.C.; Huang, H.C.; Kuo, H.C.; Chen, T.Y.; Huang, L.T.; Tain, Y.L.; Chen, C.C.; Sheen, J.M.; Lin, I.C.; et al. Identification of immunodeficient molecules in neonatal mononuclear cells by proteomic differential displays. Proteomics 2011, 11, 3491–3500. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Li, X.; Wang, X.; Johnson, G.A.; Burghardt, R.C.; Dai, Z.; Wang, J.; Wu, Z. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 2013, 45, 241–256. [Google Scholar] [CrossRef]
- Che, D.; Adams, S.; Zhao, B.; Qin, G.; Jiang, H. Effects of Dietary l-arginine Supplementation from Conception to Post- Weaning in Piglets. Curr. Protein Pept. Sci. 2019, 20, 736–749. [Google Scholar] [CrossRef] [PubMed]
- McKnight, J.R.; Satterfield, M.C.; Jobgen, W.S.; Smith, S.B.; Spencer, T.E.; Meininger, C.J.; McNeal, C.J.; Wu, G. Beneficial effects of l-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids 2010, 39, 349–357. [Google Scholar] [CrossRef]
- Yu, H.R.; Kuo, H.C.; Huang, L.T.; Chen, C.C.; Tain, Y.L.; Sheen, J.M.; Tiao, M.M.; Huang, H.C.; Yang, K.D.; Ou, C.Y.; et al. l-Arginine modulates neonatal lymphocyte proliferation through an interleukin-2 independent pathway. Immunology 2014, 143, 184–192. [Google Scholar] [CrossRef]
- Batshaw, M.L.; Wachtel, R.C.; Thomas, G.H.; Starrett, A.; Brusilow, S.W. Arginine-responsive asymptomatic hyperammonemia in the premature infant. J. Pediatr. 1984, 105, 86–91. [Google Scholar] [CrossRef]
- Becker, R.M.; Wu, G.; Galanko, J.A.; Chen, W.; Maynor, A.R.; Bose, C.L.; Rhoads, J.M. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J. Pediatr. 2000, 137, 785–793. [Google Scholar] [CrossRef]
- Zamora, S.A.; Amin, H.J.; McMillan, D.D.; Fick, G.H.; Butzner, J.D.; Parsons, H.G.; Scott, R.B. Plasma l-arginine concentration, oxygenation index, and systemic blood pressure in premature infants. Crit. Care Med. 1998, 26, 1271–1276. [Google Scholar] [CrossRef]
- Tomlinson, C.; Rafii, M.; Sgro, M.; Ball, R.O.; Pencharz, P. Arginine is synthesized from proline, not glutamate, in enterally fed human preterm neonates. Pediatr. Res. 2011, 69, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Paul, W.E.; Seder, R.A. Lymphocyte responses and cytokines. Cell 1994, 76, 241–251. [Google Scholar] [CrossRef]
- Yu, H.R.; Kuo, H.C.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Chen, Y.C.; Chang, K.A.; Tain, Y.L.; Huang, L.T. Prenatal dexamethasone exposure in rats results in long-term epigenetic histone modifications and tumour necrosis factor-alpha production decrease. Immunology 2014, 143, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.R.; Tain, Y.L.; Sheen, J.M.; Tiao, M.M.; Chen, C.C.; Kuo, H.C.; Hung, P.L.; Hsieh, K.S.; Huang, L.T. Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats. Int. J. Mol. Sci. 2016, 17, 1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.R.; Huang, L.H.; Li, S.C. Roles of microRNA in the immature immune system of neonates. Cancer Lett. 2018, 433, 99–106. [Google Scholar] [CrossRef]
- Weitzel, R.P.; Lesniewski, M.L.; Haviernik, P.; Kadereit, S.; Leahy, P.; Greco, N.J.; Laughlin, M.J. microRNA 184 regulates expression of NFAT1 in umbilical cord blood CD4+ T cells. Blood 2009, 113, 6648–6657. [Google Scholar] [CrossRef] [PubMed]
- Lederhuber, H.; Baer, K.; Altiok, I.; Sadeghi, K.; Herkner, K.R.; Kasper, D.C. MicroRNA-146: Tiny player in neonatal innate immunity? Neonatology 2011, 99, 51–56. [Google Scholar] [CrossRef]
- Charrier, E.; Cordeiro, P.; Cordeau, M.; Dardari, R.; Michaud, A.; Harnois, M.; Merindol, N.; Herblot, S.; Duval, M. Post-transcriptional down-regulation of Toll-like receptor signaling pathway in umbilical cord blood plasmacytoid dendritic cells. Cell Immunol. 2012, 276, 114–121. [Google Scholar] [CrossRef]
- Huang, H.C.; Yu, H.R.; Huang, L.T.; Huang, H.C.; Chen, R.F.; Lin, I.C.; Ou, C.Y.; Hsu, T.Y.; Yang, K.D. miRNA-125b regulates TNF-alpha production in CD14+ neonatal monocytes via post-transcriptional regulation. J. Leukoc. Biol. 2012, 92, 171–182. [Google Scholar] [CrossRef]
- Huang, H.C.; Yu, H.R.; Hsu, T.Y.; Chen, I.L.; Huang, H.C.; Chang, J.C.; Yang, K.D. MicroRNA-142-3p and let-7g Negatively Regulates Augmented IL-6 Production in Neonatal Polymorphonuclear Leukocytes. Int. J. Biol. Sci. 2017, 13, 690–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.R.; Hsu, T.Y.; Huang, H.C.; Kuo, H.C.; Li, S.C.; Yang, K.D.; Hsieh, K.S. Comparison of the Functional microRNA Expression in Immune Cell Subsets of Neonates and Adults. Front. Immunol. 2016, 7, 615. [Google Scholar] [CrossRef] [Green Version]
- Brand, S.; Kesper, D.A.; Teich, R.; Kilic-Niebergall, E.; Pinkenburg, O.; Bothur, E.; Lohoff, M.; Garn, H.; Pfefferle, P.I.; Renz, H. DNA methylation of TH1/TH2 cytokine genes affects sensitization and progress of experimental asthma. J. Allergy Clin. Immunol. 2012, 129, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lluis, A.; Illi, S.; Layland, L.; Olek, S.; von Mutius, E.; Schaub, B. T regulatory cells in cord blood—FOXP3 demethylation as reliable quantitative marker. PLoS ONE 2010, 5, e13267. [Google Scholar] [CrossRef] [Green Version]
- White, G.P.; Hollams, E.M.; Yerkovich, S.T.; Bosco, A.; Holt, B.J.; Bassami, M.R.; Kusel, M.; Sly, P.D.; Holt, P.G. CpG methylation patterns in the IFNgamma promoter in naive T cells: Variations during Th1 and Th2 differentiation and between atopics and non-atopics. Pediatr. Allergy Immunol. 2006, 17, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, A.; Huoman, J.; Soderholm, S.; Bhai Mehta, R.; Nilsson, L.; Abrahamsson, T.R.; Ernerudh, J.; Gustafsson, M.; Jenmalm, M.C. Pre- and postnatal Lactobacillus reuteri treatment alters DNA methylation of infant T helper cells. Pediatr. Allergy Immunol. 2020, 31, 544–553. [Google Scholar] [CrossRef]
- White, G.P.; Watt, P.M.; Holt, B.J.; Holt, P.G. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO− T cells. J. Immunol. 2002, 168, 2820–2827. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.R.; Tsai, C.C.; Chang, L.S.; Huang, H.C.; Cheng, H.H.; Wang, J.Y.; Sheen, J.M.; Kuo, H.C.; Hsieh, K.S.; Huang, Y.H.; et al. L-Arginine-Dependent Epigenetic Regulation of Interleukin-10, but Not Transforming Growth Factor-beta, Production by Neonatal Regulatory T Lymphocytes. Front. Immunol. 2017, 8, 487. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.H.; Kuo, H.C.; Pan, C.T.; Lin, Y.S.; Huang, Y.H.; Li, S.C. Multiomics analyses identified epigenetic modulation of the S100A gene family in Kawasaki disease and their significant involvement in neutrophil transendothelial migration. Clin. Epigenet. 2018, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Uebelhoer, L.S.; Lancioni, C.L. CD4+ T Cell Activation During the Newborn Period: Barriers Against and Pathways toward Th1 Immunity. Crit. Rev. Immunol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tsafaras, G.P.; Ntontsi, P.; Xanthou, G. Advantages and Limitations of the Neonatal Immune System. Front. Pediatr. 2020, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expression by l-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef] [Green Version]
- Schmidl, C.; Delacher, M.; Huehn, J.; Feuerer, M. Epigenetic mechanisms regulating T-cell responses. J. Allergy Clin. Immunol. 2018, 142, 728–743. [Google Scholar] [CrossRef] [Green Version]
- Martino, D.; Maksimovic, J.; Joo, J.H.; Prescott, S.L.; Saffery, R. Genome-scale profiling reveals a subset of genes regulated by DNA methylation that program somatic T-cell phenotypes in humans. Genes Immun. 2012, 13, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.R.; Kim, S.T.; Spilianakis, C.G.; Fields, P.E.; Flavell, R.A. T helper cell differentiation: Regulation by cis elements and epigenetics. Immunity 2006, 24, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, M.P.; Mecacci, F.; Sampognaro, S.; Manetti, R.; Parronchi, P.; Maggi, E.; Romagnani, S. Aeroallergen sensitization can occur during fetal life. Int. Arch. Allergy Immunol. 1993, 102, 301–303. [Google Scholar] [CrossRef]
- Chung, E.K.; Miller, R.L.; Wilson, M.T.; McGeady, S.J.; Culhane, J.F. Antenatal risk factors, cytokines and the development of atopic disease in early childhood. Arch. Dis. Child Fetal Neonatal Ed. 2007, 92, F68–F73. [Google Scholar] [CrossRef] [PubMed]
- Loibichler, C.; Pichler, J.; Gerstmayr, M.; Bohle, B.; Kisst, H.; Urbanek, R.; Szepfalusi, Z. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and pre-term deliveries perfused in vitro. Clin. Exp. Allergy 2002, 32, 1546–1551. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.J.; Jones, C.A.; Miles, E.A.; Warner, J.O.; Warner, J.A. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J. Allergy Clin. Immunol. 2000, 105, 951–959. [Google Scholar] [CrossRef]
- MacGillivray, D.M.; Kollmann, T.R. The role of environmental factors in modulating immune responses in early life. Front. Immunol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [Green Version]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Zhu, Z.; Wang, Z.; Homer, R.J.; Ma, B.; Riese, R.J., Jr.; Chapman, H.A., Jr.; Shapiro, S.D.; Elias, J.A. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Investig. 2000, 106, 1081–1093. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.B.; Rodriguez, Y.; Klimecki, W.T.; Vercelli, D. The human IL-13 locus in neonatal CD4+ T cells is refractory to the acquisition of a repressive chromatin architecture. J. Biol. Chem. 2007, 282, 700–709. [Google Scholar] [CrossRef] [Green Version]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Jacobsen, G.H.; Drake, A.J. What is the evidence in humans that DNA methylation changes link events in utero and later life disease? Clin. Endocrinol. 2013, 78, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Dzierzak, E.; Speck, N.A. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat. Immunol. 2008, 9, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkura, N.; Hamaguchi, M.; Morikawa, H.; Sugimura, K.; Tanaka, A.; Ito, Y.; Osaki, M.; Tanaka, Y.; Yamashita, R.; Nakano, N.; et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 2012, 37, 785–799. [Google Scholar] [CrossRef] [Green Version]
- Martino, D.J.; Tulic, M.K.; Gordon, L.; Hodder, M.; Richman, T.R.; Metcalfe, J.; Prescott, S.L.; Saffery, R. Evidence for age-related and individual-specific changes in DNA methylation profile of mononuclear cells during early immune development in humans. Epigenetics 2011, 6, 1085–1094. [Google Scholar] [CrossRef] [Green Version]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Bedogni, G.; Brambilla, P.; Cianfarani, S.; Nobili, V.; Pietrobelli, A.; Agostoni, C. Epigenetic mechanisms elicited by nutrition in early life. Nutr. Res. Rev. 2011, 24, 198–205. [Google Scholar] [CrossRef]


| Comparison | CpG Dicuelcotides | Gene | Enriched Pathway |
| CT vs. CC | 3797 | 1390 | 50 |
| AT vs. AC | 617 | 296 | 18 |
| AC vs. CC | 33,150 | 7730 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-R.; Hsu, T.-Y.; Tsai, C.-C.; Huang, H.-C.; Cheng, H.-H.; Lai, Y.-J.; Lin, Y.-J.; Chen, C.-C.; Li, S.-C.; Yang, K. The Functional DNA Methylation Signatures Relevant to Altered Immune Response of Neonatal T Cells with l-Arginine Supplementation. Nutrients 2021, 13, 2780. https://doi.org/10.3390/nu13082780
Yu H-R, Hsu T-Y, Tsai C-C, Huang H-C, Cheng H-H, Lai Y-J, Lin Y-J, Chen C-C, Li S-C, Yang K. The Functional DNA Methylation Signatures Relevant to Altered Immune Response of Neonatal T Cells with l-Arginine Supplementation. Nutrients. 2021; 13(8):2780. https://doi.org/10.3390/nu13082780
Chicago/Turabian StyleYu, Hong-Ren, Te-Yao Hsu, Ching-Chang Tsai, Hsin-Chun Huang, Hsin-Hsin Cheng, Yun-Ju Lai, Yu-Ju Lin, Chih-Cheng Chen, Sung-Chou Li, and Kuender Yang. 2021. "The Functional DNA Methylation Signatures Relevant to Altered Immune Response of Neonatal T Cells with l-Arginine Supplementation" Nutrients 13, no. 8: 2780. https://doi.org/10.3390/nu13082780
APA StyleYu, H.-R., Hsu, T.-Y., Tsai, C.-C., Huang, H.-C., Cheng, H.-H., Lai, Y.-J., Lin, Y.-J., Chen, C.-C., Li, S.-C., & Yang, K. (2021). The Functional DNA Methylation Signatures Relevant to Altered Immune Response of Neonatal T Cells with l-Arginine Supplementation. Nutrients, 13(8), 2780. https://doi.org/10.3390/nu13082780







