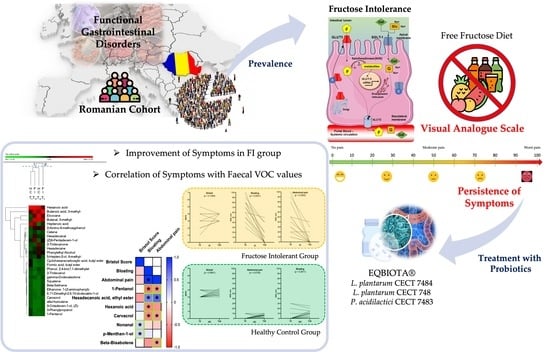
Clinical and Metabolomic Effects of Lactiplantibacillus plantarum and Pediococcus acidilactici in Fructose Intolerant Patients
Abstract
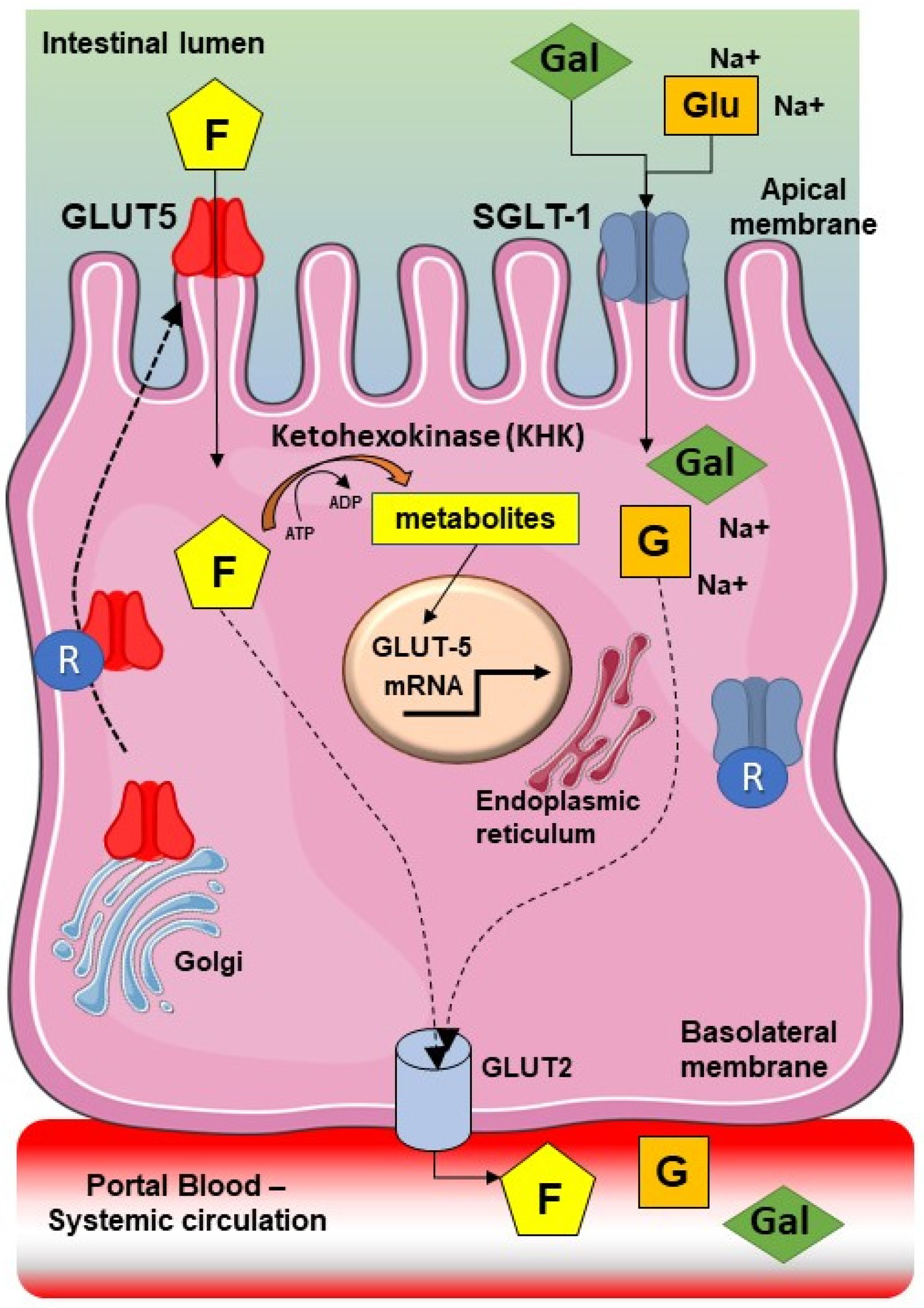
:1. Introduction
2. Materials and Methods
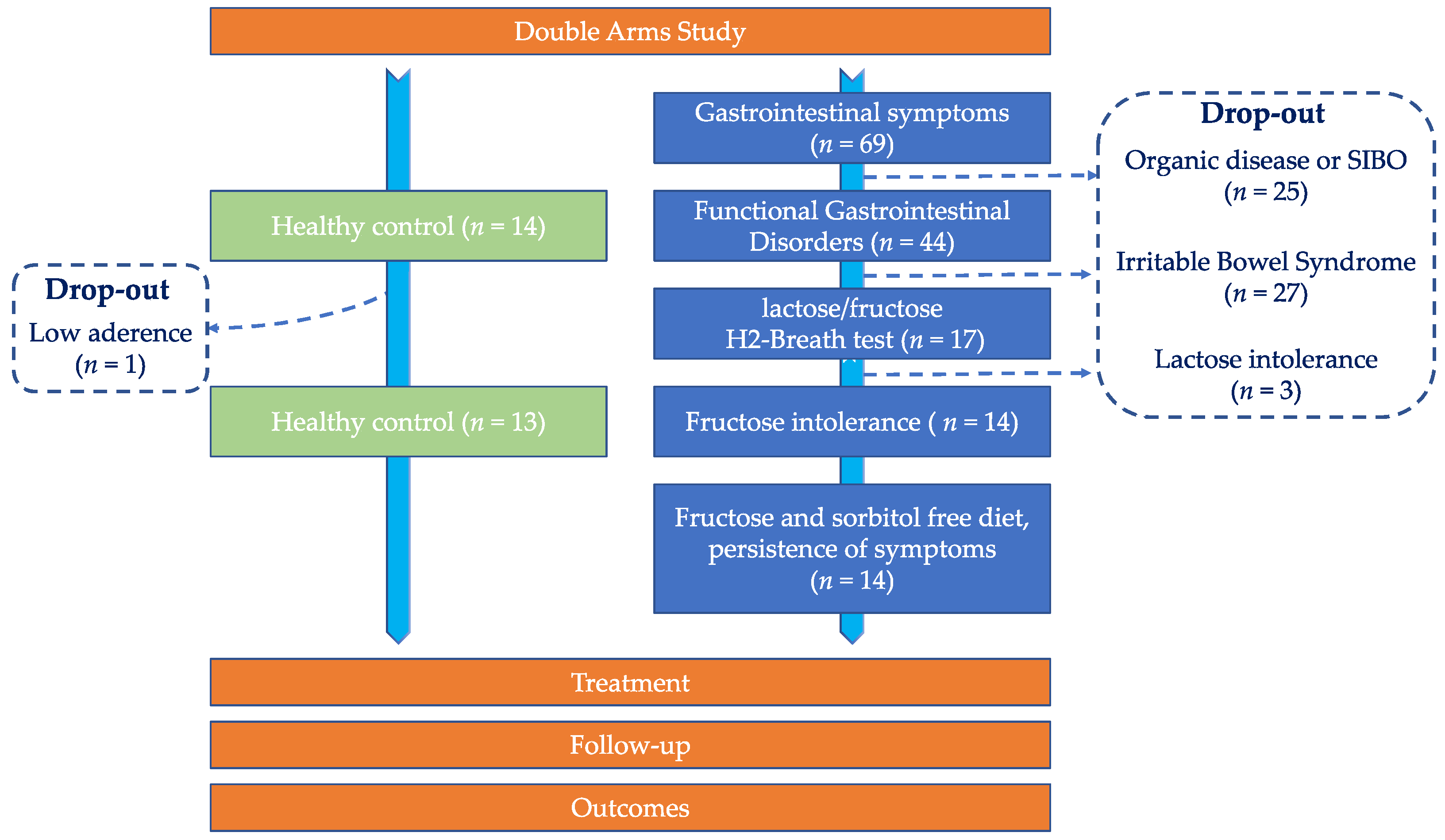
2.1. Cohort of Patients and Recruitment Criteria
2.2. Lactose and Fructose Breath Test
2.3. Probiotic Treatment with EQBIOTA
2.4. Clinical Features and Questionnaires
2.5. Fecal Collection
2.6. Fecal Metabolome
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Prevalence of Fructose Intolerance in FGIDs Cohort and Baseline Characteristics
3.2. Diet
3.3. Clinical Scores
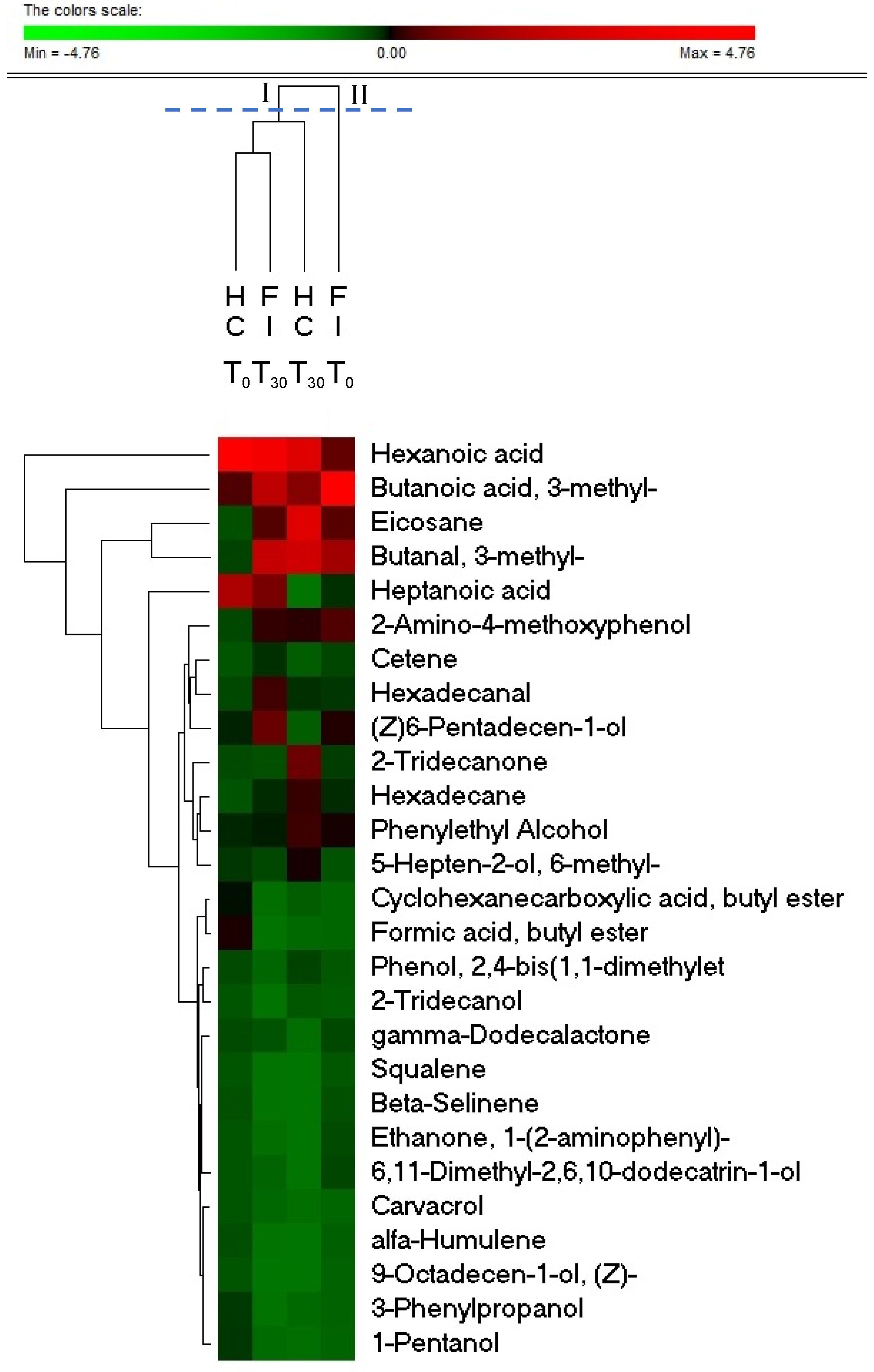
3.4. Probiotics Affects the Fecal Metabolome of FI Subjects
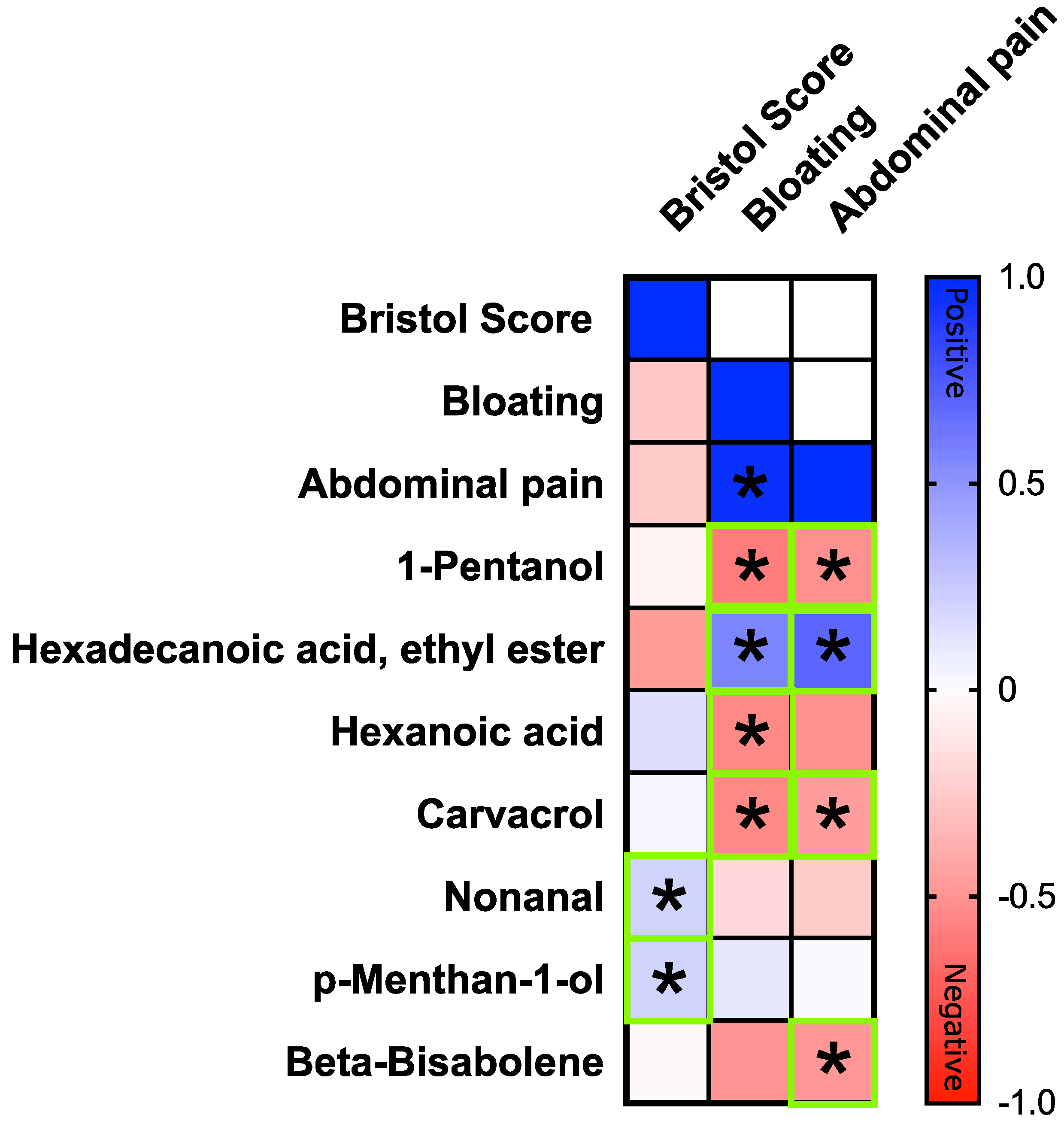
3.5. Correlation between Clinical Symptoms and VOCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basnayake, C.; Kamm, M.A.; Salzberg, M.R.; Wilson-O’Brien, A.; Stanley, A.; Thompson, A.J. Delivery of care for functional gastrointestinal disorders: A systematic review. J. Gastroenterol. Hepatol. 2020, 35, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Bonfrate, L.; Krawczyk, M.; Lembo, A.; Grattagliano, I.; Lammert, F.; Portincasa, P. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur. J. Gastroenterol. Hepatol. 2015, 27, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Moschetta, A.; Baldassarre, G.; Altomare, D.F.; Palasciano, G. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting ROME II criteria for irritable bowel syndrome. World J. Gastroenterol. 2003, 9, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst. Rev. 2006, 4, CD003664. [Google Scholar] [CrossRef]
- Leiss, O. Fiber, food intolerances, FODMAPs, gluten and functional gastrointestinal disorders--update 2014. Z. Gastroenterol. 2014, 52, 1277–1298. [Google Scholar] [CrossRef] [PubMed]
- Latulippe, M.E.; Skoog, S.M. Fructose malabsorption and intolerance: Effects of fructose with and without simultaneous glucose ingestion. Crit. Rev. Food Sci. Nutr. 2011, 51, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.S.; Irving, P.M.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment. Pharmacol. Ther. 2009, 30, 165–174. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Materna, A.; Wermelinger, C.; Schuler, J. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2013, 37, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Johlin, F.C., Jr.; Summers, R.W.; Jackson, M.; Rao, S.S. Fructose intolerance: An under-recognized problem. Am. J. Gastroenterol. 2003, 98, 1348–1353. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 554–560. [Google Scholar] [CrossRef]
- Johlin, F.C., Jr.; Panther, M.; Kraft, N. Dietary fructose intolerance: Diet modification can impact self-rated health and symptom control. Nutr. Clin. Care Off. Publ. Tufts Univ. 2004, 7, 92–97. [Google Scholar]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Noelting, J.; DiBaise, J.K. Mechanisms of Fructose Absorption. Clin. Transl. Gastroenterol. 2015, 6, e120. [Google Scholar] [CrossRef]
- Patel, C.; Douard, V.; Yu, S.; Gao, N.; Ferraris, R.P. Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J. 2015, 29, 4046–4058. [Google Scholar] [CrossRef] [Green Version]
- Cox, T.M. Aldolase B and fructose intolerance. FASEB J. 1994, 8, 62–71. [Google Scholar] [CrossRef]
- Sivaraman, K.; Senthilkumar, G.P.; Sankar, P.; Bobby, Z. Attenuation of oxidative stress, inflammation and insulin resistance by allium sativum in fructose-fed male rats. J. Clin. Diagn. Res. 2013, 7, 1860–1862. [Google Scholar] [CrossRef]
- Gomara, R.E.; Halata, M.S.; Newman, L.J.; Bostwick, H.E.; Berezin, S.H.; Cukaj, L.; See, M.C.; Medow, M.S. Fructose intolerance in children presenting with abdominal pain. J. Pediatric Gastroenterol. Nutr. 2008, 47, 303–308. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M.; Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: Present state and perspectives. Microbiology 2010, 156, 3205–3215. [Google Scholar] [CrossRef] [Green Version]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.D.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2022, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Di Ciaula, A.; Garruti, G.; Vacca, M.; De Angelis, M.; Wang, D.Q. Bile Acids and GPBAR-1: Dynamic Interaction Involving Genes, Environment and Gut Microbiome. Nutrients 2020, 12, 3709. [Google Scholar] [CrossRef]
- Fedewa, A.; Rao, S.S. Dietary Fructose Intolerance, Fructan Intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014, 16, 370. [Google Scholar] [CrossRef] [Green Version]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Leis, R.; de Castro, M.J.; de Lamas, C.; Picans, R.; Couce, M.L. Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients 2020, 12, 1487. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Zecchi, L.; Barbaro, R.; Cremon, C.; Bellacosa, L.; Marcellini, M.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J. Clin. Gastroenterol. 2012, 46, S52–S55. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Todorov, S.D.; Martin, J.H.; Senekal, M.; Dicks, L.M. The effect of prebiotics on production of antimicrobial compounds, resistance to growth at low pH and in the presence of bile, and adhesion of probiotic cells to intestinal mucus. J. Appl. Microbiol. 2006, 100, 813–820. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Staempfli, H.R.; Weese, J.S. High Doses of Halotolerant Gut-Indigenous Lactobacillus plantarum Reduce Cultivable Lactobacilli in Newborn Calves without Increasing Its Species Abundance. Int. J. Microbiol. 2017, 2017, 2439025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, F.T.; Lim, S.M.; Ramasamy, K. Pediococcus acidilactici LAB4 and Lactobacillus plantarum LAB12 assimilate cholesterol and modulate ABCA1, CD36, NPC1L1 and SCARB1 in vitro. Benef. Microbes 2017, 8, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sanchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29 (Suppl. S1), 1–49. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef]
- Serrano-Martinez, M.; Palacios, M.; Martinez-Losa, E.; Lezaun, R.; Maravi, C.; Prado, M.; Martinez, J.A.; Martinez-Gonzalez, M.A. A Mediterranean dietary style influences TNF-alpha and VCAM-1 coronary blood levels in unstable angina patients. Eur. J. Nutr. 2005, 44, 348–354. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: Implications for host-microbe interactions contributing to obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2012, 13, 799–809. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Aranias, T.; Viel, T.; Chateau, D.; Le Gall, M.; Waligora-Dupriet, A.J.; Melchior, C.; Rouxel, O.; Kapel, N.; Gourcerol, G.; et al. Intestinal invalidation of the glucose transporter GLUT2 delays tissue distribution of glucose and reveals an unexpected role in gut homeostasis. Mol. Metab. 2017, 6, 61–72. [Google Scholar] [CrossRef]
- Zubiria, M.G.; Gambaro, S.E.; Rey, M.A.; Carasi, P.; Serradell, M.L.A.; Giovambattista, A. Deleterious Metabolic Effects of High Fructose Intake: The Preventive Effect of Lactobacillus kefiri Administration. Nutrients 2017, 9, 470. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Di Luccia, B.; Crescenzo, R.; Mazzoli, A.; Cigliano, L.; Venditti, P.; Walser, J.C.; Widmer, A.; Baccigalupi, L.; Ricca, E.; Iossa, S. Rescue of Fructose-Induced Metabolic Syndrome by Antibiotics or Faecal Transplantation in a Rat Model of Obesity. PLoS ONE 2015, 10, e0134893. [Google Scholar] [CrossRef] [Green Version]
- Crescenzo, R.; Mazzoli, A.; Di Luccia, B.; Bianco, F.; Cancelliere, R.; Cigliano, L.; Liverini, G.; Baccigalupi, L.; Iossa, S. Dietary fructose causes defective insulin signalling and ceramide accumulation in the liver that can be reversed by gut microbiota modulation. Food Nutr. Res. 2017, 61, 1331657. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Villegas, A.; Sanchez-Tapia, M.; Avila-Nava, A.; Ramirez, V.; Tovar, A.R.; Torres, N. Differential Effect of Sucrose and Fructose in Combination with a High Fat Diet on Intestinal Microbiota and Kidney Oxidative Stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef]
- De Preter, V.; Machiels, K.; Joossens, M.; Arijs, I.; Matthys, C.; Vermeire, S.; Rutgeerts, P.; Verbeke, K. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 2015, 64, 447–458. [Google Scholar] [CrossRef]
- El-Far, M.; Durand, M.; Turcotte, I.; Larouche-Anctil, E.; Sylla, M.; Zaidan, S.; Chartrand-Lefebvre, C.; Bunet, R.; Ramani, H.; Sadouni, M.; et al. Upregulated IL-32 Expression and Reduced Gut Short Chain Fatty Acid Caproic Acid in People Living with HIV With Subclinical Atherosclerosis. Front. Immunol. 2021, 12, 664371. [Google Scholar] [CrossRef]
- He, Q.; Han, C.; Huang, L.; Yang, H.; Hu, J.; Chen, H.; Dou, R.; Ren, D.; Lin, H. Astragaloside IV alleviates mouse slow transit constipation by modulating gut microbiota profile and promoting butyric acid generation. J. Cell. Mol. Med. 2020, 24, 9349–9361. [Google Scholar] [CrossRef]
- Elamin, E.; Masclee, A.; Juuti-Uusitalo, K.; van Ijzendoorn, S.; Troost, F.; Pieters, H.J.; Dekker, J.; Jonkers, D. Fatty acid ethyl esters induce intestinal epithelial barrier dysfunction via a reactive oxygen species-dependent mechanism in a three-dimensional cell culture model. PLoS ONE 2013, 8, e58561. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.S.; da Silva, F.V.; Viana, A.F.; dos Santos, M.R.; Quintans-Junior, L.J.; Martins Mdo, C.; Nunes, P.H.; Oliveira Fde, A.; Oliveira Rde, C. Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 899–908. [Google Scholar] [CrossRef]
- Mooyottu, S.; Flock, G.; Upadhyay, A.; Upadhyaya, I.; Maas, K.; Venkitanarayanan, K. Protective Effect of Carvacrol against Gut Dysbiosis and Clostridium difficile Associated Disease in a Mouse Model. Front. Microbiol. 2017, 8, 625. [Google Scholar] [CrossRef]
- De Santana Souza, M.T.; Teixeira, D.F.; de Oliveira, J.P.; Oliveira, A.S.; Quintans-Junior, L.J.; Correa, C.B.; Camargo, E.A. Protective effect of carvacrol on acetic acid-induced colitis. Biomed. Pharmacother. 2017, 96, 313–319. [Google Scholar] [CrossRef]
- Lorén, V.; Manyé, J.; Fuentes, M.C.; Cabré, E.; Ojanguren, I.; Espadaler, J. Comparative effect of the I3. 1 probiotic formula in two animal models of colitis. Probiotics Antimicrob. Proteins 2017, 9, 71–80. [Google Scholar] [CrossRef]
- Chen, J.C.; Tsai, C.C.; Hsieh, C.C.; Lan, A.; Huang, C.C.; Leu, S.F. Multispecies probiotics combination prevents ovalbumin-induced airway hyperreactivity in mice. Allergol. Immunopathol. 2018, 46, 354–360. [Google Scholar] [CrossRef]
- Lim, F.T.; Lim, S.M.; Ramasamy, K. Cholesterol lowering by Pediococcus acidilactici LAB4 and Lactobacillus plantarum LAB12 in adult zebrafish is associated with improved memory and involves an interplay between npc1l1 and abca1. Food Funct. 2017, 8, 2817–2828. [Google Scholar] [CrossRef]
- Barraza-Ortiz, D.A.; Pérez-López, N.; Medina-López, V.M.; Minero-Alfaro, J.I.; Zamarripa-Dorsey, F.; Fernández-Martínez, N.D.C.; Ramos-Aguilar, G.A. Combination of a probiotic and an antispasmodic increases quality of life and reduces symptoms in patients with irritable bowel syndrome: A pilot study. Dig. Dis. 2021, 39, 294–300. [Google Scholar] [CrossRef]
- Lorenzo-Zúñiga, V.; Llop, E.; Suárez, C.; Álvarez, B.; Abreu, L.; Espadaler, J.; Serra, J.I. 31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J. Gastroenterol. 2014, 20, 8709. [Google Scholar] [CrossRef]

| Healthy Control | Fructose Intolerant | p-Value | |
|---|---|---|---|
| Number | 13 | 14 | - |
| Age (years) | 54.0 ± 16.5 (56.0) | 48.7 ± 15.5 (49.5) | n.s. |
| Males: Females | 7:6 | 6:8 | n.s. |
| BMI (Kg/m2) | 28.4 ± 5.9 (28.7) | 25.7 ± 5.2 (24.8) | n.s. |
| Bristol score (BSFS) | 3.3 ± 0.5 (3.0) | 2.9 ± 1.1 (3.0) | n.s. |
| Bloating (VAS, mm) | 6.9 ± 11.8 (0.0) | 68.6 ± 21.4 (70.0) | p = 0.0001 |
| Abdominal pain (VAS, mm) | 2.3 ± 4.4 (0.0) | 43.6 ± 28.7 (35.0) | p = 0.0001 |
| Nutrient | Healthy Control | Fructose Intolerant | p-Value |
|---|---|---|---|
| Fiber (g) | 7.2 ± 1.0 | 7.1 ± 1.1 | n. s. |
| Carbohydrates (%) | 47.0 ± 3.5 | 45.7 ± 3.8 | n. s. |
| Proteins (%) | 19.3 ± 1.2 | 18.9 ± 1.3 | n. s. |
| Animal proteins/tot (%) | 63.9 ± 5.2 | 64.8 ± 5.9 | n. s. |
| Vegetable proteins/tot (%) | 36.1 ± 5.2 | 35.2 ± 5.9 | n. s. |
| Lipids (%) | 34.9 ± 2.6 | 35.1 ± 2.8 | n. s. |
| Saturated fatty acids/tot (%) | 40.8 ± 3.5 | 41.4 ± 3.6 | n. s. |
| Vitamin B6 (mg) | 1.1 ± 0.2 | 1.2 ± 0.2 | n. s. |
| Healthy Control | Fructose Intolerant | |||||
|---|---|---|---|---|---|---|
| T0 | T30 | p-Value | T0 | T30 | p-Value | |
| Bristol Score (BSFS) | 3.3 ± 0.5 | 3.6 ± 0.4 | n.s. | 2.9 ± 1.1 | 3.4 ± 0.5 | n.s. |
| Bloating (VAS, mm) | 6.9 ± 11.8 | 11.2 ± 6.5 | n.s. | 68.6 ± 21.4 | 13.6 ± 17.8 | p = 0.0001 |
| Abdominal pain (VAS, mm) | 2.3 ± 4.4 | 5.9 ± 3.1 | n.s. | 43.6 ± 28.7 | 7.1 ± 13.3 | p = 0.0002 |
| Compounds | FIT0 | FIT30 | HCT0 | HCT30 | FIT0 vs. FIT30 | HCT0 vs. HCT30 | HCT0 vs. FIT0 | HCT30 vs. FIT30 |
|---|---|---|---|---|---|---|---|---|
| µg/g | µg/g | µg/g | µg/g | p-value * | p-value | p-value | p-value | |
| 1-Pentanol | 0.02 ± 0.045 | 0.1 ± 0.121 | 0.23 ± 0.324 | 0.05 ± 0.096 | 0.048 | n.s. | 0.044 | n.s. |
| Sulcatol (5-Hepten-2-ol, 6-methyl-) | 0.12 ± 0.232 | 0.26 ± 0.193 | 0.24 ± 0.409 | 0.48 ± 0.4 | 0.019 | n.s. | n.s. | n.s. |
| 9-Octadecen-1-ol, (Z)- | 0.03 ± 0.055 | 0.04 ± 0.043 | n.d. | n.d. | n.s. | n.s. | n.s. | 0.01 |
| Phenylethyl Alcohol | 0.39 ± 0.297 | 0.4 ± 0.478 | 0.32 ± 0.288 | 0.59 ± 0.456 | n.s. | 0.028 | n.s. | n.s. |
| 2-Tridecanol | 0.07 ± 0.157 | 0.05 ± 0.138 | n.d. | 0.19 ± 0.15 | n.s. | n.s. | n.s. | 0.041 |
| 3-Phenylpropanol | 0.03 ± 0.08 | 0.06 ± 0.096 | 0.2 ± 0.184 | 0.08 ± 0.141 | n.s. | n.s. | 0.031 | n.s. |
| (Z)6-Pentadecen-1-ol | 0.41 ± 0.439 | 0.73 ± 0.658 | 0.34 ± 0.486 | 0.16 ± 0.138 | n.s. | n.s. | n.s. | 0.022 |
| Butanal, 3-methyl- | 1.26 ± 0.812 | 1.57 ± 1.48 | 0.14 ± 0.246 | 2 ± 1.828 | n.s. | n.s. | 0.027 | n.s. |
| Hexadecanal | 0.25 ± 0.134 | 0.54 ± 0.455 | 0.12 ± 0.207 | 0.37 ± 0.199 | 0.039 | n.s. | n.s. | n.s. |
| Formic acid, butyl ester | n.d. | 0.06 ± 0.096 | 0.46 ± 0.64 | 0.08 ± 0.224 | n.s. | n.s. | 0.035 | n.s. |
| Cyclohexanecarboxylic acid, butyl ester | n.d. | 0.07 ± 0.186 | 0.4 ± 0.421 | 0.15 ± 0.267 | n.s. | n.s. | 0.013 | n.s. |
| 6,11-Dimethyl-2,6,10-dodecatrin-1-ol) | 0.18 ± 0.314 | 0.14 ± 0.179 | n.d. | n.d. | n.s. | n.s. | n.s. | 0.032 |
| Hexadecane | 0.29 ± 0.326 | 0.36 ± 0.361 | 0.03 ± 0.055 | 0.56 ± 0.447 | 0.018 | n.s. | n.s. | n.s. |
| Cetene | 0.18 ± 0.132 | 0.35 ± 0.312 | n.d. | 0.15 ± 0.125 | n.s. | n.s. | 0.024 | n.s. |
| Eicosane | 0.63 ± 1.241 | 0.61 ± 1.437 | 0.05 ± 0.051 | 2.35 ± 1.902 | n.s. | n.s. | n.s. | 0.039 |
| 2-Amino-4-methoxyphenol | 0.59 ± 0.223 | 0.5 ± 0.388 | 0.1 ± 0.108 | 0.53 ± 0.26 | n.s. | n.s. | 0.004 | n.s. |
| gamma-Dodecalactone | 0.17 ± 0.148 | 0.22 ± 0.241 | 0.09 ± 0.117 | 0.06 ± 0.044 | n.s. | n.s. | n.s. | 0.048 |
| 2-Tridecanone | 0.23 ± 0.45 | 0.23 ± 0.476 | 0.09 ± 0.104 | 0.84 ± 0.657 | n.s. | n.s. | n.s. | 0.036 |
| Ethanone, 1-(2-aminophenyl)- | 0.16 ± 0.318 | 0.08 ± 0.106 | n.d. | n.d. | n.s. | n.s. | n.s. | 0.043 |
| Butanoic acid, 3-methyl- | 3.63 ± 3.634 | 1.46 ± 1.984 | 0.75 ± 0.791 | 1.02 ± 1.618 | 0.043 | n.s. | n.s. | n.s. |
| Hexanoic acid | 0.7 ± 1.122 | 2.52 ± 2.944 | 3.84 ± 5.948 | 2.29 ± 0.62 | 0.037 | n.s. | n.s. | n.s. |
| Heptanoic acid | 0.27 ± 0.475 | 0.82 ± 1.124 | 1.99 ± 3.165 | n.d. | 0.046 | n.s. | n.s. | 0.039 |
| Phenol, 2,4-bis(1,1-dimethylethyl) | 0.1 ± 0.086 | 0.13 ± 0.081 | 0.09 ± 0.088 | 0.29 ± 0.213 | n.s. | n.s. | n.s. | 0.04 |
| alfa-Humulene | 0.05 ± 0.085 | 0.05 ± 0.065 | 0.07 ± 0.114 | n.d. | n.s. | n.s. | n.s. | 0.04 |
| Beta-Selinene | 0.13 ± 0.164 | 0.06 ± 0.073 | 0.05 ± 0.09 | n.d. | n.s. | n.s. | n.s. | 0.029 |
| Squalene | 0.1 ± 0.142 | 0.05 ± 0.093 | n.d. | n.d. | 0.043 | n.s. | n.s. | n.s. |
| Carvacrol | n.d. | 0.12 ± 0.163 | n.d. | 0.04 ± 0.115 | 0.048 | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portincasa, P.; Celano, G.; Serale, N.; Vitellio, P.; Calabrese, F.M.; Chira, A.; David, L.; Dumitrascu, D.L.; De Angelis, M. Clinical and Metabolomic Effects of Lactiplantibacillus plantarum and Pediococcus acidilactici in Fructose Intolerant Patients. Nutrients 2022, 14, 2488. https://doi.org/10.3390/nu14122488
Portincasa P, Celano G, Serale N, Vitellio P, Calabrese FM, Chira A, David L, Dumitrascu DL, De Angelis M. Clinical and Metabolomic Effects of Lactiplantibacillus plantarum and Pediococcus acidilactici in Fructose Intolerant Patients. Nutrients. 2022; 14(12):2488. https://doi.org/10.3390/nu14122488
Chicago/Turabian StylePortincasa, Piero, Giuseppe Celano, Nadia Serale, Paola Vitellio, Francesco Maria Calabrese, Alexandra Chira, Liliana David, Dan L. Dumitrascu, and Maria De Angelis. 2022. "Clinical and Metabolomic Effects of Lactiplantibacillus plantarum and Pediococcus acidilactici in Fructose Intolerant Patients" Nutrients 14, no. 12: 2488. https://doi.org/10.3390/nu14122488
APA StylePortincasa, P., Celano, G., Serale, N., Vitellio, P., Calabrese, F. M., Chira, A., David, L., Dumitrascu, D. L., & De Angelis, M. (2022). Clinical and Metabolomic Effects of Lactiplantibacillus plantarum and Pediococcus acidilactici in Fructose Intolerant Patients. Nutrients, 14(12), 2488. https://doi.org/10.3390/nu14122488