Serum Phytosterols Are Not Associated with Inflammatory Markers in Two Cross-Sectional, Swiss Population-Based Studies (The CoLaus|PsyCoLaus Study)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Sterol Assessment
2.3. Inflammatory Markers
2.4. Other Covariates
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
3.1. Sample Characteristics
3.2. Sterol Levels
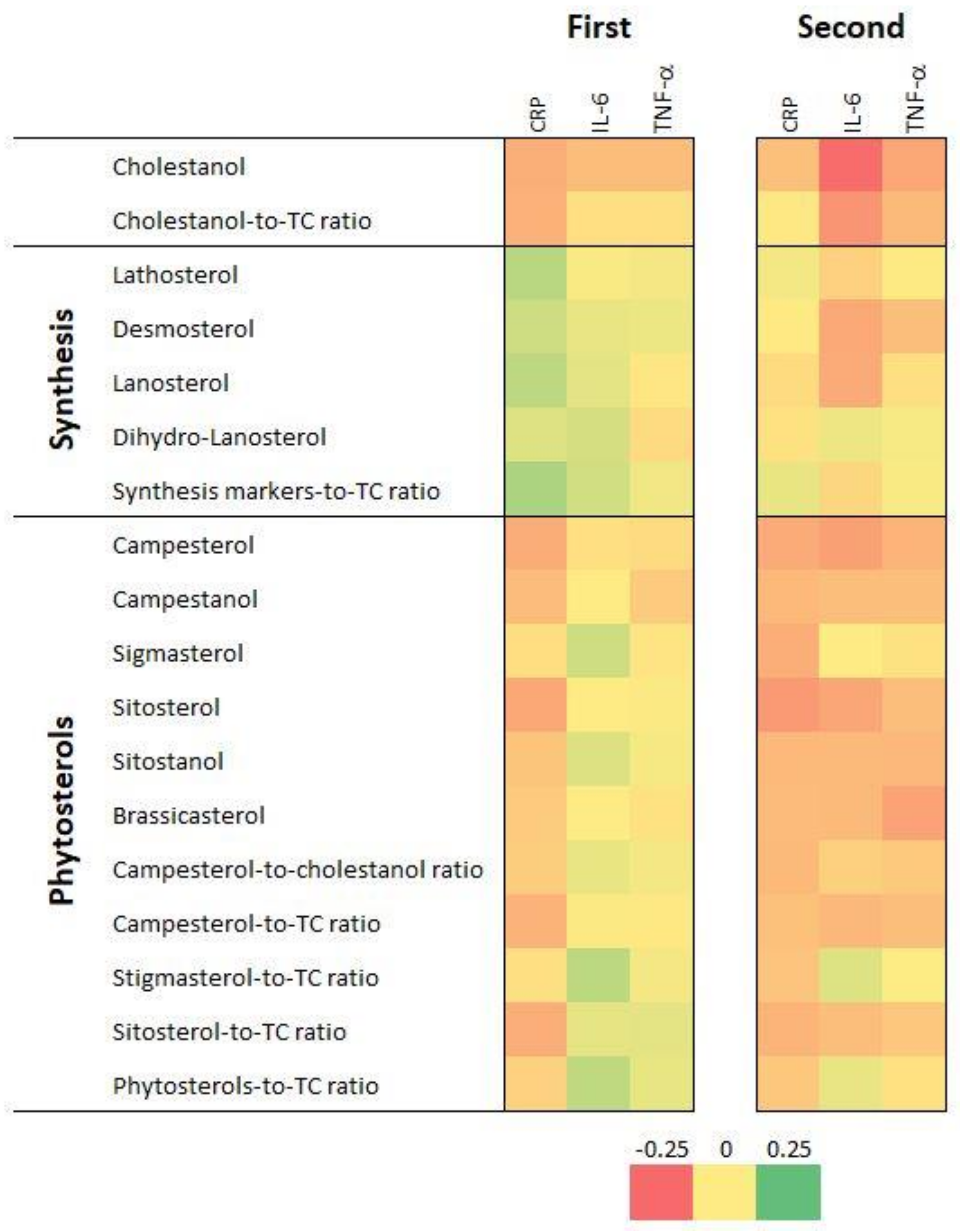
3.3. Association with Inflammatory Markers—Bivariate Analysis
3.4. Association with Inflammatory Markers—Multivariable Analysis
4. Discussion
4.1. Sterol Levels
4.2. Association between Sterol Levels and Inflammatory Markers
4.3. Implication for Public Health
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S. These Are the Top 10 Global Causes of Death—But Two Diseases Are in Decline. Available online: https://www.weforum.org/agenda/2021/02/top-10-global-causes-death/ (accessed on 7 January 2022).
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular Disease Prevention by Diet Modification: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef]
- Zampelas, A.; Magriplis, E. Dietary patterns and risk of cardiovascular diseases: A review of the evidence. Proc. Nutr. Soc. 2020, 79, 68–75. [Google Scholar] [CrossRef]
- Othman, R.A.; Moghadasian, M.H. Beyond cholesterol-lowering effects of plant sterols: Clinical and experimental evidence of anti-inflammatory properties. Nutr. Rev. 2011, 69, 371–382. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: A systematic review and meta-analysis of intervention trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingberg, S.; Andersson, H.; Mulligan, A.; Bhaniani, A.; Welch, A.; Bingham, S.; Khaw, K.T.; Andersson, S.; Ellegard, L. Food sources of plant sterols in the EPIC Norfolk population. Eur. J. Clin. Nutr. 2008, 62, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef]
- Dutta, P.C. Phytosterols as Functional Food Components and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2019; p. 450. [Google Scholar]
- Jimenez-Escrig, A.; Santos-Hidalgo, A.B.; Saura-Calixto, F. Common sources and estimated intake of plant sterols in the Spanish diet. J. Agric. Food Chem. 2006, 54, 3462–3471. [Google Scholar] [CrossRef] [Green Version]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padro, T. Phytosterols and Inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Xu, Z.; Le, K.; Moghadasian, M.H. Long-term phytosterol treatment alters gene expression in the liver of apo E-deficient mice. J. Nutr. Biochem. 2008, 19, 545–554. [Google Scholar] [CrossRef]
- Ahmad Khan, M.; Sarwar, A.; Rahat, R.; Ahmed, R.S.; Umar, S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2020, 85, 106642. [Google Scholar] [CrossRef]
- Kasirzadeh, S.; Ghahremani, M.H.; Setayesh, N.; Jeivad, F.; Shadboorestan, A.; Taheri, A.; Beh-Pajooh, A.; Azadkhah Shalmani, A.; Ebadollahi-Natanzi, A.; Khan, A.; et al. β-Sitosterol Alters the Inflammatory Response in CLP Rat Model of Sepsis by Modulation of NFkappaB Signaling. Biomed. Res. Int. 2021, 2021, 5535562. [Google Scholar] [CrossRef]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci. Rep. 2020, 40, BSR20192133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.J.; Chen, C.; Ding, L.; Shi, H.H.; Wang, C.C.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Sea cucumbers-derived sterol sulfate alleviates insulin resistance and inflammation in high-fat-high-fructose diet-induced obese mice. Pharmacol. Res. 2020, 160, 105191. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Hasegawa, K.; Kunimi, M.; Hara, M.; Yatomi, Y.; Teramoto, T.; Tsukamoto, K. Sitosterol prevents obesity-related chronic inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, M.A.; Mohammad Shahi, M.; Seyedian, S.S.; Haghighizadeh, M.H. Effects of Phytosterol Supplementation on Serum Levels of Lipid Profiles, Liver Enzymes, Inflammatory Markers, Adiponectin, and Leptin in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 651–658. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padro, T.; Sanchez-Hernandez, J.; Antonijoan, R.M.; Perez, A.; Badimon, L. Phytosterols and Omega 3 Supplementation Exert Novel Regulatory Effects on Metabolic and Inflammatory Pathways: A Proteomic Study. Nutrients 2017, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, V.Z.; Ras, R.T.; Gagliardi, A.C.; Mangili, L.C.; Trautwein, E.A.; Santos, R.D. Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 76–83. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Firmann, M.; Mayor, V.; Vidal, P.M.; Bochud, M.; Pecoud, A.; Hayoz, D.; Paccaud, F.; Preisig, M.; Song, K.S.; Yuan, X.; et al. The CoLaus study: A population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008, 8, 6. [Google Scholar] [CrossRef]
- Teunissen, C.E.; De Vente, J.; von Bergmann, K.; Bosma, H.; van Boxtel, M.P.; De Bruijn, C.; Jolles, J.; Steinbusch, H.W.; Lutjohann, D. Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. Neurobiol. Aging 2003, 24, 147–155. [Google Scholar] [CrossRef]
- Lin, X.; Racette, S.B.; Ma, L.; Wallendorf, M.; Spearie, C.A.; Ostlund, R.E., Jr. Plasma biomarker of dietary phytosterol intake. PLoS ONE 2015, 10, e0116912. [Google Scholar] [CrossRef]
- Jaceldo-Siegl, K.; Lutjohann, D.; Sirirat, R.; Mashchak, A.; Fraser, G.E.; Haddad, E. Variations in dietary intake and plasma concentrations of plant sterols across plant-based diets among North American adults. Mol. Nutr. Food Res. 2017, 61, 1600828. [Google Scholar] [CrossRef] [PubMed]
- Flower, L.; Ahuja, R.H.; Humphries, S.E.; Mohamed-Ali, V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine 2000, 12, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 2000, 243, 243–255. [Google Scholar] [CrossRef]
- Dupont, N.C.; Wang, K.; Wadhwa, P.D.; Culhane, J.F.; Nelson, E.L. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J. Reprod. Immunol. 2005, 66, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Weingartner, O.; Bogeski, I.; Kummerow, C.; Schirmer, S.H.; Husche, C.; Vanmierlo, T.; Wagenpfeil, G.; Hoth, M.; Bohm, M.; Lutjohann, D.; et al. Plant sterol ester diet supplementation increases serum plant sterols and markers of cholesterol synthesis, but has no effect on total cholesterol levels. J. Steroid Biochem. Mol. Biol. 2017, 169, 219–225. [Google Scholar] [CrossRef]
- Horenstein, R.B.; Mitchell, B.D.; Post, W.S.; Lutjohann, D.; von Bergmann, K.; Ryan, K.A.; Terrin, M.; Shuldiner, A.R.; Steinle, N.I. The ABCG8 G574R variant, serum plant sterol levels, and cardiovascular disease risk in the Old Order Amish. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 413–419. [Google Scholar] [CrossRef] [Green Version]
- de Abreu, D.; Guessous, I.; Vaucher, J.; Preisig, M.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Low compliance with dietary recommendations for food intake among adults. Clin. Nutr. 2013, 32, 783–788. [Google Scholar] [CrossRef]
- Scholz, M.; Horn, K.; Pott, J.; Gross, A.; Kleber, M.E.; Delgado, G.E.; Mishra, P.P.; Kirsten, H.; Gieger, C.; Muller-Nurasyid, M.; et al. Genome-wide meta-analysis of phytosterols reveals five novel loci and a detrimental effect on coronary atherosclerosis. Nat. Commun. 2022, 13, 143. [Google Scholar] [CrossRef]
- Silbernagel, G.; Chapman, M.J.; Genser, B.; Kleber, M.E.; Fauler, G.; Scharnagl, H.; Grammer, T.B.; Boehm, B.O.; Makela, K.M.; Kahonen, M.; et al. High intestinal cholesterol absorption is associated with cardiovascular disease and risk alleles in ABCG8 and ABO: Evidence from the LURIC and YFS cohorts and from a meta-analysis. J. Am. Coll. Cardiol. 2013, 62, 291–299. [Google Scholar] [CrossRef]
- Teupser, D.; Baber, R.; Ceglarek, U.; Scholz, M.; Illig, T.; Gieger, C.; Holdt, L.M.; Leichtle, A.; Greiser, K.H.; Huster, D.; et al. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ. Cardiovasc. Genet. 2010, 3, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Sirirat, R.; Heskey, C.; Haddad, E.; Tantamango-Bartley, Y.; Fraser, G.; Mashchak, A.; Jaceldo-Siegl, K. Comparison of phytosterol intake from FFQ with repeated 24-h dietary recalls of the Adventist Health Study-2 calibration sub-study. Br. J. Nutr. 2019, 121, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Silvestre, R.A.; Hernandez-Alvarez, E.; Granado-Lorencio, F.; Barbera, R.; Garcia-Llatas, G. A positive impact on the serum lipid profile and cytokines after the consumption of a plant sterol-enriched beverage with a milk fat globule membrane: A clinical study. Food Funct. 2018, 9, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Heggen, E.; Kirkhus, B.; Pedersen, J.I.; Tonstad, S. Effects of margarine enriched with plant sterol esters from rapeseed and tall oils on markers of endothelial function, inflammation and hemostasis. Scand. J. Clin. Lab. Investig. 2015, 75, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Chatelan, A.; Beer-Borst, S.; Randriamiharisoa, A.; Pasquier, J.; Blanco, J.M.; Siegenthaler, S.; Paccaud, F.; Slimani, N.; Nicolas, G.; Camenzind-Frey, E.; et al. Major Differences in Diet across Three Linguistic Regions of Switzerland: Results from the First National Nutrition Survey menuCH. Nutrients 2017, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
| First (2009–2012) | Second (2014–2017) | |
|---|---|---|
| Sample size | 730 | 526 |
| Women (%) | 418 (57.3) | 306 (58.2) |
| Age (years) | 70.1 ± 4.7 | 75.0 ± 4.4 |
| BMI (kg/m2) | 26.5 ± 4.6 | 26.2 ± 4.4 |
| BMI categories (%) | ||
| Normal | 295 (40.4) | 225 (42.8) |
| Overweight | 297 (40.7) | 216 (41.1) |
| Obese | 138 (18.9) | 85 (16.2) |
| Smoking status (%) | ||
| Never | 293 (40.2) | 215 (42.2) |
| Former | 328 (45.0) | 234 (45.9) |
| Current | 108 (14.8) | 61 (12.0) |
| Diabetes (%) | 120 (16.5) | 75 (14.3) |
| Treated with metformin (%) | 46 (6.3) | 50 (9.5) |
| Hypolipidemic drugs (%) | ||
| Statins | 159 (21.8) | 128 (24.3) |
| Ezetimibe | 6 (0.8) | 6 (1.1) |
| Inflammatory markers | ||
| CRP (mg/L) | 1.7 [0.8–3.2] | 1.7 [0.8–3.2] |
| IL-6 (ng/L) | 2.75 [0.97–8.97] | 2.75 [0.97–8.97] |
| TNF-α (ng/L) | 4.84 [2.75–8.60] | 4.84 [2.75–8.60] |
| First (2009–2012) | Second (2014–2017) | |||
|---|---|---|---|---|
| Average ± SD | Median [IQR] | Average ± SD | Median [IQR] | |
| Cholesterol absorption | ||||
| Cholestanol [mg/dL] | 0.31 ± 0.10 | 0.30 [0.24–0.36] | 0.41 ± 0.12 | 0.40 [0.33–0.48] |
| Cholesterol synthesis | ||||
| Lathosterol [mg/dL] | 0.23 ± 0.12 | 0.21 [0.14–0.29] | 0.20 ± 0.20 | 0.18 [0.11–0.25] |
| Desmosterol [mg/dL] | 0.15 ± 0.15 | 0.13 [0.10–0.17] | 0.16 ± 0.12 | 0.14 [0.10–0.19] |
| Lanosterol [µg/dL] | 23.9 ± 14.7 | 21.1 [13.8–29.7] | 18.8 ± 6.6 | 17.9 [14.1–22.5] |
| Dihydro-lanosterol [µg/dL] | 3.95 ± 3.26 | 2.61 [1.91–4.35] | 0.39 ± 0.30 | 0.34 [0.21–0.49] |
| Vegetal origin | ||||
| Campesterol [mg/dL] | 0.33 ± 0.20 | 0.29 [0.19–0.42] | 0.29 ± 0.16 | 0.26 [0.18–0.37] |
| Sitosterol [mg/dL] | 0.25 ± 0.13 | 0.23 [0.16–0.31] | 0.25 ± 0.12 | 0.23 [0.17–0.31] |
| Brassicasterol [µg/dL] | 19.6 ± 10.9 | 17.5 [11.9–24.3] | 20.7 ± 10.4 | 18.7 [13.9–24.8] |
| Sitostanol [µg/dL] | 7.32 ± 4.05 | 6.45 [4.62–8.96] | 4.18 ± 2.10 | 3.84 [3.31–4.52] |
| Campestanol [µg/dL] | 6.08 ± 3.92 | 5.22 [3.39–7.75] | 4.01 ± 1.50 | 3.75 [3.12–4.63] |
| Stigmasterol [µg/dL] | 6.43 ± 3.97 | 5.41 [3.40–8.43] | 7.96 ± 3.48 | 7.14 [5.65–9.31] |
| Ratios | ||||
| Cholestanol-to-TC ratio | 1.38 ± 0.35 | 1.34 [1.13–1.58] | 2.05 ± 0.52 | 2.00 [1.72–2.32] |
| Synthesis markers-to-TC ratio | 106.5 ± 62.7 | 93.6 [63.8–128.5] | 94.0 ± 30.2 | 88.0 [74.7–106.4] |
| Campesterol-to-cholestanol ratio | 1.04 ± 0.53 | 0.96 [0.68–1.28] | 0.72 ± 0.36 | 0.65 [0.47–0.87] |
| Campesterol-to-TC ratio (100×) | 1.45 ± 0.86 | 1.30 [0.86–1.85] | 1.47 ± 0.85 | 1.30 [0.90–1.80] |
| Stigmasterol-to-TC ratio | 28.8 ± 17.6 | 24.6 [15.4–37.6] | 40.1 ± 19.0 | 35.1 [27.4–47.1] |
| Sitosterol-to-TC ratio | 1.14 ± 0.56 | 1.02 [0.75–1.38] | 1.27 ± 0.67 | 1.12 [0.85–1.54] |
| Phytosterols-to-TC ratio | 64.6 ± 33.3 | 56.4 [39.2–83.3] | 64.2 ± 27.3 | 58.4 [46.8–73.4] |
| CRP | IL-6 | TNF-α | ||||
|---|---|---|---|---|---|---|
| FU1 | FU2 | FU1 | FU2 | FU1 | FU2 | |
| Cholesterol absorption | ||||||
| Cholestanol [mg/dL] | −0.554 (−1.584; 0.475) | −0.629 (−1.520; 0.262) | −0.609 (−3.281; 2.063) | −0.554 (−0.809; −0.300) | −3.375 (−6.516; −0.234) | −1.284 (−2.163; −0.405) |
| Cholesterol synthesis | ||||||
| Lathosterol [mg/dL] | 1.363 (0.489; 2.236) | −0.260 (−1.296; 0.777) | 0.088 (−2.172; 2.348) | 0.042 (−0.113; 0.196) | 1.486 (−1.197; 4.168) | −0.014 (−0.541; 0.512) |
| Desmosterol [mg/dL] | −0.258 (−0.940; 0.424) | −0.189 (−0.999; 0.620) | −0.966 (−2.745; 0.812) | −0.230 (−0.471; 0.011) | 0.339 (−1.741; 2.420) | −0.742 (−1.567; 0.083) |
| Lanosterol [µg/dL] | 0.009 (0.002; 0.016) | 0.009 (−0.009; 0.027) | 0.005 (−0.013; 0.023) | −0.004 (−0.010; 0.001) | 0.003 (−0.018; 0.024) | 0.005 (−0.014; 0.023) |
| Dihydro-lanosterol [µg/dl] | 0.015 (−0.014; 0.045) | 0.410 (0.046; 0.774) | 0.111 (0.035; 0.187) | 0.062 (−0.045; 0.168) | −0.031 (−0.121; 0.058) | 0.186 (−0.179; 0.552) |
| Vegetal origin | ||||||
| Campesterol [mg/dL] | 0.155 (−0.351; 0.660) | −0.509 (−1.178; 0.160) | 0.215 (−1.083; 1.513) | −0.140 (−0.337; 0.058) | −0.888 (−2.415; 0.639) | −0.907 (−1.573; −0.241) |
| Sitosterol [mg/dL] | −0.121 (−0.901; 0.658) | −0.690 (−1.552; 0.172) | 1.372 (−0.640; 3.385) | −0.182 (−0.438; 0.073) | −0.162 (−2.534; 2.210) | −0.714 (−1.585; 0.156) |
| Brassicasterol [µg/dL] | 0.007 (−0.002; 0.016) | −0.008 (−0.018; 0.002) | 0.013 (−0.009; 0.036) | −0.002 (−0.005; 0.001) | −0.004 (−0.031; 0.023) | −0.019 (−0.029; −0.010) |
| Sitostanol [µg/dL] | −0.010 (−0.034; 0.014) | −0.007 (−0.055; 0.041) | 0.037 (−0.024; 0.099) | −0.012 (−0.026; 0.003) | 0.041 (−0.032; 0.114) | −0.043 (−0.091; 0.006) |
| Campestanol [µg/dL] | −0.013 (−0.038; 0.012) | −0.054 (−0.122; 0.014) | −0.001 (−0.064; 0.062) | −0.012 (−0.032; 0.009) | −0.036 (−0.111; 0.039) | −0.066 (−0.135; 0.002) |
| Stigmasterol [µg/dL] | 0.009 (−0.016; 0.034) | −0.010 (−0.040; 0.020) | 0.104 (0.040; 0.168) | 0.004 (−0.004; 0.013) | −0.039 (−0.113; 0.036) | 0.011 (−0.019; 0.042) |
| Ratios | ||||||
| Cholestanol-to-TC ratio | −0.135 (−0.415; 0.144) | −0.042 (−0.235; 0.151) | 0.263 (−0.459; 0.985) | −0.128 (−0.183; −0.073) | −0.580 (−1.437; 0.276) | −0.389 (−0.581; −0.198) |
| Synthesis markers-to-TC ratio | 0.002 (0.001; 0.004) | 0.004 (0.001; 0.008) | 0.003 (−0.001; 0.007) | −0.001 (−0.002; 0.000) | 0.003 (−0.002; 0.007) | 0.000 (−0.004; 0.004) |
| Campesterol-to-cholestanol ratio | 0.048 (−0.135; 0.231) | −0.119 (−0.408; 0.171) | 0.132 (−0.338; 0.602) | 0.007 (−0.079; 0.093) | 0.019 (−0.538; 0.576) | −0.278 (−0.570; 0.015) |
| Campesterol-to-TC ratio (100×) | 0.046 (−0.070; 0.163) | −0.078 (−0.206; 0.049) | 0.154 (−0.145; 0.453) | −0.029 (−0.066; 0.009) | −0.126 (−0.479; 0.227) | −0.193 (−0.320; −0.066) |
| Stigmasterol-to-TC ratio | 0.003 (−0.003; 0.008) | 0.000 (−0.006; 0.006) | 0.032 (0.017; 0.047) | 0.001 (−0.001; 0.002) | −0.003 (−0.020; 0.014) | 0.001 (−0.005; 0.007) |
| Sitosterol-to-TC ratio | −0.024 (−0.205; 0.157) | −0.105 (−0.268; 0.058) | 0.510 (0.049; 0.972) | −0.038 (−0.087; 0.010) | 0.183 (−0.367; 0.733) | −0.168 (−0.332; −0.003) |
| Phytosterols-to-TC ratio | 0.000 (−0.003; 0.003) | 0.001 (−0.003; 0.005) | 0.013 (0.005; 0.021) | 0.000 (−0.001; 0.001) | 0.004 (−0.005; 0.013) | −0.002 (−0.006; 0.002) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanasila, L.; Marques-Vidal, P. Serum Phytosterols Are Not Associated with Inflammatory Markers in Two Cross-Sectional, Swiss Population-Based Studies (The CoLaus|PsyCoLaus Study). Nutrients 2022, 14, 2500. https://doi.org/10.3390/nu14122500
Stanasila L, Marques-Vidal P. Serum Phytosterols Are Not Associated with Inflammatory Markers in Two Cross-Sectional, Swiss Population-Based Studies (The CoLaus|PsyCoLaus Study). Nutrients. 2022; 14(12):2500. https://doi.org/10.3390/nu14122500
Chicago/Turabian StyleStanasila, Laura, and Pedro Marques-Vidal. 2022. "Serum Phytosterols Are Not Associated with Inflammatory Markers in Two Cross-Sectional, Swiss Population-Based Studies (The CoLaus|PsyCoLaus Study)" Nutrients 14, no. 12: 2500. https://doi.org/10.3390/nu14122500
APA StyleStanasila, L., & Marques-Vidal, P. (2022). Serum Phytosterols Are Not Associated with Inflammatory Markers in Two Cross-Sectional, Swiss Population-Based Studies (The CoLaus|PsyCoLaus Study). Nutrients, 14(12), 2500. https://doi.org/10.3390/nu14122500







