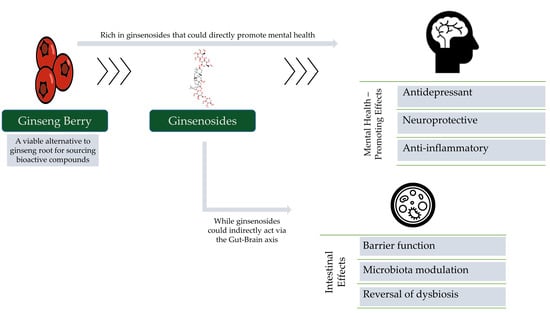
The Untapped Potential of Ginsenosides and American Ginseng Berry in Promoting Mental Health via the Gut–Brain Axis
Abstract
1. Introduction
2. The Berry Is a Highly Concentrated Source of Ginseng’s Therapeutic Compounds
3. Pharmacological Effects in The Context of Mental Health
3.1. Ginsenoside Rb3
3.2. Ginsenoside Re
3.3. Ginsenoside Rb2
3.4. Ginsenoside Rd
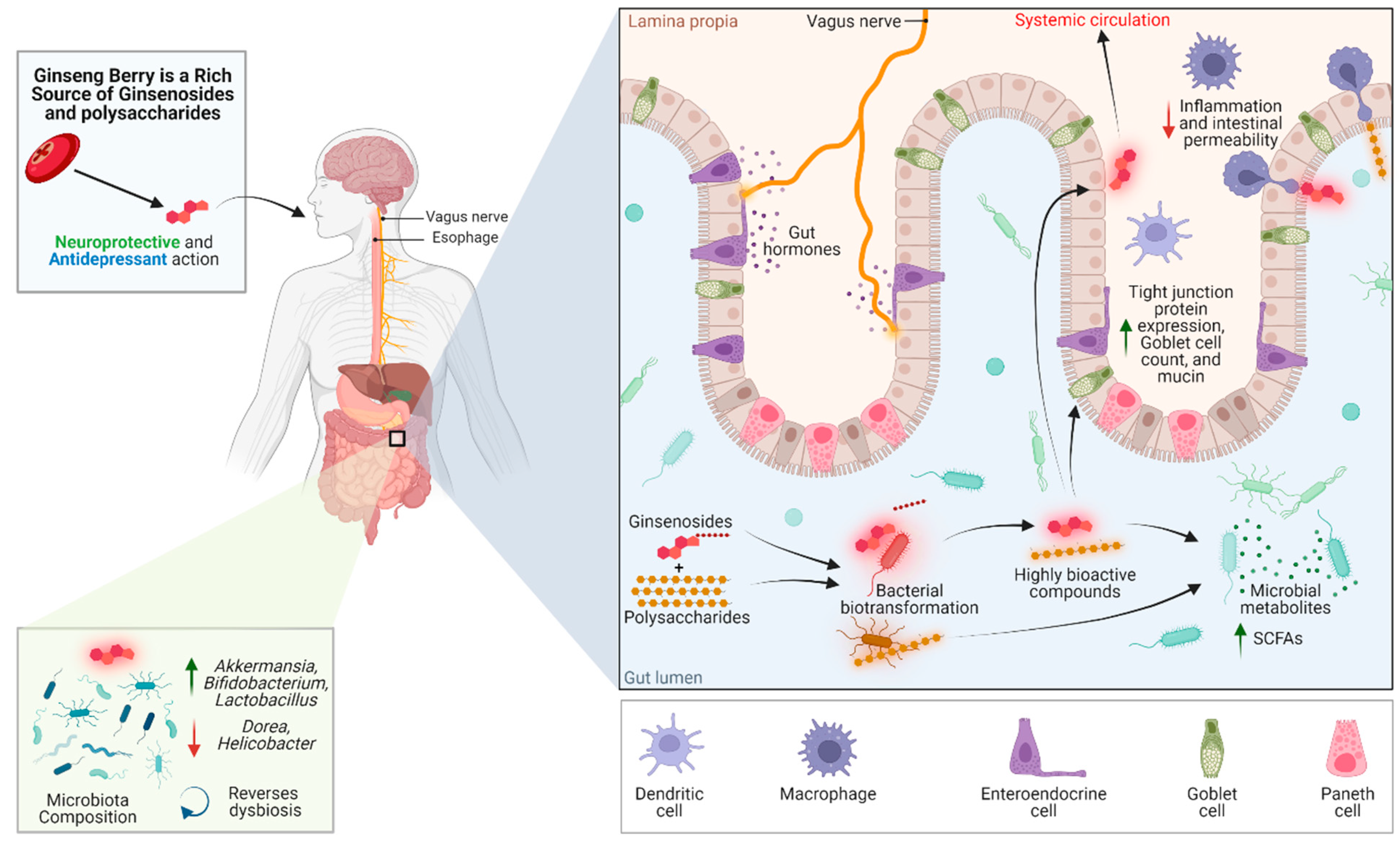
4. The Ginseng Berry and The Gut–Brain Axis
4.1. Intestinal Permeability
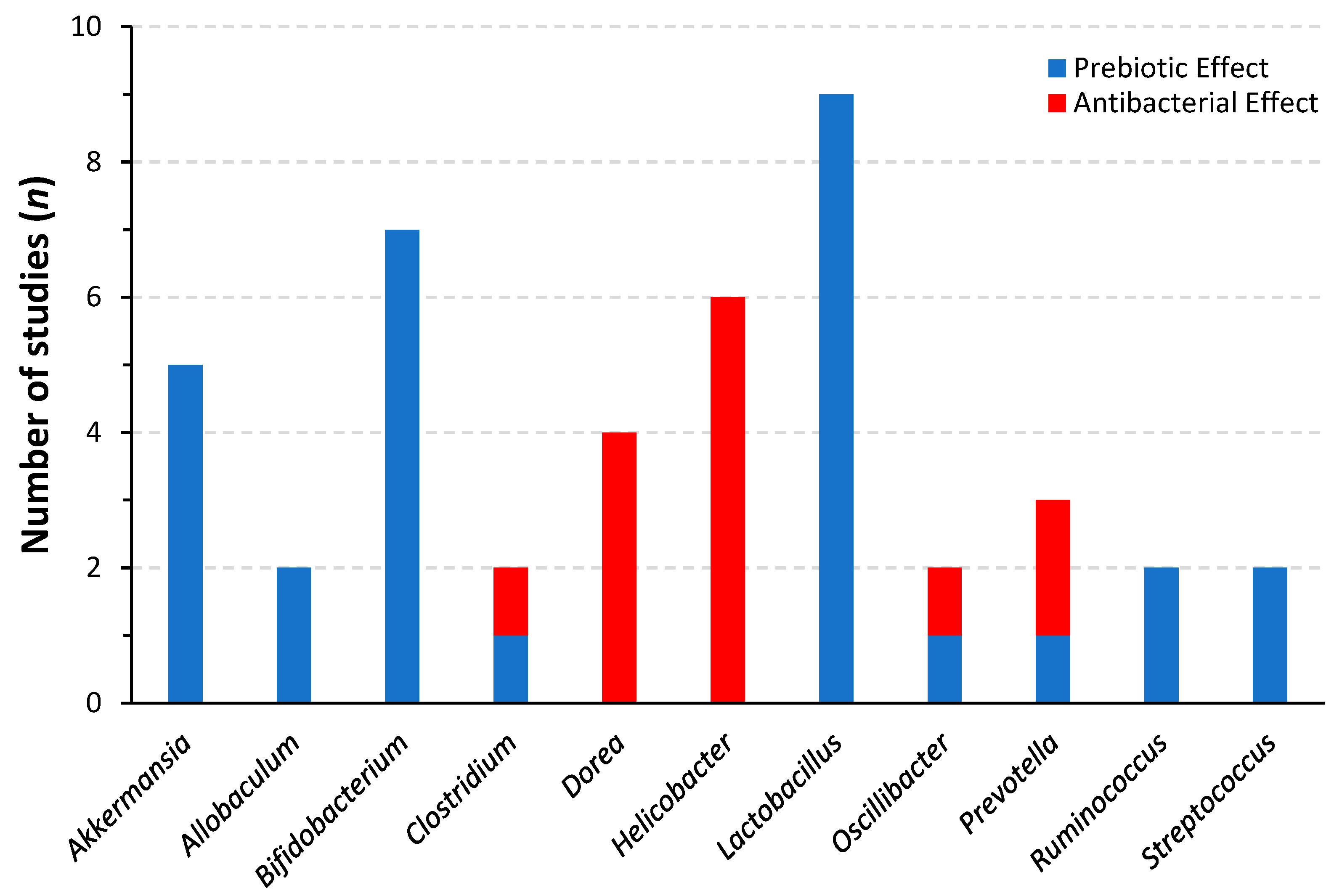
4.2. Prebiotic Effects and Modulation of The Intestinal Microbiota
4.3. Improved Health Functionality through Bioconversion
5. Safety
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, K.L.; Jacobs, P.; Ohinmaa, A.; Schopflocher, D.; Dewa, C.S. A New Population-Based Measure of the Economic Burden of Mental Illness in Canada. Chronic Dis. Can. 2008, 28, 92–98. [Google Scholar] [CrossRef]
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the True Global Burden of Mental Illness. Lancet Psychiatry 2016, 3, 171–178. [Google Scholar] [CrossRef]
- Public Health Agency of Canada Report from the Canadian Chronic Disease Surveillance System: Mood and Anxiety Disorders in Canada. 2016. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/report-canadian-chronic-disease-surveillance-system-mood-anxiety-disorders-canada-2016.html (accessed on 29 September 2021).
- Lam, R.W.; McIntosh, D.; Wang, J.; Enns, M.W.; Kolivakis, T.; Michalak, E.E.; Sareen, J.; Song, W.-Y.; Kennedy, S.H.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can. J. Psychiatry 2016, 61, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Almohammed, O.A.; Alsalem, A.A.; Almangour, A.A.; Alotaibi, L.H.; Al Yami, M.S.; Lai, L. Antidepressants and Health-Related Quality of Life (HRQoL) for Patients with Depression: Analysis of the Medical Expenditure Panel Survey from the United States. PLoS ONE 2022, 17, e0265928. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the Antimicrobial Action of Antidepressants on Gut Commensal Microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, S.-H. The Effect of Ginsenosides on Depression in Preclinical Studies: A Systematic Review and Meta-Analysis. J. Ginseng Res. 2021, 45, 420–432. [Google Scholar] [CrossRef]
- Braz, A.S.; Morais, L.C.S.; Paula, A.P.; Diniz, M.F.F.M.; Almeida, R.N. Effects of Panax Ginseng Extract in Patients with Fibromyalgia: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Rev. Bras. Psiquiatr. 2013, 35, 21–28. [Google Scholar] [CrossRef]
- Jeong, H.-G.; Ko, Y.-H.; Oh, S.-Y.; Han, C.; Kim, T.; Joe, S.-H. Effect of Korean Red Ginseng as an Adjuvant Treatment for Women with Residual Symptoms of Major Depression. Asia-Pac. Psychiatry 2015, 7, 330–336. [Google Scholar] [CrossRef]
- Lee, K.J.; Ji, G.E. The Effect of Fermented Red Ginseng on Depression Is Mediated by Lipids. Nutr. Neurosci. 2014, 17, 7–15. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Wu, J.A.; McEntee, E.; Yuan, C.-S. Saponins Composition in American Ginseng Leaf and Berry Assayed by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, J.; Zhang, L.; Cheng, R.; Wei, G.; Su, H.; Yang, J.; Qian, J.; Xu, R.; Chen, S. Rhizospheric Microbial Communities Are Driven by Panax Ginseng at Different Growth Stages and Biocontrol Bacteria Alleviates Replanting Mortality. Acta Pharm. Sin. B 2018, 8, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Raza, S.H.; Maryam, A.; Setzer, W.N.; Braidy, N.; Nabavi, S.F.; de Oliveira, M.R.; Nabavi, S.M. Ginsenoside Rb1 as a Neuroprotective Agent: A Review. Brain Res. Bull. 2016, 125, 30–43. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an Anti-Diabetic Agent and Its Underlying Mechanism Analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; Sun, Y.; Dai, Z.; Li, G.; Sun, G.; Sun, X. Ginsenoside Rb1 and Mitochondria: A Short Review of the Literature. Mol. Cell. Probes 2019, 43, 1–5. [Google Scholar] [CrossRef]
- Kochan, E.; Kołodziej, B.; Gadomska, G.; Chmiel, A. Ginsenoside Contents in Panax Quinquefolium Organs from Field Cultivation. Z. Für Nat. C 2008, 63, 91–95. [Google Scholar] [CrossRef]
- Sritularak, B.; Morinaga, O.; Yuan, C.-S.; Shoyama, Y.; Tanaka, H. Quantitative Analysis of Ginsenosides Rb1, Rg1, and Re in American Ginseng Berry and Flower Samples by ELISA Using Monoclonal Antibodies. J. Nat. Med. 2009, 63, 360–363. [Google Scholar] [CrossRef]
- Son, T.; Eguchi, T.; Shoyama, Y.; Tanaka, H. ELISA for the Detection of Marker Compound for Crop Fertilizer Use of Various Medicinal Crop Extracts Using Bacterium. J.-Fac. Agric. Kyushu Univ. 2019, 64, 27–32. [Google Scholar] [CrossRef]
- Miao, L.; Yang, Y.; Li, Z.; Fang, Z.; Zhang, Y.; Han, C. Ginsenoside Rb2: A Review of Pharmacokinetics and Pharmacological Effects. J. Ginseng Res. 2022, 46, 206–213. [Google Scholar] [CrossRef]
- Jin, Y.; Hao, Y.; Zhang, H.; Qu, Z.; Wang, Y.; Piao, X. Dynamic Changes of Ginsenosides in Panax Quinquefolium Fruit at Different Development Stages Measured Using UHPLC-Orbitrap MS. Rapid Commun. Mass Spectrom. 2022, 36, e9270. [Google Scholar] [CrossRef]
- Li, W.; Duan, Y.; Yan, X.; Liu, X.; Fan, M.; Wang, Z. A Mini-Review on Pharmacological Effects of Ginsenoside Rb3, a Marked Saponin from Panax genus. Biocell 2022, 46, 1417–1423. [Google Scholar] [CrossRef]
- Bae, E.-A.; Choo, M.-K.; Park, E.-K.; Park, S.-Y.; Shin, H.-Y.; Kim, D.-H. Metabolism of Ginsenoside Rc by Human Intestinal Bacteria and Its Related Antiallergic Activity. Biol. Pharm. Bull. 2002, 25, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, C.H.; Park, D.; Choi, Y.J.; Park, M.H.; Chung, K.W.; Kim, S.R.; Lee, J.S.; Chung, H.Y. Ginsenoside Rc Modulates Akt/FoxO1 Pathways and Suppresses Oxidative Stress. Arch. Pharm. Res. 2014, 37, 813–820. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Y.; Kwak, Y.-S.; Song, G.G.; Kim, M.-Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax Ginseng Exerts Anti-Inflammatory Activity by Targeting TANK-Binding Kinase 1/Interferon Regulatory Factor-3 and P38/ATF-2. J. Ginseng Res. 2017, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Su, H.; Qi, B.; Wang, Y.; Yan, K.; Wang, X.; Li, X.; Zhao, D. A SIRT1 Activator, Ginsenoside Rc, Promotes Energy Metabolism in Cardiomyocytes and Neurons. J. Am. Chem. Soc. 2021, 143, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Liu, Q.-P.; An, P.; Jia, M.; Luan, X.; Tang, J.-Y.; Zhang, H. Ginsenoside Rd: A Promising Natural Neuroprotective Agent. Phytomedicine 2022, 95, 153883. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.-L.; Zhang, B.; Fan, X.-X.; Tang, Q.; Yu, X.; Li, L.-N.; Fan, A.-R.; Chang, H.-S.; Zhang, L.-Z. Antidepressant-Like Effect and Mechanism of Ginsenoside Rd on Rodent Models of Depression. Drug Des. Devel. Ther. 2022, 16, 843–861. [Google Scholar] [CrossRef]
- Peng, L.; Sun, S.; Xie, L.-H.; Wicks, S.M.; Xie, J.-T. Ginsenoside Re: Pharmacological Effects on Cardiovascular System. Cardiovasc. Ther. 2012, 30, e183–e188. [Google Scholar] [CrossRef]
- Madhi, I.; Kim, J.-H.; Shin, J.E.; Kim, Y. Ginsenoside Re Exhibits Neuroprotective Effects by Inhibiting Neuroinflammation via CAMK/MAPK/NF-κB Signaling in Microglia. Mol. Med. Rep. 2021, 24, 698. [Google Scholar] [CrossRef]
- Wang, H.; Lv, J.; Jiang, N.; Huang, H.; Wang, Q.; Liu, X. Ginsenoside Re Protects against Chronic Restraint Stress-Induced Cognitive Deficits through Regulation of NLRP3 and Nrf2 Pathways in Mice. Phytother. Res. 2021, 35, 2523–2535. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effect of Ginsenoside Re on Depression- and Anxiety-like Behaviors and Cognition Memory Deficit Induced by Repeated Immobilization in Rats. J. Microbiol. Biotechnol. 2012, 22, 708–720. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yu, C.; Liu, T.; Jia, H. Ginsenoside Rg1 as an Effective Regulator of Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yao, G. Ginsenoside Rg1 as a Potential Regulator of Hematopoietic Stem/Progenitor Cells. Stem Cells Int. 2021, 2021, 4633270. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Wang, J.; Li, X.; Li, J.; Chu, S.; Li, L.; Chen, N.; Zhang, L. Ginsenoside Rg1 Prevent and Treat Inflammatory Diseases: A Review. Int. Immunopharmacol. 2020, 87, 106805. [Google Scholar] [CrossRef]
- Jiang, B.; Xiong, Z.; Yang, J.; Wang, W.; Wang, Y.; Hu, Z.-L.; Wang, F.; Chen, J.-G. Antidepressant-like Effects of Ginsenoside Rg1 Are Due to Activation of the BDNF Signalling Pathway and Neurogenesis in the Hippocampus. Br. J. Pharmacol. 2012, 166, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, N.; Wang, Z. Ginsenoside Rg2 Attenuates Myocardial Fibrosis and Improves Cardiac Function after Myocardial Infarction via AKT Signaling Pathway. Biosci. Biotechnol. Biochem. 2020, 84, 2199–2206. [Google Scholar] [CrossRef]
- Liu, G.; Qi, X.; Li, X.; Sun, F. Ginsenoside Rg2 Protects Cardiomyocytes against Trastuzumab-Induced Toxicity by Inducing Autophagy. Exp. Ther. Med. 2021, 21, 473. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, W.; Yu, X.; Xu, H.; Sui, D.; Wang, Y. Ginsenoside Rg2 Alleviates Myocardial Fibrosis by Regulating TGF-Β1/Smad Signalling Pathway. Pharm. Biol. 2021, 59, 106–113. [Google Scholar] [CrossRef]
- Gou, D.; Pei, X.; Wang, J.; Wang, Y.; Hu, C.; Song, C.; Cui, S.; Zhou, Y. Antiarrhythmic Effects of Ginsenoside Rg2 on Calcium Chloride-Induced Arrhythmias without Oral Toxicity. J. Ginseng Res. 2020, 44, 717–724. [Google Scholar] [CrossRef]
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective Effects of Ginsenoside Rg2 against Memory Impairment and Neuronal Death Induced by Aβ25-35 in Rats. J. Ethnopharmacol. 2021, 266, 113466. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Zhang, B.; Song, W.-X.; Wang, A.; Ni, M.; Luo, X.; Aung, H.H.; Xie, J.-T.; Tong, R.; He, T.-C.; et al. Steamed American Ginseng Berry: Ginsenoside Analyses and Anticancer Activities. J. Agric. Food Chem. 2006, 54, 9936–9942. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wu, W.W.; Yi, P. The Efficacy of Ginsenoside Rg3 Combined with First-Line Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2020, 11, 630825. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Anti-Angiogenic Properties of Ginsenoside Rg3. Molecules 2020, 25, 4905. [Google Scholar] [CrossRef]
- Kim, J.; Shim, J.; Lee, S.; Cho, W.-H.; Hong, E.; Lee, J.H.; Han, J.-S.; Lee, H.J.; Lee, K.W. Rg3-Enriched Ginseng Extract Ameliorates Scopolamine-Induced Learning Deficits in Mice. BMC Complement. Altern. Med. 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.E.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Med. 2018, 84, 139–152. [Google Scholar] [CrossRef]
- Zhang, H.; Park, S.; Huang, H.; Kim, E.; Yi, J.; Choi, S.-K.; Ryoo, Z.; Kim, M. Anticancer Effects and Potential Mechanisms of Ginsenoside Rh2 in Various Cancer Types (Review). Oncol. Rep. 2021, 45, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Park, C.W.; Lee, S.J.; Park, H.-R.; Kim, S.H.; Son, S.-U.; Park, J.; Shin, K.-S. Anti-Cancer Effects of Panax Ginseng Berry Polysaccharides via Activation of Immune-Related Cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Hou, L.; Wan, J.-Y.; Yao, H.; Yuan, J.; Zeng, J.; Park, C.W.; Kim, S.H.; Seo, D.B.; Shin, K.-S.; et al. Ginseng Berry Polysaccharides on Inflammation-Associated Colon Cancer: Inhibiting T-Cell Differentiation, Promoting Apoptosis, and Enhancing the Effects of 5-Fluorouracil. J. Ginseng Res. 2020, 44, 282–290. [Google Scholar] [CrossRef]
- Xie, J.-T.; Wang, C.-Z.; Zhang, B.; Mehendale, S.R.; Li, X.-L.; Sun, S.; Han, A.H.; Du, W.; He, T.-C.; Yuan, C.-S. In Vitro and in Vivo Anticancer Effects of American Ginseng Berry: Exploring Representative Compounds. Biol. Pharm. Bull. 2009, 32, 1552–1558. [Google Scholar] [CrossRef][Green Version]
- Xie, J.T.; Wang, C.Z.; Ni, M.; Wu, J.A.; Mehendale, S.R.; Aung, H.H.; Foo, A.; Yuan, C.S. American Ginseng Berry Juice Intake Reduces Blood Glucose and Body Weight in Ob/Ob Mice. J. Food Sci. 2007, 72, S590–S594. [Google Scholar] [CrossRef]
- Ko, S.K.; Bae, H.M.; Cho, O.S.; Im, B.O.; Chung, S.H.; Lee, B.Y. Analysis of Ginsenoside Composition of Ginseng Berry and Seed. Food Sci. Biotechnol. 2008, 17, 1379–1382. [Google Scholar]
- Hou, W.; Wang, Y.; Zheng, P.; Cui, R. Effects of Ginseng on Neurological Disorders. Front. Cell. Neurosci. 2020, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax Ginseng Action as an Antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Zhou, Z.; Yang, H.; Zhong, Z.; Lou, C. Antidepressant-like Effects of Ginsenosides: A Comparison of Ginsenoside Rb3 and Its Four Deglycosylated Derivatives, Rg3, Rh2, Compound K, and 20(S)-Protopanaxadiol in Mice Models of Despair. Pharmacol. Biochem. Behav. 2016, 140, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, J.; Zhang, L.; Geng, Q.; Ge, Y. Antidepressant-like Effects of Ginseng Fruit Saponin in Myocardial Infarction Mice. Biomed. Pharmacother. 2019, 115, 108900. [Google Scholar] [CrossRef]
- He, D.-F.; Ren, Y.-P.; Liu, M.-Y. Effects of Ginseng Fruit Saponins on Serotonin System in Sprague-Dawley Rats with Myocardial Infarction, Depression, and Myocardial Infarction Complicated with Depression. Chin. Med. J. 2016, 129, 2913–2919. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Ren, Y.-P.; Zhang, L.-J.; Ding, J.Y. Pretreatment with Ginseng Fruit Saponins Affects Serotonin Expression in an Experimental Comorbidity Model of Myocardial Infarction and Depression. Aging Dis. 2016, 7, 680–686. [Google Scholar] [CrossRef][Green Version]
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng Berry Aqueous Extract Prevents Scopolamine-Induced Memory Impairment in Mice. Exp. Ther. Med. 2019, 18, 4388–4396. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, Z.; Jiang, S.; Jiang, Z. Protective effects of ginsenoside RB3 on hypoxic/ischemic brain injury and involved mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2006, 22, 302–306. [Google Scholar]
- Wang, T.; Yu, X.; Qu, S.; Xu, H.; Han, B.; Sui, D. Effect of Ginsenoside Rb3 on Myocardial Injury and Heart Function Impairment Induced by Isoproterenol in Rats. Eur. J. Pharmacol. 2010, 636, 121–125. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Huangpu, H.; Yao, F. Ginsenoside Rb3 Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury via Activating the Antioxidation Signaling Pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019, 109, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front. Pharmacol. 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fang, D.-F.; Chen, Y. Involvement of N-Methyl-D-Aspartic Acid Receptor and DL-α-Amino-3-Hydroxy-5- Methyl-4-Isoxazole Propionic Acid Receptor in Ginsenosides Rb1 and Rb3 against Oxygen-Glucose Deprivation-Induced Injury in Hippocampal Slices from Rat. Pharmacology 2018, 101, 133–139. [Google Scholar] [CrossRef]
- Peng, L.-L.; Shen, H.-M.; Jiang, Z.-L.; Li, X.; Wang, G.-H.; Zhang, Y.-F.; Ke, K.-F. Inhibition of NMDA Receptors Underlies the Neuroprotective Effect of Ginsenoside Rb3. Am. J. Chin. Med. 2009, 37, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Miao, B.; Song, X.; Jiang, Z. Inactivation of GABA(A) Receptor Reduces Ginsenoside Rb3 Neuroprotection in Mouse Hippocampal Slices after Oxygen-Glucose Deprivation. J. Ethnopharmacol. 2011, 133, 914–916. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, L.; Xiang, H. Ginsenoside Rb3 Exerts Antidepressant-like Effects in Several Animal Models. J. Psychopharmacol. 2012, 26, 697–713. [Google Scholar] [CrossRef]
- Kim, M.S.; Yu, J.M.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Jang, S.K.; Choi, Y.W.; Lee, D.I.; Joo, S.S. Ginsenoside Re and Rd Enhance the Expression of Cholinergic Markers and Neuronal Differentiation in Neuro-2a Cells. Biol. Pharm. Bull. 2014, 37, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 Suppresses the Glutamate-Mediated Oxidative Stress and Neuronal Cell Death in HT22 Cells. J. Ginseng Res. 2019, 43, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Joo, M.-K.; Kim, J.-K.; Jeung, W.; Kang, H.; Kim, D.-H. Bifidobacteria-Fermented Red Ginseng and Its Constituents Ginsenoside Rd and Protopanaxatriol Alleviate Anxiety/Depression in Mice by the Amelioration of Gut Dysbiosis. Nutrients 2020, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-M.; Lu, T.-C.; Sun, M.-L.; Jia, W.-Y.; Ji, X.; Luo, Y.-G. Ginsenoside Rd Inhibits Glioblastoma Cell Proliferation by Up-Regulating the Expression of MiR-144-5p. Biol. Pharm. Bull. 2020, 43, 1534–1541. [Google Scholar] [CrossRef]
- Kim, C.; Spencer, B.; Rockenstein, E.; Yamakado, H.; Mante, M.; Adame, A.; Fields, J.A.; Masliah, D.; Iba, M.; Lee, H.-J.; et al. Immunotherapy Targeting Toll-like Receptor 2 Alleviates Neurodegeneration in Models of Synucleinopathy by Modulating α-Synuclein Transmission and Neuroinflammation. Mol. Neurodegener. 2018, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sundquist, K.; Hedelius, A.; Palmér, K.; Memon, A.A.; Sundquist, J. Circulating MicroRNA-144-5p Is Associated with Depressive Disorders. Clin. Epigenetics 2015, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, K.; Memon, A.A.; Palmér, K.; Sundquist, J.; Wang, X. Inflammatory Proteins and MiRNA-144-5p in Patients with Depression, Anxiety, or Stress- and Adjustment Disorders after Psychological Treatment. Cytokine 2021, 146, 155646. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K. A Recent Overview on Ginsenosides as MicroRNA Modulators in the Treatment of Human Diseases. EXCLI J. 2021, 20, 1453–1457. [Google Scholar] [CrossRef]
- Toledo, A.R.L.; Monroy, G.R.; Salazar, F.E.; Lee, J.-Y.; Jain, S.; Yadav, H.; Borlongan, C.V. Gut-Brain Axis as a Pathological and Therapeutic Target for Neurodegenerative Disorders. Int. J. Mol. Sci. 2022, 23, 1184. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Guijarro, L.G.; Lahera, G.; Monserrat, J.; Valls, P.; Mora, F.; Rodríguez-Jiménez, R.; et al. Gut Microbiota Metabolites in Major Depressive Disorder-Deep Insights into Their Pathophysiological Role and Potential Translational Applications. Metabolites 2022, 12, 50. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Nutritional and Therapeutic Approaches for Protecting Human Gut Microbiota from Psychotropic Treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110182. [Google Scholar] [CrossRef]
- Grootjans, J.; Thuijls, G.; Verdam, F.; Derikx, J.P.; Lenaerts, K.; Buurman, W.A. Non-Invasive Assessment of Barrier Integrity and Function of the Human Gut. World J. Gastrointest. Surg. 2010, 2, 61–69. [Google Scholar] [CrossRef]
- Rodrigues, F.T.S.; de Souza, M.R.M.; Lima, C.N.d.C.; da Silva, F.E.R.; Costa, D.V.d.S.; Dos Santos, C.C.; Miyajima, F.; de Sousa, F.C.F.; Vasconcelos, S.M.M.; Barichello, T.; et al. Major Depression Model Induced by Repeated and Intermittent Lipopolysaccharide Administration: Long-Lasting Behavioral, Neuroimmune and Neuroprogressive Alterations. J. Psychiatr. Res. 2018, 107, 57–67. [Google Scholar] [CrossRef]
- Grigoleit, J.-S.; Kullmann, J.S.; Wolf, O.T.; Hammes, F.; Wegner, A.; Jablonowski, S.; Engler, H.; Gizewski, E.; Oberbeck, R.; Schedlowski, M. Dose-Dependent Effects of Endotoxin on Neurobehavioral Functions in Humans. PLoS ONE 2011, 6, e28330. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C. The Gut-Brain Barrier in Major Depression: Intestinal Mucosal Dysfunction with an Increased Translocation of LPS from Gram Negative Enterobacteria (Leaky Gut) Plays a Role in the Inflammatory Pathophysiology of Depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar] [PubMed]
- Ren, D.-D.; Li, S.-S.; Lin, H.-M.; Xia, Y.-S.; Li, Z.-M.; Bo, P.-P.; Mu, R.; Zhao, L.-J.; Sun, Y.-S. Panax Quinquefolius Polysaccharides Ameliorate Antibiotic-Associated Diarrhoea Induced by Lincomycin Hydrochloride in Rats via the MAPK Signaling Pathways. J. Immunol. Res. 2022, 2022, 4126273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, X.; Qi, Y.; Ren, D.; Li, Z.; Qu, D.; Sun, Y. The Protective Effects of Ginseng Polysaccharides and Their Effective Subfraction against Dextran Sodium Sulfate-Induced Colitis. Foods 2022, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, S.; Ai, Z.; Chen, Y.; Wang, Y.; Li, Y.; Li, X.; Xiao, S.; Wang, Y. Fermented Ginseng Attenuates Lipopolysaccharide-Induced Inflammatory Responses by Activating the TLR4/MAPK Signaling Pathway and Remediating Gut Barrier. Food Funct. 2021, 12, 852–861. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Jiang, S.; Zhang, J.; Liu, Z.; Hou, J.; Gong, X.; Wang, Y.; Wang, Z.; Li, W. Panax Quinquefolium Saponins Protect against Cisplatin Evoked Intestinal Injury via ROS-Mediated Multiple Mechanisms. Phytomedicine 2021, 82, 153446. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, H.-Y.; Bae, C.-H.; Lee, Y.; Kim, S. Korean Red Ginseng Regulates Intestinal Tight Junction and Inflammation in the Colon of a Parkinson’s Disease Mouse Model. J. Med. Food 2020, 23, 1231–1237. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Han, Q.; Lan, J.; Chen, G.; Cao, G.; Yang, C. Effects of Astragalus and Ginseng Polysaccharides on Growth Performance, Immune Function and Intestinal Barrier in Weaned Piglets Challenged with Lipopolysaccharide. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1096–1105. [Google Scholar] [CrossRef]
- He, L.-X.; Wang, J.-B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.-Q.; Ren, J.; Liu, R.; et al. Suppression of TNF-α and Free Radicals Reduces Systematic Inflammatory and Metabolic Disorders: Radioprotective Effects of Ginseng Oligopeptides on Intestinal Barrier Function and Antioxidant Defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, P.; Chen, X.; Yan, J.; Yao, J.; Yu, Z.; Chen, X. Ginsenoside Rb1 Protects the Intestinal Mucosal Barrier Following Peritoneal Air Exposure. Exp. Ther. Med. 2016, 12, 2563–2567. [Google Scholar] [CrossRef]
- Lee, E.-J.; Song, M.-J.; Kwon, H.-S.; Ji, G.E.; Sung, M.-K. Oral Administration of Fermented Red Ginseng Suppressed Ovalbumin-Induced Allergic Responses in Female BALB/c Mice. Phytomedicine 2012, 19, 896–903. [Google Scholar] [CrossRef]
- Seong, M.A.; Woo, J.K.; Kang, J.-H.; Jang, Y.S.; Choi, S.; Jang, Y.S.; Lee, T.H.; Jung, K.H.; Kang, D.K.; Hurh, B.S.; et al. Oral Administration of Fermented Wild Ginseng Ameliorates DSS-Induced Acute Colitis by Inhibiting NF-ΚB Signaling and Protects Intestinal Epithelial Barrier. BMB Rep. 2015, 48, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-S.; Balan, P.; Hong, H.-D.; Choi, W.-I.; Cho, C.-W.; Lee, Y.-C.; Moughan, P.J.; Singh, H. Korean Ginseng Modulates the Ileal Microbiota and Mucin Gene Expression in the Growing Rat. Food Funct. 2014, 5, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, H.; Deng, J.; Fan, D. Ginsenoside Rk3 Ameliorates Obesity-Induced Colitis by Regulating of Intestinal Flora and the TLR4/NF-ΚB Signaling Pathway in C57BL/6 Mice. J. Agric. Food Chem. 2021, 69, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, B.; Li, Y.; Fang, B.; Zhu, X.; Xu, B.; Zhang, J.; Wang, M.; Fang, J. New Insight into 20(S)-Ginsenoside Rh2 against T-Cell Acute Lymphoblastic Leukemia Associated with the Gut Microbiota and the Immune System. Eur. J. Med. Chem. 2020, 203, 112582. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Khan, I.; Li, X.; Chen, L.; Leong, W.; Ho, L.T.; Hsiao, W.L.W. Ginsenosides Rb3 and Rd Reduce Polyps Formation While Reinstate the Dysbiotic Gut Microbiota and the Intestinal Microenvironment in ApcMin/+ Mice. Sci. Rep. 2017, 7, 12552. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Ma, X.; Fan, D. Ginsenoside Rk3 Suppresses Hepatocellular Carcinoma Development through Targeting the Gut-Liver Axis. J. Agric. Food Chem. 2021, 69, 10121–10137. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Duan, Z.; Wang, P.; Zhu, C.; Fan, D. Ginsenoside Rk3 Alleviates Gut Microbiota Dysbiosis and Colonic Inflammation in Antibiotic-Treated Mice. Food Res. Int. 2021, 146, 110465. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, H.; Zhu, C.; Deng, J.; Fan, D. Hypoglycemic Effect of Ginsenoside Rg5 Mediated Partly by Modulating Gut Microbiota Dysbiosis in Diabetic Db/Db Mice. J. Agric. Food Chem. 2020, 68, 5107–5117. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Gut-Liver Axis Modulation of Panax Notoginseng Saponins in Nonalcoholic Fatty Liver Disease. Hepatol. Int. 2021, 15, 350–365. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Duan, Z.; Liu, Y.; Zhu, C.; Fan, D. Ginsenoside Rh4 Alleviates Antibiotic-Induced Intestinal Inflammation by Regulating the TLR4-MyD88-MAPK Pathway and Gut Microbiota Composition. Food Funct. 2021, 12, 2874–2885. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides from American Ginseng (Panax Quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 6650901. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Liu, X.-K.; Kang, Z.-P.; Wang, M.-X.; Zhao, H.-M.; Huang, J.-Q.; Xiao, Q.-P.; Liu, D.-Y.; Zhong, Y.-B. Ginsenoside Rg1 Ameliorated Experimental Colitis by Regulating the Balance of M1/M2 Macrophage Polarization and the Homeostasis of Intestinal Flora. Eur. J. Pharmacol. 2022, 917, 174742. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhou, K.; Jian, P.; Chang, Z.; Zhang, Q.; Liu, Y.; Xiao, S.; Zhang, L. Ginsenosides Improve Nonalcoholic Fatty Liver Disease via Integrated Regulation of Gut Microbiota, Inflammation and Energy Homeostasis. Front. Pharmacol. 2021, 12, 622841. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, S.; Wei, R.; Xie, X.; Wang, C.; Fan, S.; Zhang, X.; Su, J.; Liu, J.; Jia, W.; et al. Metabolome and Gut Microbiota Variation with Long-Term Intake of Panax Ginseng Extracts on Rats. Food Funct. 2018, 9, 3547–3556. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Xia, X.; Fu, C.; Wang, X.; Zhao, Y. Dietary Ginsenoside T19 Supplementation Regulates Glucose and Lipid Metabolism via AMPK and PI3K Pathways and Its Effect on Intestinal Microbiota. J. Agric. Food Chem. 2020, 68, 14452–14462. [Google Scholar] [CrossRef]
- Qu, Q.; Yang, F.; Zhao, C.; Liu, X.; Yang, P.; Li, Z.; Han, L.; Shi, X. Effects of Fermented Ginseng on the Gut Microbiota and Immunity of Rats with Antibiotic-Associated Diarrhea. J. Ethnopharmacol. 2021, 267, 113594. [Google Scholar] [CrossRef]
- Han, K.-H.; Enomoto, M.; Pelpolage, S.; Nagata, R.; Fukuma, N.; Fukushima, M. In Vitro Fermentation Potential of the Residue of Korean Red Ginseng Root in a Mixed Culture of Swine Faecal Bacteria. Food Funct. 2020, 11, 6202–6214. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Y.; You, Y.; Ai, Z.; Dai, W.; Piao, C.; Liu, J.; Wang, Y. Fermented Ginseng Improved Alcohol Liver Injury in Association with Changes in the Gut Microbiota of Mice. Food Funct. 2019, 10, 5566–5573. [Google Scholar] [CrossRef]
- Yang, C.M.; Han, Q.J.; Wang, K.L.; Xu, Y.L.; Lan, J.H.; Cao, G.T. Astragalus and Ginseng Polysaccharides Improve Developmental, Intestinal Morphological, and Immune Functional Characters of Weaned Piglets. Front. Physiol. 2019, 10, 418. [Google Scholar] [CrossRef]
- Qi, Y.-L.; Li, S.-S.; Qu, D.; Chen, L.-X.; Gong, R.-Z.; Gao, K.; Sun, Y.-S. Effects of ginseng neutral polysaccharide on gut microbiota in antibiotic-associated diarrhea mice. Zhongguo Zhong Yao Za Zhi 2019, 44, 811–818. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhang, X.; Wang, X.-Y.; Jia, W. Effect of long-term intake of ginseng extracts on gut microbiota in rats. Zhongguo Zhong Yao Za Zhi 2018, 43, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, R.; Li, N.; Zheng, F.; Dai, Y.; Ge, Y.; Yue, H.; Yu, S. Mechanism of Antidiabetic and Synergistic Effects of Ginseng Polysaccharide and Ginsenoside Rb1 on Diabetic Rat Model. J. Pharm. Biomed. Anal. 2018, 158, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gao, X.-J.; Li, T.; Jing, W.-H.; Han, B.-L.; Jia, Y.-M.; Hu, N.; Yan, Z.-X.; Li, S.-L.; Yan, R. Ginseng Polysaccharides Enhanced Ginsenoside Rb1 and Microbial Metabolites Exposure through Enhancing Intestinal Absorption and Affecting Gut Microbial Metabolism. J. Ethnopharmacol. 2018, 216, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Huang, W.-H.; Zhang, C.-F.; Wan, J.-Y.; Wang, Y.; Yu, C.; Williams, S.; He, T.-C.; Du, W.; Musch, M.W.; et al. Role of Intestinal Microbiome in American Ginseng-Mediated Colon Cancer Protection in High Fat Diet-Fed AOM/DSS Mice. Clin. Transl. Oncol. 2018, 20, 302–312. [Google Scholar] [CrossRef]
- Wang, D.; Shao, S.; Zhang, Y.; Zhao, D.; Wang, M. Insight Into Polysaccharides from Panax ginseng C. A. Meyer in Improving Intestinal Inflammation: Modulating Intestinal Microbiota and Autophagy. Front. Immunol. 2021, 12, 683911. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Zhou, J.; Xu, J.-D.; Shen, H.; Kong, M.; Yip, K.-M.; Han, Q.-B.; Zhao, Z.-Z.; Xu, J.; Chen, H.-B.; et al. Ginseng Ameliorates Exercise-Induced Fatigue Potentially by Regulating the Gut Microbiota. Food Funct. 2021, 12, 3954–3964. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Wu, Y.; Li, F.; Han, M.; Dai, Y.; Zheng, F.; Yue, H. In Vitro Transformation of Protopanaxadiol Saponins in Human Intestinal Flora and Its Effect on Intestinal Flora. Evid.-Based Complement. Altern. Med. 2021, 2021, 1735803. [Google Scholar] [CrossRef]
- Song, M.-Y.; Kim, B.-S.; Kim, H. Influence of Panax Ginseng on Obesity and Gut Microbiota in Obese Middle-Aged Korean Women. J. Ginseng Res. 2014, 38, 106–115. [Google Scholar] [CrossRef]
- Hong, J.T.; Lee, M.-J.; Yoon, S.J.; Shin, S.P.; Bang, C.S.; Baik, G.H.; Kim, D.J.; Youn, G.S.; Shin, M.J.; Ham, Y.L.; et al. Effect of Korea Red Ginseng on Nonalcoholic Fatty Liver Disease: An Association of Gut Microbiota with Liver Function. J. Ginseng Res. 2021, 45, 316–324. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax Notoginseng Saponins Modulate the Gut Microbiota to Promote Thermogenesis and Beige Adipocyte Reconstruction via Leptin-Mediated AMPKα/STAT3 Signaling in Diet-Induced Obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef]
- Bai, Y.; Bao, X.; Mu, Q.; Fang, X.; Zhu, R.; Liu, C.; Mo, F.; Zhang, D.; Jiang, G.; Li, P.; et al. Ginsenoside Rb1, Salvianolic Acid B and Their Combination Modulate Gut Microbiota and Improve Glucolipid Metabolism in High-Fat Diet Induced Obese Mice. PeerJ 2021, 9, e10598. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Song, F.; Xing, J.; Zheng, Z.; Liu, S.; Liu, Z. Comprehensive Fecal Metabolomics and Gut Microbiota for the Evaluation of the Mechanism of Panax Ginseng in the Treatment of Qi-Deficiency Liver Cancer. J. Ethnopharmacol. 2022, 292, 115222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhang, M.-Y.; Wu, Y.-X.; Wang, Y.-Z.; Li, F.-T.; Han, M.-X.; Dai, Y.-L.; Yue, H. Biotransformation of Ginsenosides (Rb1, Rb2, Rb3, Rc) in Human Intestinal Bacteria and Its Effect on Intestinal Flora. Chem. Biodivers. 2021, 18, e2100296. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Lin, J.; Isnard, S.; Fombuena, B.; Peng, X.; Marette, A.; Routy, B.; Messaoudene, M.; Chen, Y.; Routy, J.-P. The Bacterium Akkermansia Muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation. Front. Immunol. 2020, 11, 645. [Google Scholar] [CrossRef]
- Cheng, D.; Xie, M.Z. A Review of a Potential and Promising Probiotic Candidate-Akkermansia Muciniphila. J. Appl. Microbiol. 2021, 130, 1813–1822. [Google Scholar] [CrossRef]
- Mennigen, R.; Bruewer, M. Effect of Probiotics on Intestinal Barrier Function. Ann. N. Y. Acad. Sci. 2009, 1165, 183–189. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, H.; Kang, Y.; Tian, Y.; Li, L.; Kang, X.; Yang, H.; Wang, Y.; Tian, J.; Zhang, F.; et al. Probiotics Alleviate Autoimmune Hepatitis in Mice through Modulation of Gut Microbiota and Intestinal Permeability. J. Nutr. Biochem. 2021, 98, 108863. [Google Scholar] [CrossRef]
- Uusitupa, H.-M.; Rasinkangas, P.; Lehtinen, M.J.; Mäkelä, S.M.; Airaksinen, K.; Anglenius, H.; Ouwehand, A.C.; Maukonen, J. Bifidobacterium Animalis Subsp. Lactis 420 for Metabolic Health: Review of the Research. Nutrients 2020, 12, 892. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium Longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Yang, X.; Li, W.; Si, J.; Yang, X. The Antidepressant Potential of Lactobacillus Casei in the Postpartum Depression Rat Model Mediated by the Microbiota-Gut-Brain Axis. Neurosci. Lett. 2022, 774, 136474. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The Epigenetic Effects of Butyrate: Potential Therapeutic Implications for Clinical Practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.M.; Leonel, A.J.; Melo, M.A.; Santos, R.R.G.; Cara, D.C.; Cardoso, V.N.; Correia, M.I.T.D.; Alvarez-Leite, J.I. Oral Supplementation of Butyrate Reduces Mucositis and Intestinal Permeability Associated with 5-Fluorouracil Administration. Lipids 2012, 47, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutvin, S.A.L.W.; Troost, F.J.; Hamer, H.M.; Lindsey, P.J.; Koek, G.H.; Jonkers, D.M.A.E.; Kodde, A.; Venema, K.; Brummer, R.J.M. Butyrate-Induced Transcriptional Changes in Human Colonic Mucosa. PLoS ONE 2009, 4, e6759. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Zhang, Q.; Yun, Y.; An, H.; Zhao, W.; Ma, T.; Wang, Z.; Yang, F. Gut Microbiome Composition Associated with Major Depressive Disorder and Sleep Quality. Front. Psychiatry 2021, 12, 645045. [Google Scholar] [CrossRef]
- Kim, D.-H. Gut Microbiota-Mediated Pharmacokinetics of Ginseng Saponins. J. Ginseng Res. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.; Kim, D.; Lee, J.; Yoo, J.; Koh, B. Studies on Absorption, Distribution and Metabolism of Ginseng in Humans after Oral Administration. J. Ethnopharmacol. 2009, 122, 143–148. [Google Scholar] [CrossRef]
- Dong, W.-W.; Zhao, J.; Zhong, F.-L.; Zhu, W.-J.; Jiang, J.; Wu, S.; Yang, D.-C.; Li, D.; Quan, L.-H. Biotransformation of Panax Ginseng Extract by Rat Intestinal Microflora: Identification and Quantification of Metabolites Using Liquid Chromatography-Tandem Mass Spectrometry. J. Ginseng Res. 2017, 41, 540–547. [Google Scholar] [CrossRef]
- Chen, W.; Yao, P.; Vong, C.T.; Li, X.; Chen, Z.; Xiao, J.; Wang, S.; Wang, Y. Ginseng: A Bibliometric Analysis of 40-Year Journey of Global Clinical Trials. J. Adv. Res. 2021, 34, 187–197. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.; Kim, M.J.; Kim, M.-S.; Kim, J.; Park, C.-W.; Seo, D.; Shin, S.S.; Oh, S.W. Efficacy and Safety of Panax Ginseng Berry Extract on Glycemic Control: A 12-Wk Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. J. Ginseng Res. 2018, 42, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.D.; Park, C.W.; Jang, J.; Kim, S.H.; Jeon, H.Y.; Kim, W.G.; Lee, S.J.; Chung, W.S. Effects of Korean Ginseng Berry Extract on Sexual Function in Men with Erectile Dysfunction: A Multicenter, Placebo-Controlled, Double-Blind Clinical Study. Int. J. Impot. Res. 2013, 25, 45–50. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Pharmacological Effects | Content (Berry vs. Root, mg/g Dry Weight) | |
|---|---|---|---|
| Ginsenosides | Rb1 | Neuroprotective [14], anti-diabetic [15], mitochondrial antioxidant [16] | *B< [12,17] 0.86 ± 0.09 vs. 25.36 ± 1.67 [17] 9.03 ± 0.60 vs. ND [18] ND vs. 48.51 ± 1.79 [19] ND vs. 47.96 ± 1.04 [19] |
| Rb2 | Anti-diabetic, anti-viral, cardioprotective, neuroprotective [20] | *B> [12,17,21] 1.54 ± 0.95 vs. 0.3 ± 0.02 [17] | |
| Rb3 | Anti-diabetic, anticonvulsant, antitumor, cardioprotective, antidepressant [22] | *B> [12,21] | |
| Rc | Antiallergic [23], antioxidant [24], anti-inflammatory [25], SIRT1 activation [26] | *B> [12] *B< [17] 1.51 ± 0.11 vs. 7.03 ± 2.15 [17] | |
| Rd | Neuroprotective, antioxidant, anti-inflammatory, neuroprotective [27], antidepressant [28] | B~ [12] *B< [17] 0.48 ± 0.1 vs. 3.16 ± 0.98 [17] | |
| Re | Cardioprotective [29], Neuroprotective [30,31], antidepressant [32] | *B> [12] *B< [17] 5.30 ± 0.54 vs. 17.45 ± 1.6 [17] 8.42 ± 0.19 vs. ND [18] | |
| Rg1 | Stem cell regulation [33,34], anti-inflammatory [35], antidepressant [36] | *B< [12,17] 0.53 ± 0.09 vs. 2.39 ± 1.01 [17] 0.390 ± 0.010 vs. ND [18] ND vs. 3.15 ± 0.23 [19] ND vs. 2.49 ± 0.04 [19] | |
| Rg2 | Cardioprotective [37,38,39,40], neuroprotective [41] | *B> [12] | |
| 20(R)-Rg2 | Insufficient data | nil [12,42] | |
| Rg3 | Anticancer [43,44], neuroprotective [45] | *B> [12] | |
| Rh1 | Anti-inflammatory, antioxidant, immunomodulatory, neuroprotective [46] | B~ [12] | |
| Rh2 | Anti-cancer [47] | nil [12,42] | |
| Polysaccharides | Anti-cancer [48,49] | ||
| Compounds | Models | Mechanism(s) | Significant Effects (p < 0.05) |
|---|---|---|---|
| American Ginseng Root Polysaccharides | Antibiotic-associated Diarrhea in Rats (Lincomycin Hydrochloride) | MAPK Signaling | Reduces colonic IL-1β, IL-6, IL-17A and TNF-α and increases IL-4 and IL-10. Increases Claudin-1 and Occludin expression [83] |
| Korean Ginseng Root Polysaccharides | DSS-induced Colitis in Rats | TLR4/MyD88/NF-κB-signaling pathway inhibition | Alleviates colitis symptoms, downregulates IL-1β, IL-2, IL-6, IL-17A, upregulates ZO-1 and Occludin [84] |
| Fermented Korean Ginseng Root Ginsenosides | Intraperitoneal LPS Injection in Mice | TLR4/MAPK | Attenuates LPS-induced increases in IL-6, TNF-α and IL-1β. Attenuates LPS-induced increases in ALT and AST, increases LPS-induced expression of Claudin-1 [85] |
| American Ginseng Ginsenosides | Cisplatin-induced intestinal injury in Mice | Decreased NF-κB activity | Attenuates cisplatin-induced increases in TNF-α and IL-1β. Attenuates cisplatin-induced decreases in ZO-1 and Occludin [86] |
| Korean Red Ginseng Root | MPTP-induced Intestinal Permeability in Mice | - | Prevents MPTP-induced decrease in Occludin and ZO-1, and MPTP-induced colonic increase in TNF-α and IL-1β [87] |
| Ginseng Polysaccharides (Unspecified Variety) | Intraperitoneal LPS Injection in Piglets | Decreased LPS-induced NF-κB activity | Increases jejunal villus height and expression of Occludin and Claudin in both LPS-treated and control groups. Alleviates LPS-induced increases in ALT, AST, TNF-α, and IL-1β [88] |
| Korean Ginseng Root Oligopeptides | Irradiation induced intestinal injury in mice | - | Decreases serum LPS levels and decreases plasma FITC-dextran. Pretreatment prevented plasma IL-6 decrease and TNF-α increase. Treatment dose-dependently increases ZO-1 and Occludin post-radiation injury [89] |
| Ginsenoside Rb1 | Peritoneal air exposure intestinal damage in Rats | - | Dose-dependently reduces serum D-lactate and intestinal clearance of FITC-dextran [90] |
| Fermented and Unfermented Korean Red Ginseng Root | Ovalbumin-induced allergy in sensitized mice | Th1/Th2 balance, IgE suppression | Both treatments decrease IL-4 and TNF-α mRNA expression. Both treatments prevented an allergy-induced increase in serum beta-lactoglobulin after gastric administration [91] |
| Fermented Wild Ginseng Root | DSS-induced colitis Mouse Model | Decreased DSS-induced NF-κB activity | Alleviates colitis, prevents DSS-induced loss of ZO-1, downregulates DSS-induced IL-1β, IL-6, TNF-α, and IFN-γ mRNA expression. Decreases colonic levels of TNF-α [92] |
| Korean Ginseng | Healthy Mouse Model | - | Increased Muc2 expression [93] |
| Ginsenoside Rk3 | High-fat diet Mouse Model | TLR4/NF-κB signaling pathway inhibition | Reduced colonic inflammatory cytokines and oxidative stress. Increases ZO-1, Occludin, and Claudin expression [94] |
| Ginsenoside Rh2 | T-cell acute lymphoblastic leukemia mouse model | Decreased TLR4/MyD88 expression | Decreased IL-1β, IL-6, and TNF-α. Increased IL-10 and TGF-β. Increased mRNA expression of ZO-1, Claudin, and Occludin [95] |
| Ginsenosides Rb3 and Rd | ApcMin/+ mice (colon cancer model) | - | Increased Goblet and Paneth cell count [96] |
| Ginsenoside Rk3 | DEN- and CCl4-induced Hepatocellular carcinoma mouse model | TLR4 pathway inhibition | Visual restoration of the intestinal barrier, increased expression of ZO-1, Occludin, and Claudin [97] |
| Ginsenoside Rk3 | Lincomycin-treated mice | - | Increased expression of ZO-1, Occludin, and Claudin-1, and reversed structural changes to the epithelium. Prevented increased IL-1β, IL-6, IL-17, IFN- γ and TNF-α and prevented decreased IL-10 [98] |
| Ginsenoside Rg5 | db/db diabetes mouse model | TLR4/NF-κB signaling pathway inhibition | Increased Occludin and ZO-1 protein expression, decreased serum LPS [99] |
| Panax Notoginseng saponins | Lepob mice on a high-fat diet | TLR4 pathway inhibition | Increased expression of ZO-1 and Claudin-1 [100] |
| Ginsenoside Rh4 | Antibiotic intestinal inflammation mouse model | Decreased TLR4/NF-κB /MyD88 expression | Increased expression of ZO-1 and Claudin-1. Decreased IL-1β, IL-6, IL-17, IFN- γ and TNF-α. Prevented increase in IL-10. Reduced serum LPS [101] |
| American ginseng polysaccharides and ginsenosides | Cyclophosphamide-Induced Intestinal Damage in Mice | - | Both ginsenosides and polysaccharides independently increased mucin area, goblet cell count, and increased expression of ZO-1 and Occludin, but the combination had higher effect [102] |
| Ginsenoside Rg1 | DSS-induced colitis mouse model | - | Decreased levels of IL-6, IL-33, TNF-α and increased IL-4 and IL-10 [103] |
| Korean Ginseng Ginsenosides | Mice on a high-fat diet | - | Increased expression of ZO-1 and Occludin mRNA expression. Decreased serum LPS [104] |
| Compounds | Models | Significant Effects (p < 0.05) |
|---|---|---|
| American Ginseng Root Polysaccharides | Antibiotic-associated Diarrhea in Rats (Lincomycin Hydrochloride) | Increased production of acetate and propionate, improved the relative richness of Lactobacillus and Bacteroides, and reduced the relative richness of Blautia and Coprococcus [83] |
| Korean Ginseng | Healthy Mouse Model | Increased total bacterial count and Lactobacillus count [93] |
| Ginsenoside Rk3 | High-fat diet Mouse Model | Increased abundance of Bacteroides and Bifidobacteria, decreased abundance of Firmicutes [94] |
| 25-hydroxyl-protopanaxatriol | High-fat diet Mouse Model with streptozotocin | Partly reversed an increase in Firmicutes/Bacteroides ratio, increased relative abundance of Lachnospiraceae [106] |
| Fermented Wild Ginseng root | Antibiotic-associated diarrhea mouse model | Increased recovery of total bacteria counts after antibiotic treatment. Increased recovery of Lactobacillus murinus, Bifidobacterium, Enterobacteriaceae bacterium, and Enterococcus faecium [107] |
| Ginsenoside Rh2 | T-cell acute lymphoblastic leukemia mouse model | Increased abundance of Bacteroides and Verrucomicrobia, decreased abundance of Firmicutes and Proteobacteria. Increased relative abundance of Akkermansia, Lactobacillus, and Lachnospiraceae [95] |
| Korean red ginseng root insoluble fiber | In vitro colon-simulated fermentation using swine fecal bacteria | Increased production of short-chain fatty acids, decreased alpha-diversity, and increased relative abundance of Bifidobacterium and Prevotella compared to control fermentation with cellulose [108] |
| Fermented Korean Ginseng Root | Alcoholic injury mice (ethanol diet) | Prevented relative abundance loss of Akkermansia and Allobaculum. Decreased relative abundance of Parabacteroides [109] |
| Ginseng Root Polysaccharides (Unspecified variety) | Healthy Piglets with supplemented diet | Increased colonic acetic acid, isobutyric acid, and butyrate. Decreased abundance of Malainabacteria [110] |
| Water Soluble Neutral Ginseng Polysaccharides | Antibiotic-associated Diarrhea in Mice (Lincomycin Hydrochloride) | Increased abundance of Lactobacillus, decreased abundance of Bacteroides, Streptococcus, Ochrobactrum, and Pseudomonas [111] |
| Unspecified Ginseng Extracts (Article in Chinese) | Healthy Rats | Increased abundance of Bifidobacterium, Lactobacillus, Allobaculum, and Clostridium. Decreased abundance of Butyricimonas, Parabacteroides, Alistipes, and Helicobacter [112] |
| Korean Red Ginseng Root Polysaccharides and Ginsenoside Rb1 | Streptozotocin-Induced Diabetes Mouse Model | Polysaccharide treatment reversed the dysbiosis caused by the treatment, as evidenced by reversal of loss of relative abundance of Firmicutes and reversal of increase of the relative abundance of Bacteroides [113] |
| Ginseng Root Polysaccharides | DSS-induced Colitis Mouse Model | Reverses DSS-induced changes; increases abundance of Bifidobacterium, Lactobacillus, and the bacteria Clostridium leptum and Clostridium coccoides. Reduces abundance of Enterobacteriaceae and the bacterium Bacteroides fragilis [114] |
| Ginsenosides Rb3 and Rd | ApcMin/+ mice (colon cancer model) | Decreased abundance of Dysgonomonas, Porphyromonas, and Parabacteroides. Increased abundance of Prevotella and Paraprevotella (Rd only). Increased richness of family Bacteroidaceae; promoted growth of Bacteroides vulgatus, Bacteroides xylanisolvens, Bacteroides gallinarum, and Bacteroides acidifiaciens [96] |
| American Ginseng Root | AOM/DSS intestinal inflammation and tumorigenesis mouse model | Gradual reversal of loss of alpha-diversity and beta-diversity following DSS treatment. Reversed increase in Bacteroidaceae, Porphyromonadaceae, Enterobacteriaceae, and Verrucomicrobiaceae, and reversed the decrease in Clostridiaceae, Catabacteriaceae, Lachnospiraceae, and Ruminococcaceae [115] |
| Ginsenoside Rk3 | DEN- and CCl4-induced Hepatocellular carcinoma mouse model | Reversed decrease in Bacteroidetes and increase in Firmicutes. Reversed decrease in Lachnospiraceae and Bifidobacteriaceae. Reversed increase in Ruminococcaceae. Reversed increase in Helicobacter and reversed the decrease in Akkermansia, Lactobacillus, Oscillibacter, and Bifidobacterium [97] |
| Korean Ginseng Root Polysaccharides | DSS-induced colitis in Mice | Restored loss of alpha diversity (Shannon Index). Reversed relative increase in Bacteroidetes, Verrucomicrobia, Proteobacteria, Tenericutes, Cyanobacteria, Prevotella and Deferribacteres and reversed loss of Firmicutes and Akkermansia [116] |
| Ginsenoside Rk3 | Lincomycin-treated mice | Preserved Simpson, Shannon, ACE and Chao1 index at levels of control. Increased levels Bacillaceae, Bacteroidaceae and Prevotellaceae. Increased levels of Anaerostipes, Alloprevotella, Lachnoclostridium and Blautia. Decreased loss of acetic acid production, prevented decrease of propionic acid, butyric acid, isobutyric acid, and valeric acid production [98] |
| Ginseng Root Water-Soluble Extract (Unspecified Variety) | Exercise-Fatigue Mouse Model | Reversed relative loss of Bacteroidetes and reversed relative increase of Firmicutes. Increased Lactobacillus and Bacteroides, decreased Anaerotruncus. Reversed loss of Bifidobacterium, Streptococcus, Coprpcoccus, and Clostridium [117] |
| Protopanaxadiol-type Ginsenosides Extracted from Korean Ginseng Root | Human Fecal Microbiota In Vitro Fermentation | Increased relative abundance of Escherichia-Shigella, decreased relative abundance of Dorea, Prevotella, and Megasphaera. Increased abundance of Lachnospiraceae, Streptococcaceae… (Abridged) [118] |
| Korean Ginseng Root | Middle-Aged Korean Women with Obesity | Decreased relative abundance of Anaerostipes [119] |
| Korean Red Ginseng Root | Patients with non-alcoholic steatohepatitis | Increased Lactobacillus in subgroup who experienced improvements in ALT [120] |
| Ginsenoside Rg5 | db/db diabetes mouse model | Reversed decrease in abundance of Alloprevotella, Barnesiella, Coprobacter, Lactobacillus, Lactococcus, and Parasutterella, reversed increase in abundance of Oscillibacter, Clostridium, Helicobacter, and Dorea (abridged) [99] |
| Panax notoginseng saponins | Diet-induced obesity mice | Increased abundance of Akkermansia muciniphila and Parabacteroides distasonis [121] |
| Ginsenoside Rb1 | Diet-induced obesity mice | Decreased Helicobacteraceae and Ruminococcaceae, and enriched Rikenellaceae. Decreased abundance of Dorea, Helicobacter and Oscillospira [122] |
| Panax Notoginseng saponins | Lepob mice on High-fat diet | Increased fecal acetic acid, butyric acid, propionic acid, isobutyric acid, valeric acid and isovaleric acid [100] |
| Ginsenoside Rh4 | Antibiotic intestinal inflammation mouse model | Decreased Firmicutes/Bacteroidetes ratio. Increased fecal acetic acid, butyric acid, propionic acid, isobutyric acid, valeric acid and isovaleric acid [101] |
| American ginseng polysaccharides and ginsenosides | Cyclophosphamide-Induced Intestinal Damage in Mice | The combination increased abundance of Clostridiales, Bifidobacterium, and Lachnospiraceae, and decreased abundance of Escherichia-Shigella and Peptococcaceae (reversing detrimental changes in microbiota). Polysaccharides and ginsenosides had different and synergistic effects [102] |
| Korean Ginseng polysaccharides and ginsenosides | Exhaustion by forced swimming and human hepatoma HepG2 cells xenograft | The combination reversed the changes in microbiota. Polysaccharides and ginsenosides had different and synergistic effects [123] |
| Ginsenosides | Human Fecal Microbiota In Vitro Fermentation | Increased relative abundance of Firmicutes and Proteobacteria and decreased relative abundance of Bacteroidetes. Increased abundance of Escherichia, Streptococcus and Ruminococcus. Decreased abundance of Dorea, Sutterella, Prevotella and Megasphaera [124] |
| Ginsenoside Rg1 | DSS-induced colitis mouse model | Increased relative abundance of Lachnospiraceae and decrease of Staphylococcus, Bacteroide and Ruminococcaceae [103] |
| Korean Ginseng Ginsenosides | Mice on High-fat diet | Increased abundance of Parabacteroides, Muribaculaceae, Akkermansia, and Ruminococcus. Decreased abundance of Lachnospiraceae and Helicobacter [104] |
| Korean Ginseng | Healthy Rats | Increased abundance of Bifidobacterium and Lactobacillus [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
St-Laurent, T.; Hammami, R. The Untapped Potential of Ginsenosides and American Ginseng Berry in Promoting Mental Health via the Gut–Brain Axis. Nutrients 2022, 14, 2523. https://doi.org/10.3390/nu14122523
St-Laurent T, Hammami R. The Untapped Potential of Ginsenosides and American Ginseng Berry in Promoting Mental Health via the Gut–Brain Axis. Nutrients. 2022; 14(12):2523. https://doi.org/10.3390/nu14122523
Chicago/Turabian StyleSt-Laurent, Tristan, and Riadh Hammami. 2022. "The Untapped Potential of Ginsenosides and American Ginseng Berry in Promoting Mental Health via the Gut–Brain Axis" Nutrients 14, no. 12: 2523. https://doi.org/10.3390/nu14122523
APA StyleSt-Laurent, T., & Hammami, R. (2022). The Untapped Potential of Ginsenosides and American Ginseng Berry in Promoting Mental Health via the Gut–Brain Axis. Nutrients, 14(12), 2523. https://doi.org/10.3390/nu14122523