Short-Chain Fatty Acids Modulate Permeability, Motility and Gene Expression in the Porcine Fetal Jejunum Ex Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Short-Chain Fatty Acid Solution
2.3. Measurement of Jejunal Motility
2.4. Measurement of Gut Electrophysiological Parameters
2.5. Analysis of SCFA
2.6. Measurement of the Gene Expression
2.7. Statistical Analysis
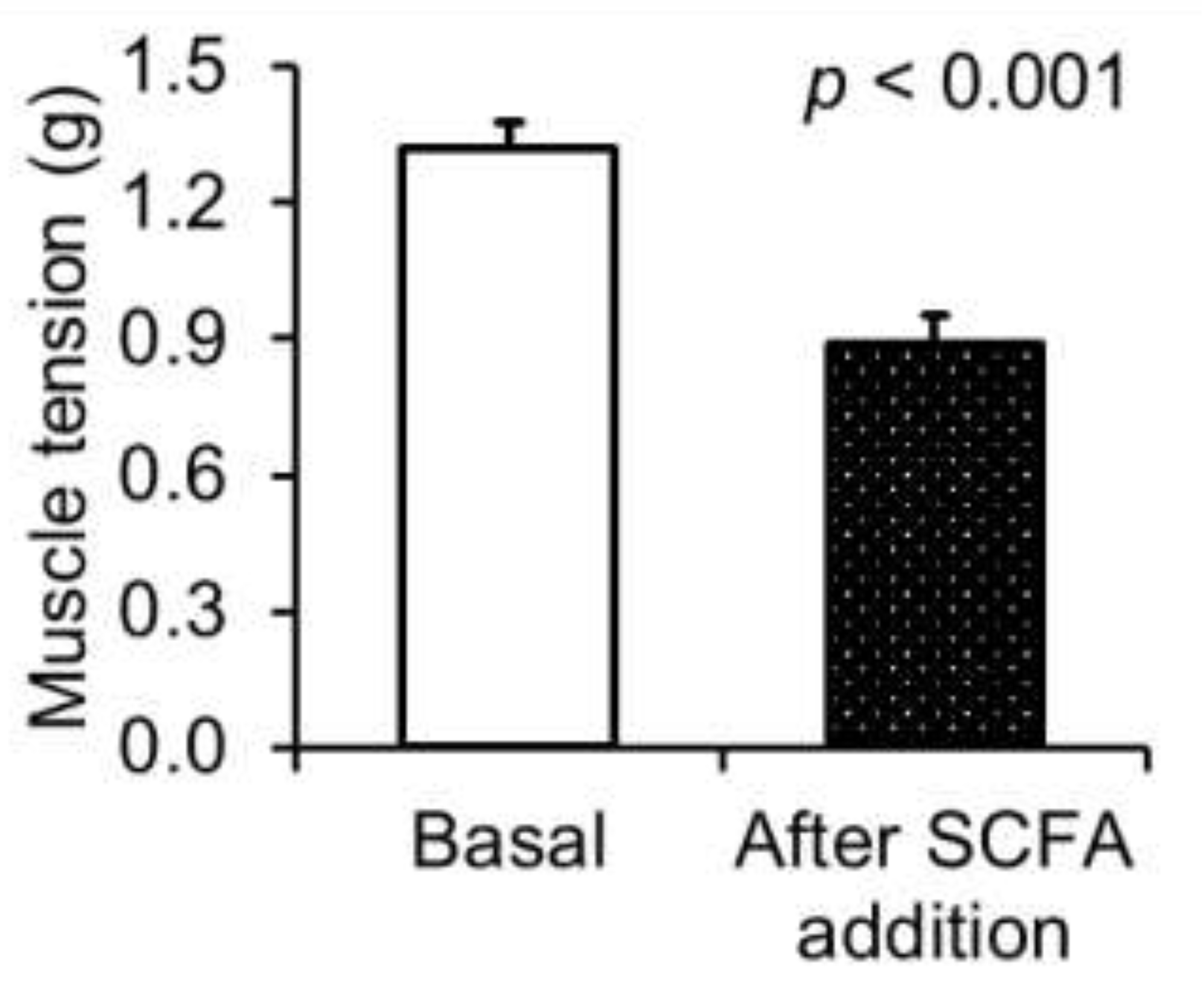
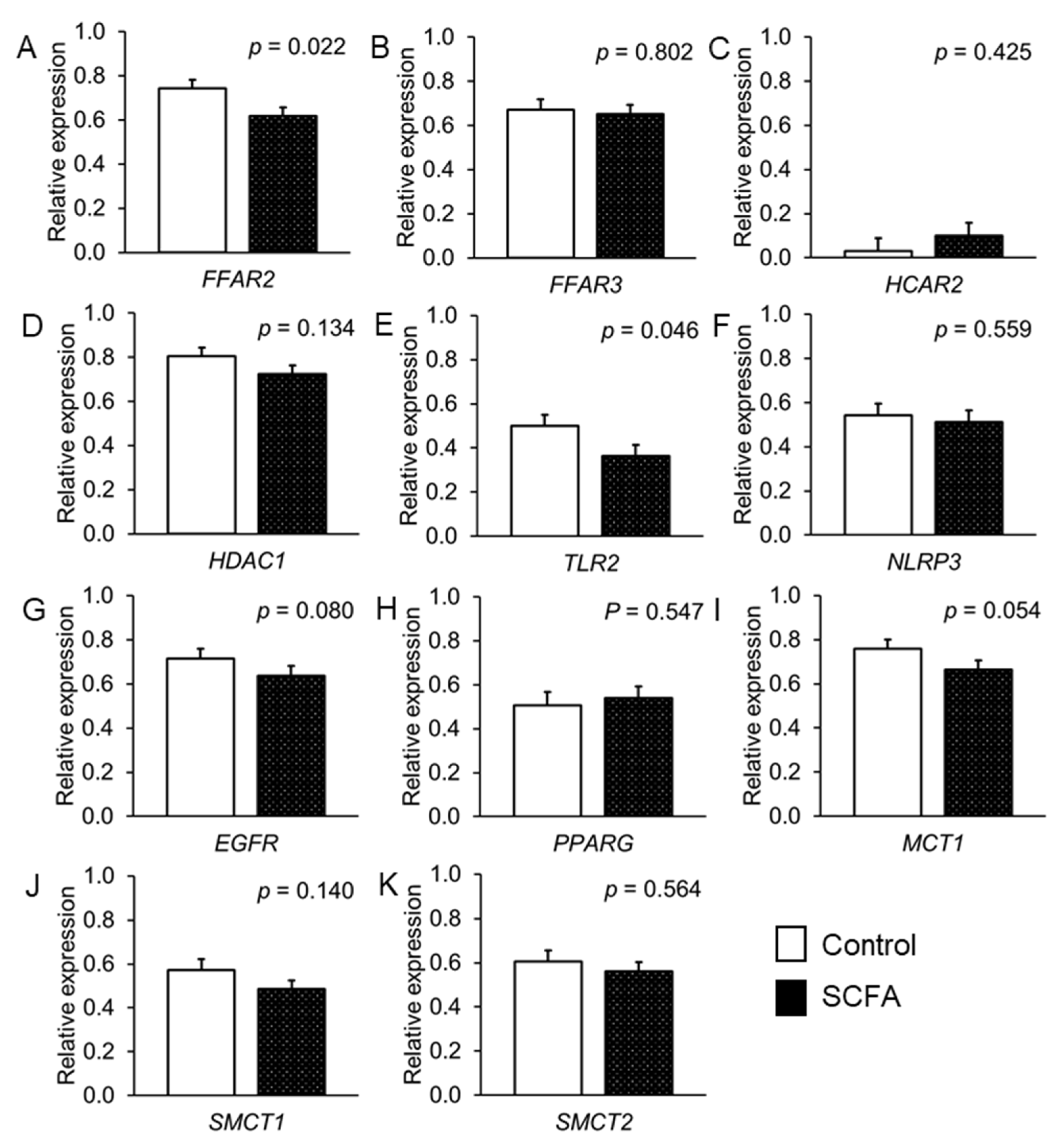
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metzler-Zebeli, B.U.; Lang, I.S.; Görs, S.; Brüssow, K.P.; Hennig, U.; Nürnberg, G.; Rehfeldt, C.; Otten, W.; Metges, C.C. High-protein-low-carbohydrate diet during pregnancy alters maternal plasma amino acid concentration and placental amino acid extraction but not fetal plasma amino acids in pigs. Br. J. Nutr. 2012, 108, 2176–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, M.; Nagura, H.; Ueno, S.; Nakashima, A. Amino acid composition of amniotic fluid during the perinatal period reflects mother’s fat and carbohydrate intake. Nutrients 2021, 13, 2136. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Holman, D.B.; Schmidt, K.; McCarthy, K.L.; Dorsam, S.T.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; Caton, J.S.; Sedivec, K.K.; et al. Characterization of the microbiota associated with 12-week-old bovine fetuses exposed to divergent in utero nutrition. Front. Microbiol. 2022, 12, 771832. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, E.D.; Bajaj, T. Embryology, Amniotic Fluid; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- He, Q.; Kwok, L.Y.; Xi, X.; Zhong, Z.; Ma, T.; Xu, H.; Meng, H.; Zhao, F.; Zhang, H. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 2020, 12, 1794266. [Google Scholar] [CrossRef]
- Shrestha, A.; Metzler-Zebeli, B.U.; Karembe, H.; Sperling, D.; Koger, S.; Joachim, A. Shifts in the fecal microbial community of Cystoisospora suis infected piglets in response to toltrazuril. Front. Microbiol. 2020, 11, 983. [Google Scholar] [CrossRef]
- Kirschner, S.K.; Ten Have, G.A.M.; Engelen, M.P.K.J.; Deutz, N.E.P. Transorgan short-chain fatty acid fluxes in the fasted and postprandial state in the pig. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E665–E673. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Theil, P.K.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Zhuo, Y.; Wu, F.; Jiang, X.; et al. The differences in energy metabolism and redox status between sows with short and long farrowing duration. Animal 2021, 15, 100355. [Google Scholar] [CrossRef]
- Nakatani, M.; Inoue, R.; Tomonaga, S.; Fukuta, K.; Tsukahara, T. Production, absorption, and blood flow dynamics of short-chain fatty acids produced by fermentation in piglet hindgut during the suckling-weaning period. Nutrients 2018, 10, 1220. [Google Scholar] [CrossRef] [Green Version]
- Metzler-Zebeli, B.U.; Sener-Aydemir, A.; Sharma, S.; Lerch, F. Postnatal development of gut microbial activity and their importance for jejunal motility in piglets. J. Anim. Sci. 2021, 99, skab171. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U. Chapter 8: Porcine Gut Microbiota and Host Interactions during the Transition from the Suckling to Post-Weaning Phase. In Gut Microbiota, Immunity, and Health in Production Animals; Kogut, M.H., Zhang, G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 147–178. [Google Scholar]
- Lerch, F.; Vötterl, J.; Koger, S.; Ehmig, J.; Verhovsek, D.; Metzler-Zebeli, B.U. Age rather than creep feeding modified jejunal and caecal gene expression for fatty acid signaling and immune response in neonatal piglets. Anim.-Sci. Proc. 2022, 13, 210–211. [Google Scholar] [CrossRef]
- Huang, S.; Li, N.; Liu, C.; Li, T.; Wang, W.; Jiang, L.; Li, Z.; Han, D.; Tao, S.; Wang, J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Ferenc, K.; Pietrzak, P.; Godlewski, M.M.; Piwowarski, J.; Kiliańczyk, R.; Guilloteau, P.; Zabielski, R. Intrauterine growth retarded piglet as a model for humans--studies on the perinatal development of the gut structure and function. Reprod. Biol. 2014, 14, 51–60. [Google Scholar] [CrossRef]
- Kreutzmann, H.; Stadler, J.; Knecht, C.; Sassu, E.L.; Ruczizka, U.; Zablotski, Y.; Vatzia, E.; Balka, G.; Zaruba, M.; Chen, H.-W.; et al. Phenotypic characterization of a virulent PRRSV-1 isolate in a reproductive model with and without prior heterologous modified live PRRSV-1 vaccination. Front. Vet. Sci. 2022, 9, 820233. [Google Scholar] [CrossRef]
- Baskara, A.P.; Sharma, S.; Sener-Aydemir, A.; Koger, S.; Ariyadi, B.; Dono, N.D.; Zuprizal, Z.; Metzler-Zebeli, B.U. Cinnamon bark oil and coconut oil emulsions modified small intestinal motility and barrier function in laying hens in an ex vivo experiment. Br. Poult. Sci. 2021, 62, 435–442. [Google Scholar] [CrossRef]
- Yosi, F.; Sharma, S.; Sener-Aydemir, A.; Koger, S.; Baskara, A.P.; Metzler-Zebeli, B.U. Short-chain fatty acids promote jejunal barrier function and caecal muscle contractibility in laying hens ex vivo. Br. Poult. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Vötterl, J.; Klinsoda, J.; Zebeli, Q.; Hennig-Pauka, I.; Kandler, W.; Metzler-Zebeli, B. Dietary phytase and lactic acid-treated cereal grains differently affected calcium and phosphorus homeostasis from intestinal uptake to systemic metabolism in a pig model. Nutrients 2020, 12, 1542. [Google Scholar] [CrossRef]
- Klinsoda, J.; Vötterl, J.; Zebeli, Q.; Metzler-Zebeli, B.U. Alterations of the viable ileal microbiota of the gut mucosa-lymph node axis in pigs fed phytase and lactic acid-treated cereals. Appl. Environ. Microbiol. 2020, 86, e02128-19. [Google Scholar] [CrossRef]
- Newman, M.A.; Petri, R.M.; Grüll, D.; Zebeli, Q.; Metzler-Zebeli, B.U. Transglycosylated starch modulates the gut microbiome and expression of genes related to lipid synthesis in liver and adipose tissue of pigs. Front. Microbiol. 2018, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 16, 2645465. [Google Scholar] [CrossRef] [Green Version]
- Metzler-Zebeli, B.U.; Klinsoda, J.; Vötterl, J.; Sharma, S.; Koger, S.; Sener-Aydemir, A. Short-, medium-, and long-chain fatty acid profiles and signaling is responsive to dietary phytase and lactic acid treatment of cereals along the gastrointestinal tract of growing pigs. J. Anim. Sci. 2021, 99, skab117. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-chain fatty acid transporters: Role in colonic homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef] [Green Version]
- Ichida, S.; Oka, H.; Masada, A.; Fujisue, T.; Hata, T.; Matsuda, N. Effects of synthetic omega-conotoxin on the contractile responses of segments of rat ileum, stomach fundus and uterus and guinea pig taenia coli. Jpn. J. Pharmacol. 1988, 48, 395–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherbut, C.; Aubé, A.C.; Blottière, H.M.; Pacaud, P.; Scarpignato, C.; Galmiche, J.P. In vitro contractile effects of short chain fatty acids in the rat terminal ileum. Gut 1998, 38, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, N.; Mehmood, M.H.; Al-Rehaily, A.J.; Mothana, R.A.; Gilani, A.J. Species and tissue-specificity of prokinetic, laxative and spasmodic effects of Fumaria parviflora. BMC Compl. Altern. Med. 2012, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Blakeney, B.A.; Crowe, M.S.; Mahavadi, S.; Murthy, K.S.; Grider, J.R. Branched short-chain fatty acid isovaleric acid causes colonic smooth muscle relaxation via cAMP/PKA pathway. Dig. Dis. Sci. 2018, 64, 1171–1181. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Lan, J.; Han, J.; Yang, Y.; Shan, A. TLR2/EGFR Are Two sensors for pBD3 and pEP2C induction by sodium butyrate independent of HDAC inhibition. J. Agric. Food Chem. 2020, 68, 512–522. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Kibbie, J.J.; Dillon, S.M.; Thompson, T.A.; Purba, C.M.; McCarter, M.D.; Wilson, C.C. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 2021, 226, 152126. [Google Scholar] [CrossRef]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef]


| Components | Concentration (g/L) | Molarity (mM) |
|---|---|---|
| Krebs–Henseleit buffer used in organ bath | ||
| NaCl | 6.90 | 118.1 |
| NaHCO3 | 2.10 | 25.0 |
| KCl | 0.35 | 4.7 |
| MgSO4 | 0.30 | 1.2 |
| CaCl2 | 0.17 | 1.2 |
| KH2PO4 | 0.16 | 1.2 |
| D-Glucose | 1.50 | 8.3 |
| Modified Krebs–Henseleit buffer used in Ussing chamber | ||
| NaCl | 6.72 | 115.0 |
| NaHCO3 | 2.10 | 25.0 |
| Na2HPO4.2H2O | 0.42 | 2.4 |
| KCl | 0.37 | 5.0 |
| CaCl2.2H2O | 0.17 | 1.2 |
| MgCl2 | 0.11 | 1.2 |
| NaH2PO4.H2O | 0.05 | 0.4 |
| Mannitol | 0.36 | 2.0 |
| D-Glucose | 1.80 | 10.0 |
| HEPES | 1.19 | 5.0 |
| Kanamycin sulphate | 0.10 | 0.2 |
| Item | Concentration (µmol/mL Buffer in Chamber) | Proportion (%) |
|---|---|---|
| Total SCFA 1 | 70.53 | - |
| Acetate | 50.36 | 71.40 |
| Propionate | 7.72 | 10.95 |
| Isobutyrate | 0.5 | 0.71 |
| Butyrate | 10.18 | 14.43 |
| Isovalerate | 0.52 | 0.74 |
| Valerate | 0.99 | 1.40 |
| Caproate | 0.26 | 0.37 |
| Item 1 | Mean 2 | SD 3 |
|---|---|---|
| Total SCFA | 0.35 | 0.18 |
| Acetate | 0.34 | 0.19 |
| Propionate | 0.01 | 0.02 |
| Item | Control | SCFA | SE | p-Value |
|---|---|---|---|---|
| Basal ISC (µA/cm2) | −37.5 | −32.4 | 6.68 | 0.395 |
| Post-additional ISC (µA/cm2) | −37.2 | 5.1 | 4.77 | <0.001 |
| Difference in ISC (µA/cm2) | 0.4 | 37.5 | 4.78 | <0.001 |
| Difference in ISC (% of basal ISC) | 1.0 | 126.8 | 4.77 | <0.001 |
| Basal GT (mS/cm2) | 20.0 | 19.9 | 1.50 | 0.947 |
| Post-additional GT (mS/cm2) | 19.0 | 11.9 | 1.21 | <0.001 |
| Difference in GT (mS/cm2) | −1.05 | −8.02 | 0.53 | <0.001 |
| Difference in GT (% of basal GT) | −5.1 | −40.8 | 1.60 | <0.001 |
| Gene of Interest | Mean | SE 1 |
|---|---|---|
| FFAR2 | 1.6 | 0.02 |
| FFAR3 | 2.1 | 0.02 |
| HCAR2 | 0.2 | 0.08 |
| HDAC1 | 3.8 | 0.01 |
| TLR2 | 2.6 | 0.03 |
| NLRP3 | 2.4 | 0.03 |
| EGFR | 3.6 | 0.02 |
| PPARG | 2.4 | 0.03 |
| SMCT1 | 3.8 | 0.03 |
| SMCT2 | 2.9 | 0.04 |
| MCT1 | 4.0 | 0.02 |
| ZO1 | 3.8 | 0.02 |
| OCLN | 3.9 | 0.03 |
| CLDN1 | 1.8 | 0.05 |
| CLDN4 | 4.9 | 0.04 |
| CDH1 | 4.1 | 0.02 |
| JAML | 0.3 | 0.04 |
| NFKB | 0.9 | 0.17 |
| BD3 | 2.3 | 0.02 |
| EP2C | 2.0 | 0.01 |
| IL10 | 2.4 | 0.02 |
| IL18 | 3.0 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzler-Zebeli, B.U.; Koger, S.; Sharma, S.; Sener-Aydemir, A.; Ruczizka, U.; Kreutzmann, H.; Ladinig, A. Short-Chain Fatty Acids Modulate Permeability, Motility and Gene Expression in the Porcine Fetal Jejunum Ex Vivo. Nutrients 2022, 14, 2524. https://doi.org/10.3390/nu14122524
Metzler-Zebeli BU, Koger S, Sharma S, Sener-Aydemir A, Ruczizka U, Kreutzmann H, Ladinig A. Short-Chain Fatty Acids Modulate Permeability, Motility and Gene Expression in the Porcine Fetal Jejunum Ex Vivo. Nutrients. 2022; 14(12):2524. https://doi.org/10.3390/nu14122524
Chicago/Turabian StyleMetzler-Zebeli, Barbara U., Simone Koger, Suchitra Sharma, Arife Sener-Aydemir, Ursula Ruczizka, Heinrich Kreutzmann, and Andrea Ladinig. 2022. "Short-Chain Fatty Acids Modulate Permeability, Motility and Gene Expression in the Porcine Fetal Jejunum Ex Vivo" Nutrients 14, no. 12: 2524. https://doi.org/10.3390/nu14122524
APA StyleMetzler-Zebeli, B. U., Koger, S., Sharma, S., Sener-Aydemir, A., Ruczizka, U., Kreutzmann, H., & Ladinig, A. (2022). Short-Chain Fatty Acids Modulate Permeability, Motility and Gene Expression in the Porcine Fetal Jejunum Ex Vivo. Nutrients, 14(12), 2524. https://doi.org/10.3390/nu14122524






