Fluoride Content of Matcha Tea Depending on Leaf Harvest Time and Brewing Conditions
Abstract
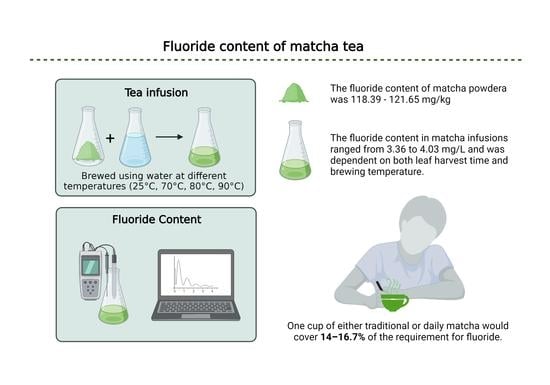
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Infusion
2.3. Determination of the Fluoride Content in Prepared Samples
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farooq, S.; Sehgal, A. Antioxidant Activity of Different Forms of Green Tea: Loose Leaf, Bagged and Matcha. Curr. Res. Nutr. Food Sci. J. 2018, 6, 35–40. [Google Scholar] [CrossRef]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J.A. Healthy Properties of Green and White Teas: An Update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, H.; Ema, K.; Kadokawa, O. Chemical Components of Matcha and Powdered Green Tea. J. Jpn. Soc. Cook. Sci. 2017, 50, 182–188. [Google Scholar] [CrossRef]
- Schröder, L.; Marahrens, P.; Koch, J.G.; Heidegger, H.; Vilsmeier, T.; Phan-Brehm, T.; Hofmann, S.; Mahner, S.; Jeschke, U.; Richter, D.U. Effects of Green Tea, Matcha Tea and Their Components Epigallocatechin Gallate and Quercetin on MCF-7 and MDA-MB-231 Breast Carcinoma Cells. Oncol. Rep. 2019, 41, 387–396. [Google Scholar] [PubMed]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green Tea Preparation and Its Influence on the Content of Bioactive Compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Patel, S.H. Camellia Sinensis: Historical Perspectives and Future Prospects. J. Agromed. 2005, 10, 57–64. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of Shading Intensity on Morphological and Color Traits and on Chemical Components of New Tea (Camellia Sinensis L.) Shoots under Direct Covering Cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Sharangi, A.B. Medicinal and Therapeutic Potentialities of Tea (Camellia Sinensis L.)—A Review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics Analysis Reveals the Compositional Differences of Shade Grown Tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Horie, H.; Nakamura, Y. Stress-Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials. Nutrients 2018, 10, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurleto, K.; Kurowski, G.; Laskowska, B.; Malinowska, M.; Sikora, E.; Vogt, O. Wpływ Warunków Parzenia Na Zawartość Antyoksydantow w Naparach Różnych Rodzajów Herbat. Wiad. Chem. 2013, 67, 11–12. [Google Scholar]
- Peluso, I.; Serafini, M. Antioxidants from Black and Green Tea: From Dietary Modulation of Oxidative Stress to Pharmacological Mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafini, M.; Del Rio, D.; Yao, D.N.; Bettuzzi, S.; Peluso, I. Health Benefits of Tea. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-0713-2. [Google Scholar]
- Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Protective Effects of Green Tea and Its Main Constituents against Natural and Chemical Toxins: A Comprehensive Review. Food Chem. Toxicol. 2017, 100, 115–137. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2021, 26, 85. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Głowniak, K. Catechin Composition and Antioxidant Activity of Black Teas in Relation to Brewing Time. J. AOAC Int. 2017, 100, 1694–1699. [Google Scholar] [CrossRef]
- Maleki, A.; Daraei, H.; Mohammadi, E.; Zandi, S.; Teymouri, P.; Mahvi, A.H.; Gharibi, F. Daily Fluoride Intake from Iranian Green Tea: Evaluation of Various Flavorings on Fluoride Release. Environ. Health Insights 2016, 10, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Kolasa-Wołosiuk, A.; Tarnowski, M.; Baranowska-Bosiacka, I.; Gutowska, I. Long-Term Exposure to Fluoride as a Factor Promoting Changes in the Expression and Activity of Cyclooxygenases (COX1 and COX2) in Various Rat Brain Structures. NeuroToxicology 2019, 74, 81–90. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a Factor Initiating and Potentiating Inflammation in THP1 Differentiated Monocytes/Macrophages. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2015, 29, 1661–1668. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Siennicka, A. Fluoride and generation of pro-inflammatory factors in human macrophages. Res. Rep. Fluoride 2011, 44, 125–134. [Google Scholar]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular Mechanisms of Fluoride Toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Chlubek, D. Fluoride and Oxidative Stress. Fluoride 2003, 36, 217–228. [Google Scholar]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Infusions from edible flowers as a source of fluoride in diet. Fluoride 2019, 52, 319–329. [Google Scholar]
- Belete, Y.; Chandravanshi, B.S.; Zewgea, F. Levels of the fluoride ion in six traditional alcoholic fermented beverages commonly consumed in Ethiopia. Fluoride 2014, 50, 79. [Google Scholar]
- Łukomska, A.; Jakubczyk, K.; Maciejewska, D.; Baranowska-Bosiacka, I.; Janda, K.; Goschorska, M.; Chlubek, D.; Bosiacka, B.; Gutowska, I. The Fluoride Content of Yerba Mate Depending on the Country of Origin and the Conditions of the Infusion. Biol. Trace Elem. Res. 2015, 167, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.; Wong, M.H. Aluminium and Fluoride Concentrations of Three Tea Varieties Growing at Lantau Island, Hong Kong. Environ. Geochem. Health 2003, 25, 219–232. [Google Scholar] [CrossRef]
- Emekli-Alturfan, E.; Yarat, A.; Akyuz, S. Fluoride Levels in Various Black Tea, Herbal and Fruit Infusions Consumed in Turkey. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 1495–1498. [Google Scholar] [CrossRef]
- Chan, J.T.; Koh, S.H. Fluoride Content in Caffeinated, Decaffeinated and Herbal Teas. Caries Res. 1996, 30, 88–92. [Google Scholar] [CrossRef]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of Fluoride Concentration and Daily Intake by Human from Tea and Herbal Infusions. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 1055–1061. [Google Scholar] [CrossRef]
- Gupta, P.; Sandesh, N. Estimation of Fluoride Concentration in Tea Infusions, Prepared from Different Forms of Tea, Commercially Available in Mathura City. J. Int. Soc. Prev. Community Dent. 2012, 2, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolska, J.; Janda, K.; Jakubczyk, K.; Szymkowiak, M.; Chlubek, D.; Gutowska, I. Levels of Antioxidant Activity and Fluoride Content in Coffee Infusions of Arabica, Robusta and Green Coffee Beans in According to Their Brewing Methods. Biol. Trace Elem. Res. 2017, 179, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esfehani, M.; Ghasemzadeh, S.; Mirzadeh, M. Comparison of Fluoride Ion Concentration in Black, Green and White Tea Research Article. Int. J. Ayurvedic Med. 2018, 9, 263–265. [Google Scholar] [CrossRef]
- Dębia, K.; Janda, K.; Siwiec, E.; Wolska, J. Baranowska-Bosiacka Do Brewing Temperature and the Morphological Part of the Ground Elder Plant Have an Influence on the Fluoride Content of Ground Elder Infusions? Available online: https://www.researchgate.net/publication/326840008_Do_brewing_temperature_and_the_morphological_part_of_the_ground_elder_plant_have_an_influence_on_the_fluoride_content_of_ground_elder_infusions (accessed on 14 January 2020).
- Trautner, K.; Siebert, G. An Experimental Study of Bio-Availability of Fluoride from Dietary Sources in Man. Arch. Oral Biol. 1986, 31, 223–228. [Google Scholar] [CrossRef]
- Regelson, S.; Dehghan, M.; Tantbirojn, D.; Almoazen, H. Evaluation of Fluoride Levels in Commercially Available Tea in the United States. Gen. Dent. 2021, 69, 17–20. [Google Scholar]
- Satou, R.; Oka, S.; Sugihara, N. Risk Assessment of Fluoride Daily Intake from Preference Beverage. J. Dent. Sci. 2021, 16, 220–228. [Google Scholar] [CrossRef]
- Štepec, D.; Tavčar, G.; Ponikvar-Svet, M. Surprisingly High Fluorine Content in Some Exotic Superfoods. J. Fluor. Chem. 2020, 234, 109521. [Google Scholar] [CrossRef]
| Fluoride Content in Matcha Powder [mg/kg] | |||||
|---|---|---|---|---|---|
| Traditional | Daily | ||||
| 118.39 * | ± | 0.10 | 121.65 * | ± | 2.39 |
| Fluoride Content in Matcha Tea [mg/L] | ||||||
|---|---|---|---|---|---|---|
| Temp. | Traditional | Daily | ||||
| 25 °C A | 3.36 *,B,D | ± | 0.11 | 3.38 *,B,D | ± | 0.22 |
| 70 °C B | 3.96 *,A | ± | 0.26 | 4.03 *,A | ± | 0.24 |
| 80 °C C | 3.77 | ± | 0.02 | 3.67 | ± | 0.33 |
| 90 °C D | 3.95 *,A | ± | 0.62 | 4.00 *,A | ± | 0.05 |
| Temp. | Traditional Matcha Tea Cups Per Day (1 cup = 125 mL) | |||||
|---|---|---|---|---|---|---|
| Fluoride Content [mg] | Recommended Dietary Allowances (RDA) [%] | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 25 °C | 0.42 | 0.84 | 1.26 | 14.0 | 28.0 | 42.0 |
| 70 °C | 0.50 | 1.0 | 1.5 | 16.7 | 33.4 | 66.8 |
| 80 °C | 0.47 | 0.94 | 1.41 | 15.7 | 31.4 | 47.5 |
| 90 °C | 0.49 | 0.98 | 1.47 | 16.3 | 32.6 | 48.9 |
| Temp. | Daily Matcha Tea Cups Per Day (1 cup = 125 mL) | |||||
|---|---|---|---|---|---|---|
| Fluoride Content [mg] | Recommended Dietary Allowances (RDA) [%] | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 25 °C | 0.42 | 0.84 | 1.26 | 14.0 | 28.0 | 42.0 |
| 70 °C | 0.50 | 1.0 | 1.5 | 16.7 | 33.4 | 66.8 |
| 80 °C | 0.46 | 0.92 | 1.38 | 15.3 | 31.0 | 45.9 |
| 90 °C | 0.50 | 1.0 | 1.5 | 16.7 | 33.4 | 66.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, K.; Ligenza, A.; Gutowska, I.; Janda-Milczarek, K. Fluoride Content of Matcha Tea Depending on Leaf Harvest Time and Brewing Conditions. Nutrients 2022, 14, 2550. https://doi.org/10.3390/nu14122550
Jakubczyk K, Ligenza A, Gutowska I, Janda-Milczarek K. Fluoride Content of Matcha Tea Depending on Leaf Harvest Time and Brewing Conditions. Nutrients. 2022; 14(12):2550. https://doi.org/10.3390/nu14122550
Chicago/Turabian StyleJakubczyk, Karolina, Alicja Ligenza, Izabela Gutowska, and Katarzyna Janda-Milczarek. 2022. "Fluoride Content of Matcha Tea Depending on Leaf Harvest Time and Brewing Conditions" Nutrients 14, no. 12: 2550. https://doi.org/10.3390/nu14122550
APA StyleJakubczyk, K., Ligenza, A., Gutowska, I., & Janda-Milczarek, K. (2022). Fluoride Content of Matcha Tea Depending on Leaf Harvest Time and Brewing Conditions. Nutrients, 14(12), 2550. https://doi.org/10.3390/nu14122550