Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Spontaneous Activity Measurement
2.3. Effort and Endurance Exercise Test
2.4. Anatomical Measurements and Histological Stanning
2.5. Study of Mitochondrial Function
2.6. RNA Isolation, Reverse Transcription, and Real-Time Quantitative PCR
2.7. Western Blotting and Antibodies
2.8. Statistical Analyses
3. Results
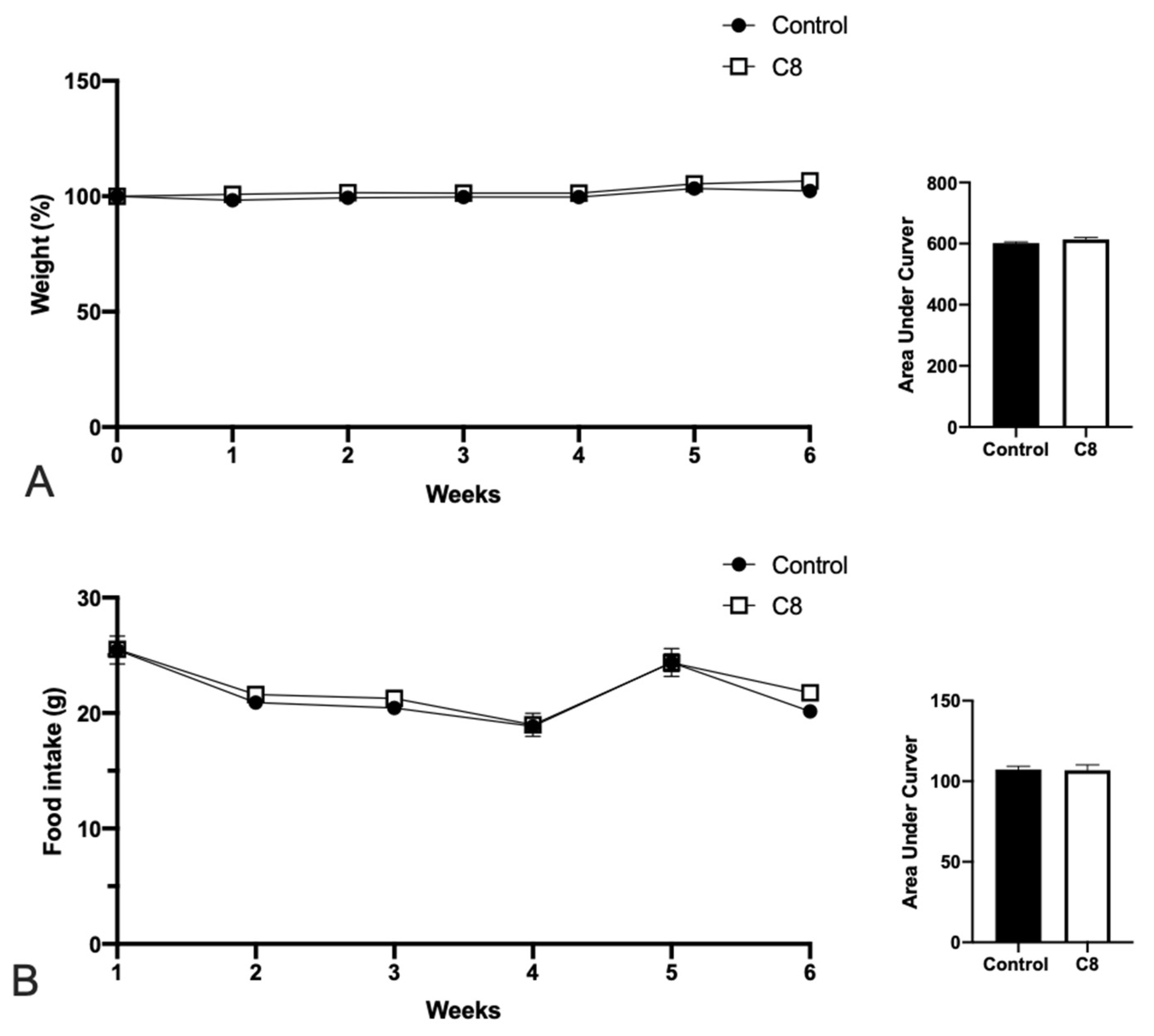
3.1. C8 Enrichment Did Not Affect Body Weight
3.2. C8 Increased Endurance Capacity and Spontaneous Activity
3.3. C8 Enrichment Increased Mitochondrial Biogenesis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bach, A.C.; Ingenbleek, Y.; Frey, A. The usefulness of dietary medium-chain triglycerides in body weight control: Fact or fancy? J. Lipid Res. 1996, 37, 708–726. [Google Scholar] [CrossRef]
- Berning, J.R. The Role of Medium-Chain Triglycerides in Exercise. Int. J. Sport Nutr. 1996, 6, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Bournat, J.C.; Brown, C.W. Mitochondrial Dysfunction in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.M. Ketogenic low-CHO, high-fat diet: The future of elite endurance sport? J. Physiol. 2021, 599, 819–843. [Google Scholar] [CrossRef] [PubMed]
- Charlot, A.; Zoll, J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Collège des Enseignants de Nutrition. Nutrition: Enseignement Intégré—UE Nutrition; Elsevier Masson: Paris, France, 2014. [Google Scholar]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E268–E275. [Google Scholar] [CrossRef]
- Erickson, A.; Moreau, R. The regulation of FGF21 gene expression by metabolic factors and nutrients. Horm. Mol. Biol. Clin. Investig. 2017, 30. [Google Scholar] [CrossRef]
- Fushiki, T.; Matsumoto, K.; Inoue, K.; Kawada, T.; Sugimoto, E. Swimming endurance capacity of mice is increased by chronic consumption of medium-chain triglycerides. J. Nutr. 1995, 125, 531–539. [Google Scholar] [CrossRef]
- Galpin, A.J.; Raue, U.; Jemiolo, B.; Trappe, T.A.; Harber, M.P.; Minchev, K.; Trappe, S. Human skeletal muscle fiber type specific protein content. Anal. Biochem. 2012, 425, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Goedecke, J.H.; Christie, C.; Wilson, G.; Dennis, S.C.; Noakes, T.D.; Hopkins, W.G.; Lambert, E.V. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism 1999, 48, 1509–1517. [Google Scholar] [CrossRef]
- Goedecke, J.H.; Clark, V.R.; Noakes, T.D.; Lambert, E.V. The Effects of Medium-Chain Triacylglycerol and Carbohydrate Ingestion on Ultra-Endurance Exercise Performance. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, P.D.; Bayly, W.M.; Hodgson, D.R. Exercise intensity, training, diet, and lactate concentration in muscle and blood. Med. Sci. Sports Exerc. 1986, 18, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Hämäläinen, N.; Pette, D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J. Histochem. Cytochem. 1993, 41, 733–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Hoppeler, H.; Baum, O.; Lurman, G.; Mueller, M. Molecular Mechanisms of Muscle Plasticity with Exercise. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; Volume 1, pp. 1383–1412. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef] [Green Version]
- Howlett, R.A.; Gonzalez, N.C.; Wagner, H.E.; Fu, Z.; Britton, S.L.; Koch, L.G.; Wagner, P.D. Selected Contribution: Skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J. Appl. Physiol. 2003, 94, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Jäger, S.; Handschin, C.; St.-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A. A Step Towards Personalized Sports Nutrition: Carbohydrate Intake During Exercise. Sports Med. 2014, 44 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeukendrup, A.E.; Aldred, S. Fat supplementation, health, and endurance performance. Nutrition 2004, 20, 678–688. [Google Scholar] [CrossRef]
- Jeukendrup, A.; Saris, W.; Wagenmakers, A. Fat Metabolism During Exercise: A Review—Part II: Regulation of Metabolism and the Effects of Training. Int. J. Sports Med. 1998, 19, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-S.; Joe, Y.; Choi, H.-S.; Back, S.H.; Park, J.W.; Chung, H.T.; Roh, E.; Kim, M.-S.; Ha, T.Y.; Yu, R. Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J. Inflamm. 2019, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Knottnerus, S.J.; van Harskamp, D.; Schierbeek, H.; Bleeker, J.C.; Crefcoeur, L.L.; Ferdinandusse, S.; van Goudoever, J.B.; Houtkooper, R.H.; Ijlst, L.; Langeveld, M.; et al. Exploring the metabolic fate of medium-chain triglycerides in healthy individuals using a stable isotope tracer. Clin. Nutr. 2021, 40, 1396–1404. [Google Scholar] [CrossRef]
- Koh, H.-J.; Brandauer, J.; Goodyear, L.J. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Lambert, E.V.; Goedecke, J.H.; van Zyl, C.; Murphy, K.; Hawley, J.A.; Dennis, S.C.; Noakes, T.D. High-Fat Diet versus Habitual Diet Prior to Carbohydrate Loading: Effects on Exercise Metabolism and Cycling Performance. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 209–225. [Google Scholar] [CrossRef]
- Loon, L.J.C.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A.J.M. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Loyd, C.; Magrisso, I.J.; Haas, M.; Balusu, S.; Krishna, R.; Itoh, N.; Sandoval, D.A.; Perez-Tilve, D.; Obici, S.; Habegger, K.M. Fibroblast growth factor 21 is required for beneficial effects of exercise during chronic high-fat feeding. J. Appl. Physiol. 2016, 121, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Lyons, C.N.; Mathieu-Costello, O.; Moyes, C.D. Regulation of Skeletal Muscle Mitochondrial Content During Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Lyudinina, A.Y.; Ivankova, G.E.; Bojko, E.R. Priority use of medium-chain fatty acids during high-intensity exercise in cross-country skiers. J. Int. Soc. Sports Nutr. 2018, 15, 57. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, J.; Tselykh, T.V.; Maiorana, F.; Eriksson, O.; Do, H.T.; Mudò, G.; Korhonen, L.T.; Belluardo, N.; Lindholm, D. Fibroblast growth factor-21 enhances mitochondrial functions and increases the activity of PGC-1α in human dopaminergic neurons via Sirtuin-1. SpringerPlus 2014, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Malapaka, R.R.V.; Khoo, S.; Zhang, J.; Choi, J.H.; Zhou, X.E.; Xu, Y.; Gong, Y.; Li, J.; Yong, E.-L.; Chalmers, M.J.; et al. Identification and Mechanism of 10-Carbon Fatty Acid as Modulating Ligand of Peroxisome Proliferator-activated Receptors. J. Biol. Chem. 2012, 287, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Mallard, J.; Hucteau, E.; Charles, A.; Bender, L.; Baeza, C.; Pélissie, M.; Trensz, P.; Pflumio, C.; Kalish-Weindling, M.; Gény, B.; et al. Chemotherapy impairs skeletal muscle mitochondrial homeostasis in early breast cancer patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.H.; Dalsky, G.P.; Hurley, B.F.; Matthews, D.E.; Bier, D.M.; Hagberg, J.M.; Rogers, M.A.; King, D.S.; Holloszy, J.O. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 1993, 265, E708–E714. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brown, S.H.J.; Small, L.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J. Lipid Res. 2013, 54, 3322–3333. [Google Scholar] [CrossRef] [Green Version]
- Moxnes, J.F.; Sandbakk, Ø. The kinetics of lactate production and removal during whole-body exercise. Theor. Biol. Med. Model. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Y.-Q.; Ling, P.-R.; Qu, J.Z.; Bistrian, B.R. Effects of Medium-Chain Triglycerides, Long-Chain Triglycerides, or 2-Monododecanoin on Fatty Acid Composition in the Portal Vein, Intestinal Lymph, and Systemic Circulation in Rats. J. Parenter. Enter. Nutr. 2008, 32, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.-X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARδ Agonists Are Exercise Mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Pendergast, D.R.; Leddy, J.J.; Venkatraman, J.T. A Perspective on Fat Intake in Athletes. J. Am. Coll. Nutr. 2000, 19, 345–350. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. FGF21 activates AMPK signaling: Impact on metabolic regulation and the aging process. J. Mol. Med. 2017, 95, 123–131. [Google Scholar] [CrossRef]
- Sander, H.; Wallace, S.; Plouse, R.; Tiwari, S.; Gomes, A.V. Ponceau S waste: Ponceau S staining for total protein normalization. Anal. Biochem. 2019, 575, 44–53. [Google Scholar] [CrossRef]
- Shang, H.; Xia, Z.; Bai, S.; Zhang, H.; Gu, B.; Wang, R. Downhill Running Acutely Elicits Mitophagy in Rat Soleus Muscle. Med. Sci. Sports Exerc. 2019, 51, 1396–1403. [Google Scholar] [CrossRef]
- Sidossis, L.S.; Wolfe, R.R.; Coggan, A.R. Regulation of fatty acid oxidation in untrained vs. Trained men during exercise. Am. J. Physiol. Endocrinol. Metab. 1998, 274, E510–E515. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Jones, P.J.H. Physiological Effects of Medium-Chain Triglycerides: Potential Agents in the Prevention of Obesity. J. Nutr. 2002, 132, 329–332. [Google Scholar] [CrossRef]
- Takikawa, M.; Kumagai, A.; Hirata, H.; Soga, M.; Yamashita, Y.; Ueda, M.; Ashida, H.; Tsuda, T. 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5’-AMP-activated protein kinase in L6 myotubes and mice. Mol. Nutr. Food Res. 2013, 57, 1794–1802. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. [Google Scholar] [CrossRef]
- Lambert, E.; Hawley, J.A.; Goedecke, J.; Noakes, T.D.; Dennis, S.C. Nutritional strategies for promoting fat utilization and delaying the onset of fatigue during prolonged exercise. J. Sports Sci. 1997, 15, 315–324. [Google Scholar] [CrossRef]
- Venables, M.C.; Achten, J.; Jeukendrup, A.E. Determinants of fat oxidation during exercise in healthy men and women: A cross-sectional study. J. Appl. Physiol. 2005, 98, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1α. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, M.; Puntschart, A.; Howald, H.; Mueller, B.; Mannhart, C.; Gfeller-Tuescher, L.; Mullis, P.; Hoppeler, H. Effects of Dietary Fat on Muscle Substrates, Metabolism, and Performance in Athletes. Med. Sci. Sports Exerc. 2003, 35, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Qin, Z.; Wang, P.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Han, Y.; Xu, J.; Huang, W.; Li, Z. Medium Chain Triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism. PLoS ONE 2018, 13, e0191182. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-X.; Zhang, C.-L.; Yu, R.T.; Cho, H.K.; Nelson, M.C.; Bayuga-Ocampo, C.R.; Ham, J.; Kang, H.; Evans, R.M. Regulation of Muscle Fiber Type and Running Endurance by PPARδ. PLoS Biol. 2004, 2, e294. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.J.; Steinberg, G.R.; Chen, Z.-P.; Kemp, B.E.; Febbraio, M.A. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J. Physiol. 2006, 574, 139–147. [Google Scholar] [CrossRef]







| Diet Composition (g/kg) | Control Diet | C8 Diet |
|---|---|---|
| Acid Casein | 200.0 | 200.0 |
| Corn starch | 367.5 | 367.5 |
| Maltodextrin | 132.0 | 132.0 |
| Sugar | 100.0 | 100.0 |
| Cellulose (Arbocel B600) | 50.0 | 50.0 |
| Soya oil | 100.0 | 20.0 |
| Caprylic acid (Neobee 895®) | - | 80.0 |
| Vitamin mix AIN-93 | 10.0 | 10.0 |
| Mineral mix AIN-93G | 35.0 | 35.0 |
| L-Cystine | 3.0 | 3.0 |
| Choline bitartrate | 2.5 | 2.5 |
| Tert.butyl hydroquinone | 0.014 | 0.014 |
| Total | 1000.0 | 1000.0 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 36B4 | GAGGAATCAGATGAGGATATGGGA | AAGCAGGCTGACTTGGTTGC |
| ACADL | GAAGATGTCCGATTGCCAGC | AGTTTATGCTGCACCGTCTGT |
| ACADM | ATGACAAAAGCGGGGAGTACC | CCATACGCCAACTCTTCGGT |
| PFK | GGTTTGGAAGCCTCTCCTCC | GCAGCATTCATACCTTGGGC |
| PGC1a | GACCGCTTTGAAGTTTTTGG | AGCAGGGTCAAAATCGTCTG |
| PGC1b | AGATTGTAGAGTGCCAGGTGCTGA | TGCTCTGAACACCGGAAGGTGATA |
| PPARa | ACTACGGAGTTCACGCATGTG | TTGTCGTACACCAGCTTCAGC |
| TFAM | CCGTATTGCGTGAGACGAAC | TGAAAGTTTTGCATCTGGGTGT |
| COX4 | GTCTTGGTCTTCCGGTTGCG | TTCACAACACTCCCATGTGCT |
| CS | CGGTTTGTCTACCCTTCCCC | GGCAGGATGAGTTCTTGGCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlot, A.; Morel, L.; Bringolf, A.; Georg, I.; Charles, A.-L.; Goupilleau, F.; Geny, B.; Zoll, J. Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice. Nutrients 2022, 14, 2721. https://doi.org/10.3390/nu14132721
Charlot A, Morel L, Bringolf A, Georg I, Charles A-L, Goupilleau F, Geny B, Zoll J. Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice. Nutrients. 2022; 14(13):2721. https://doi.org/10.3390/nu14132721
Chicago/Turabian StyleCharlot, Anouk, Lucas Morel, Anthony Bringolf, Isabelle Georg, Anne-Laure Charles, Fabienne Goupilleau, Bernard Geny, and Joffrey Zoll. 2022. "Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice" Nutrients 14, no. 13: 2721. https://doi.org/10.3390/nu14132721
APA StyleCharlot, A., Morel, L., Bringolf, A., Georg, I., Charles, A.-L., Goupilleau, F., Geny, B., & Zoll, J. (2022). Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice. Nutrients, 14(13), 2721. https://doi.org/10.3390/nu14132721







