Micronutrient Deficiencies Presenting with Optic Disc Swelling Associated with or without Intracranial Hypertension: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Data Extraction
3. Results
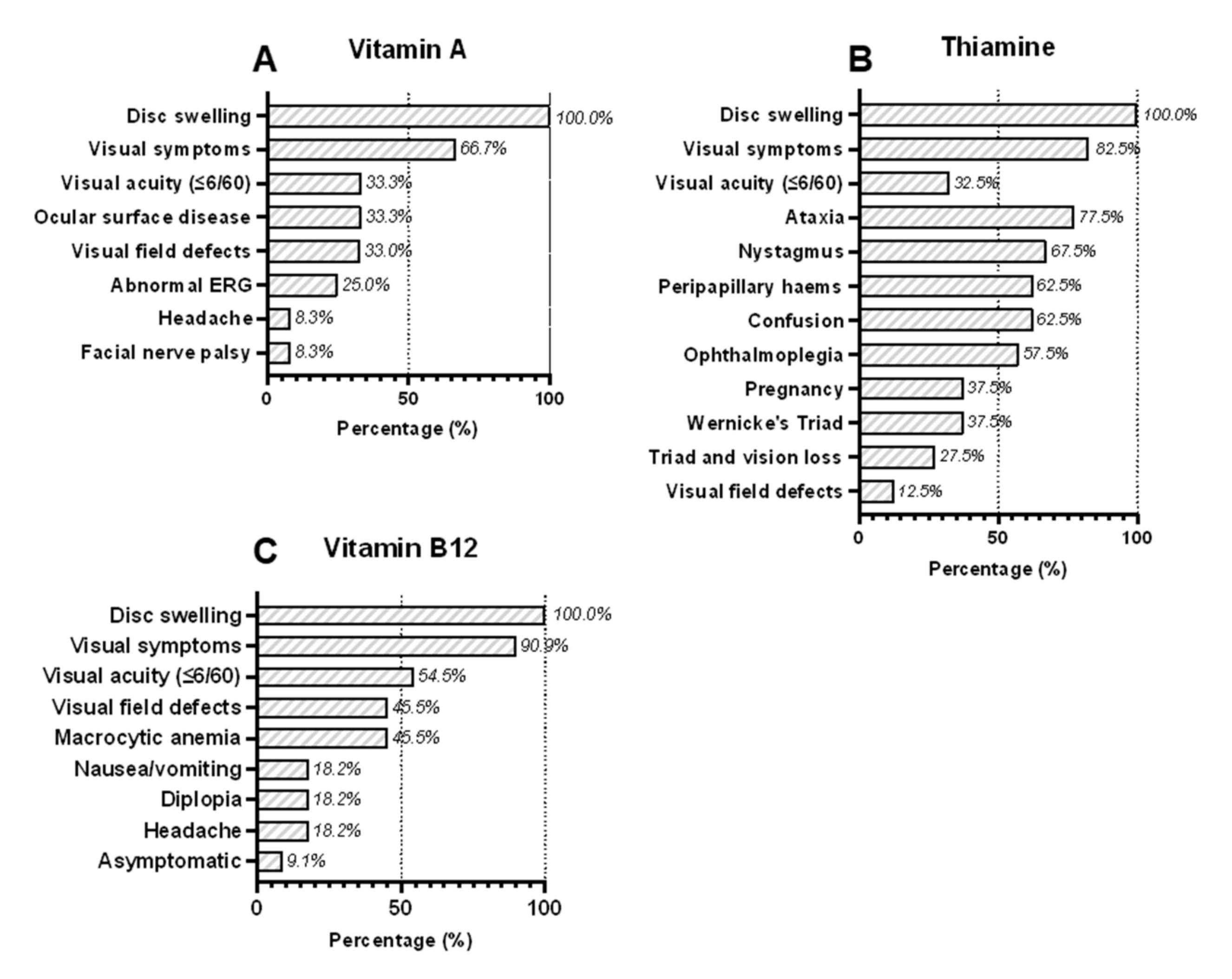
3.1. Vitamin A Deficiency
3.2. Vitamin B1 (Thiamine) Deficiency
3.3. Vitamin B12 (Cobalamin) Deficiency
3.4. Presumed Nutritional Deficiencies
3.5. Risk of Bias Assessment
4. Discussion
5. Conclusions
Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Best, J.; Silvestri, G.; Burton, B.; Foot, B.; Acheson, J. The Incidence of Blindness Due to Idiopathic Intracranial Hypertension in the UK. Open Ophthalmol. J. 2013, 7, 26–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesner, D.; Rosenman, R.; Lobb, B.M.; Tanne, E. Idiopathic Intracranial Hypertension in the USA: The Role of Obesity in Establishing Prevalence and Healthcare Costs: Prevalence and Healthcare Costs in IIH. Obes. Rev. 2011, 12, e372–e380. [Google Scholar] [CrossRef] [PubMed]
- Mollan, S.P.; Aguiar, M.; Evison, F.; Frew, E.; Sinclair, A.J. The Expanding Burden of Idiopathic Intracranial Hypertension. Eye 2019, 33, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, D.I.; Liu, G.T.; Digre, K.B. Revised Diagnostic Criteria for the Pseudotumor Cerebri Syndrome in Adults and Children. Neurology 2013, 81, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; Uldall, M.; Botfield, H.; Cato, L.D.; Miah, M.A.; Hassan-Smith, G.; Jensen, R.H.; Gonzalez, A.M.; Sinclair, A.J. Idiopathic Intracranial Hypertension, Hormones, and 11β-Hydroxysteroid Dehydrogenases. J. Pain Res. 2016, 2016, 223–232. [Google Scholar]
- Kupersmith, M.J.; Gamell, L.; Turbin, R.; Peck, V.; Spiegel, P.; Wall, M. Effects of Weight Loss on the Course of Idiopathic Intracranial Hypertension in Women. Neurology 1998, 50, 1094–1098. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, M.; Liu, B.; Du, Y.; Rong, S.; Xu, G.; Snetselaar, L.G.; Bao, W. Inverse Association between Serum Vitamin B12 Concentration and Obesity Among Adults in the United States. Front. Endocrinol. 2019, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Maffoni, S.; De Giuseppe, R.; Stanford, F.C.; Cena, H. Folate Status in Women of Childbearing Age with Obesity: A Review. Nutr. Res. Rev. 2017, 30, 265–271. [Google Scholar] [CrossRef]
- Cepeda-Lopez, A.C.; Melse-Boonstra, A.; Zimmermann, M.B.; Herter-Aeberli, I. In Overweight and Obese Women, Dietary Iron Absorption Is Reduced and the Enhancement of Iron Absorption by Ascorbic Acid Is One-Half That in Normal-Weight Women. Am. J. Clin. Nutr. 2015, 102, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Rahimi, F.; Boschetti, S.; Devecchi, A.; De Francesco, A.; Mancino, M.V.; Toppino, M.; Morino, M.; Fanni, G.; Ponzo, V.; et al. Pre-Operative Micronutrient Deficiencies in Patients with Severe Obesity Candidates for Bariatric Surgery. J. Endocrinol. Investig. 2020, 44, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Rucker, J.C.; Vignal, C.; Crassard, I.; Katz, B.J.; Newman, N.J. Anemia and Papilledema. Am. J. Ophthalmol. 2003, 135, 437–446. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern Iron Replacement Therapy: Clinical and Pathophysiological Insights. Int. J. Hematol. 2018, 107, 16–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.W.; Waisberg, E.; Kwok, J.M.; Micieli, J.A. Anemia and Idiopathic Intracranial Hypertension: A Systematic Review and Meta-Analysis. J. Neuroophthalmol. 2022, 42, e78. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. Glob. Adv. Health Med. 2013, 2, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotan, G.; Goldstein, M.; Stolovitch, C.; Kesler, A. Pediatric Pseudotumor Cerebri Associated with Low Serum Levels of Vitamin A. J. Child Neurol. 2013, 28, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Osunkunle, O.A.; Purbrick, R.M.; Downes, S.M. Visual Loss in Raised Intracranial Pressure Associated with Severe Vitamin A Deficiency. Ann. Clin. Case Rep. 2017, 2, 1261. [Google Scholar]
- Lewis, C.D.; Traboulsi, E.I.; Rothner, A.D.; Jeng, B.H. Xerophthalmia and Intracranial Hypertension in an Autistic Child with Vitamin A Deficiency. J. Pediatr. Ophthalmol. Strabismus 2011, 48, e1–e3. [Google Scholar] [CrossRef]
- Kinlin, L.M.; Vresk, L.; Friedman, J.N. Vision Loss in a Child with Autism Spectrum Disorder. Paediatr. Child Health 2019, 24, 148–150. [Google Scholar] [CrossRef]
- McSweeney, N.; Johnson, N.; Moore, W.; Robinson, R. Optic Neuritis and Facial Nerve Palsies in Autism. Dev. Med. Child Neurol. 2009, 51, 33–34. [Google Scholar]
- Panozzo, G.; Babighian, S.; Bonora, A. Association of Xerophthalmia, Flecked Retina, and Pseudotumor Cerebri Caused by Hypovitaminosis A. Am. J. Ophthalmol. 1998, 125, 708–710. [Google Scholar] [CrossRef]
- Lucidi, V.; Di Capua, M.; Rosati, P.; Papadatou, B.; Castro, M. Benign Intracranial Hypertension in an Older Child with Cystic Fibrosis. Pediatr. Neurol. 1993, 9, 494–495. [Google Scholar] [CrossRef]
- Sulaiman, W.; Othman, A.; Mohamad, M.; Salleh, H.R.; Mushahar, L. Wernicke’s Encephalopthy Associated with Hyperemesis Gravidarum—A Case Report. Malays. J. Med. Sci. 2002, 9, 43–46. [Google Scholar]
- Namasivayam, B.; Arthanari, A.; Senthilkumaran, S.; Chellammal, P.; Siddharthan, V.; Manikam, R. Loss of Vision: A Rare Presentation in Hyperemesis Gravidarum Induced Wernicke’s Encephalopathy. Internet J. Genom. Proteom. 2012, 6, 3. [Google Scholar]
- Wilson, R.K.; Kuncl, R.W.; Corse, A.M. Wernicke’s Encephalopathy: Beyond Alcoholism. Nat. Clin. Pract. Neurol. 2006, 2, 54–58. [Google Scholar] [CrossRef]
- Ferdinands, M.D.; Seneviratne, J.; White, O. Visual Deterioration in Hyperemesis Gravidarum. Med. J. Aust. 2005, 182, 585–586. [Google Scholar] [CrossRef]
- Mathew, N.R.; Menon, S.G.; Mathew, M. Ocular Manifestations in a Case of Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum. Oman J. Ophthalmol. 2018, 11, 85–87. [Google Scholar] [PubMed]
- Mumford, C.J. Papilloedema Delaying Diagnosis of Wernicke’s Encephalopathy in a Comatose Patient. Postgrad. Med. J. 1989, 65, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, V.V.; Prijesh, J.; Praveenkumar, R.; Saifudheen, K. Wernicke’s Encephalopathy Due to Hyperemesis Gravidarum: Clinical and Magnetic Resonance Imaging Characteristics. J. Postgrad. Med. 2016, 62, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Kantor, S.; Prakash, S.; Chandwani, J.; Gokhale, A.; Sarma, K.; Albahrani, M.J. Wernicke’s Encephalopathy Following Hyperemesis Gravidarum. Indian J. Crit. Care Med. 2014, 18, 164–166. [Google Scholar] [CrossRef] [Green Version]
- Di Gangi, S.; Gizzo, S.; Patrelli, T.S.; Saccardi, C.; D’Antona, D.; Nardelli, G.B. Wernicke’s Encephalopathy Complicating Hyperemesis Gravidarum: From the Background to the Present. J. Matern. Fetal Neonatal Med. 2012, 25, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Chiossi, G.; Neri, I.; Cavazzuti, M.; Basso, G.; Facchinetti, F. Hyperemesis Gravidarum Complicated by Wernicke Encephalopathy: Background, Case Report, and Review of the Literature. Obstet. Gynecol. Surv. 2006, 61, 255–268. [Google Scholar] [CrossRef]
- Tesfaye, S.; Achari, V.; Yang, Y.C.; Harding, S.; Bowden, A.; Vora, J.P. Pregnant, Vomiting, and Going Blind. Lancet 1998, 352, 1594. [Google Scholar] [CrossRef]
- Mun-Wei, L.; Gayathri, G.; Kwang Hwee, G.; Ruban, K.; Suresh Kumar, V.; Shatriah, I. Optic Discs Swelling Procrastinates Wernicke’s Encephalopathy Associated with Hyperemesis Gravidarum: A Case Report and Review of Literature. Cureus 2018, 10, e2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palakkuzhiyil, N.; Rehiman, S.; Manoj, P.P.B.; Hameed, S.; Uvais, N.A. Visual Loss and Optic Neuropathy Associated with Wernicke’s Encephalopathy in Hyperemesis Gravidarum. J. Fam. Med. Prim. Care 2019, 8, 1243–1245. [Google Scholar]
- Galloway, P.J. Wernicke’s Encephalopathy and Hyperemesis Gravidarum. BMJ 1992, 305, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitra, S.; Lath, K.V.S. Wernicke’s Encephalopathy with Visual Loss in a Patient with Hyperemesis Gravidarum. J. Assoc. Physicians India 2012, 60, 53–56. [Google Scholar] [PubMed]
- Lulla, P.D.; Lu, L. The Deficient B-Vitamin, Causing a “Very-Very” Bizzare Presentation. J. Gen. Intern. Med. 2011, 26, S537–S538. [Google Scholar]
- Sia, P.I.; Sia, D.I.T.; Crompton, J.L.; Casson, R.J. Nerve Fiber Layer Infarcts in Thiamine Deficiency. J. Neuro Ophthalmol. 2015, 35, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Taniguchi, A.; Sogabe, Y. Significant Visual Impairment in an Alcoholic Patient. J. Gen. Intern. Med. 2019, 34, S634. [Google Scholar]
- Lineback, C.; Brahmbhatt, N.; Abbott, S. A Case of Non-Alcoholic Wernicke’s Encephalopathy and Dry Beri-Beri with GI Involvement Following a Period of Prolonged Gastrointestinal Illness. Neurology 2020, 94, 1266. [Google Scholar]
- Gratton, S.M.; Lam, B.L. Visual Loss and Optic Nerve Head Swelling in Thiamine Deficiency without Prolonged Dietary Deficiency. Clin. Ophthalmol. 2014, 8, 1021–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth-Mysore, C.; Marky, B. Oculomotor Dysfunction Due to Idiopathic Intracranial Hypertension and Thiamine Deficiency. Neurology 2014, 82, 302. [Google Scholar]
- Lukas, R.V.; Piantino, J.; Nichols, J.; Cohen, E.; Haraf, D.J.; Rezania, K. Thiamine Deficiency Presenting with Encephalopathy and Optic Neuropathy after Chemoradiation for Tongue Cancer. Neurology 2010, 74, A466. [Google Scholar]
- Cooke, C.A.; Hicks, E.; Page, A.B.; Mc Kinstry, S. An Atypical Presentation of Wernicke’s Encephalopathy in an 11-Year-Old Child. Eye 2006, 20, 1418–1420. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Kumanomido, T. Optic Neuropathy from Thiamine Deficiency. Intern. Med. 1997, 36, 532. [Google Scholar] [CrossRef] [Green Version]
- Yeh, W.Y.; Lian, L.M.; Chang, A.; Cheng, C.K. Thiamine-Deficient Optic Neuropathy Associated with Wernicke’s Encephalopathy in Patients with Chronic Diarrhea. J. Formos. Med. Assoc. 2013, 112, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Sparacia, G.; Banco, A.; Lagalla, R. Reversible MRI Abnormalities in an Unusual Paediatric Presentation of Wernicke’s Encephalopathy. Pediatr. Radiol. 1999, 29, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Lee, A.G.; Holstein, S.A.; Warner, J.E.A. You Are What You Eat. Surv. Ophthalmol. 2005, 50, 389–393. [Google Scholar] [CrossRef]
- Kamasak, T.; Kul, S.; Tusat, M.; Ozgun, N.; Cansu, A. A Case of Wernicke Encephalopathy Developing after Ileal Bypass Surgery. Pediatr. Emerg. Care 2018, 34, e223–e225. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.M.; Silva, K.P.; Vidal, G.; Silva, A.F.; Domingues, R.C.; Berditchevsky, C.R. Early Diagnosis of Pediatric Wernicke’s Encephalopathy. Pediatr. Neurol. 1999, 20, 289–294. [Google Scholar] [CrossRef]
- Miller, D. Blind, Deaf and Confused: An Unusual Case of Wernicke’s Encephalopathy in a Young Adult. Neurology 2016, 86, 12. [Google Scholar]
- Kramer, L.D.; Locke, G.E. Wernicke’s Encephalopathy. Complication of Gastric Plication. J. Clin. Gastroenterol. 1987, 9, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Longmuir, R.; Lee, A.G.; Rouleau, J. Visual Loss Due to Wernicke Syndrome Following Gastric Bypass. Semin. Ophthalmol. 2007, 22, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lawton, A.W.; Frisard, N.E. Visual Loss, Retinal Hemorrhages, and Optic Disc Edema Resulting from Thiamine Deficiency Following Bariatric Surgery Complicated by Prolonged Vomiting. Ochsner J. 2017, 17, 112–114. [Google Scholar] [PubMed]
- Gokce, M.; Bulbuloglu, E.; Tuncel, D.; Ozdemir, G.; Kale, I.T. Nonalcoholic Wernicke’s Encephalopathy with Prominent Astasia and Optic Neuropathy. Med. Princ. Pract. 2005, 14, 438–440. [Google Scholar] [CrossRef]
- Bohnsack, B.L.; Patel, S.S. Peripapillary Nerve Fiber Layer Thickening, Telangiectasia, and Retinal Hemorrhages in Wernicke Encephalopathy. J. Neuroophthalmol. 2010, 30, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Carbunar, O. Wernicke Encephalopathy Mimicking Idiopathic Intracranial Hypertension. Neurology 2019, 92, 9–22. [Google Scholar]
- Hwang, P.; Chalia, M. Eyes Make Brain Go Crazy. Neurology 2019, 92, 9–44. [Google Scholar]
- Serlin, T.; Moisseiev, E. Fundus Findings in Wernicke Encephalopathy. Case Rep. Ophthalmol. 2017, 8, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.; Usmani, H.A.; Khan, M.I.; Blakely, E.L.; Taylor, R.W.; Vassallo, G.; Ashworth, J. Bilateral Paediatric Optic Neuropathy Precipitated by Vitamin B12 Deficiency and a Novel Mitochondrial DNA Mutation. Int. Ophthalmol. 2013, 33, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, S.; Derman, O.; Dogan, M. A Pediatric Patient with Recurrent Pseudotumor Cerebri and Vitamin B 12 Deficiency. Pediatr. Hematol. Oncol. 2006, 23, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Monferrer-Adsuara, C.; Garcia-Villanueva, C.; Mata-Moret, L.; Ortiz-Salvador, M.; Remoli-Sargues, L.; Cervera-Taulet, E. Case Report: Nutritional and Toxic Optic Neuropathy: A Diagnostic Dilemma. Optom. Vis. Sci. 2020, 97, 477–481. [Google Scholar] [CrossRef]
- Incecik, F.; Herguner, M.O.; Karagun, B.; Altunbasak, S. Pseudotumor Cerebri Associated with Vitamin B12 Deficiency. Eur. J. Gen. Med. 2014, 11, 121–122. [Google Scholar] [CrossRef] [Green Version]
- Chiarello, F.; Marini, E.; Ballerini, A.; Ricca, V. Optic Neuropathy Due to Nutritional Deficiency in a Male Adolescent with Avoidant/Restrictive Food Intake Disorder: A Case Report. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2018, 23, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Singanamalla, B.; Madaan, P.; Saini, L.; Sankhyan, N. Vitamin B12 Deficiency: An Association or Etiology of Pseudotumor Cerebri in An Infant. Indian J. Pediatr. 2020, 87, 658–659. [Google Scholar] [CrossRef]
- Sethi, H.S.; Naik, M.; Gandhi, A. Megaloblastic Anemia and Bilateral Disc Edema: An Enigma… Have We Figured It out Yet? Taiwan J. Ophthalmol. 2020, 10, 71–75. [Google Scholar] [PubMed]
- Chu, C.; Scanlon, P. Vitamin B12 Deficiency Optic Neuropathy Detected by Asymptomatic Screening. BMJ Case Rep. 2011, bcr0220113823. [Google Scholar] [CrossRef] [Green Version]
- Ata, F.; Bint I Bilal, A.; Javed, S.; Shabir Chaudhry, H.; Sharma, R.; Fatima Malik, R.; Choudry, H.; Bhaskaran Kartha, A. Optic Neuropathy as a Presenting Feature of Vitamin B-12 Deficiency: A Systematic Review of Literature and a Case Report. Ann. Med. Surg. 2020, 60, 316–322. [Google Scholar] [CrossRef]
- Petramfar, P.; Hosseinzadeh, F.; Mohammadi, S.S. Pseudo-Foster Kennedy Syndrome as a Rare Presentation of Vitamin B12 Deficiency. Iran. Red Crescent Med. J. 2016, 18, e24610. Available online: https://sites.kowsarpub.com/ircmj/articles/16576.html (accessed on 29 September 2020). [CrossRef] [PubMed] [Green Version]
- Ornek, N.; Onaran, Z.; Ornek, K.; Buyuktortop, N. Bilateral Consecutive Optic Neuropathy in a Patient with Thrombophilia. BMJ Case Rep. 2013, bcr2013009389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, M.R.; Talbot, K. Functional Vitamin B12 Deficiency. Pract. Neurol. 2009, 9, 37–45. [Google Scholar] [CrossRef]
- Rani, U.; Imdad, A.; Beg, M. Rare Neurological Manifestation of Celiac Disease. Case Rep. Gastroenterol. 2015, 9, 200–205. [Google Scholar] [CrossRef]
- Pathmanandavel, K.; Gupta, S.; Dutt, S.; Wong, M.; Williams, A. Unusual Presentation of Coeliac Disease with Idiopathic Intracranial Hypertension. J. Paediatr. Child Health 2021, 57, 1321–1322. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Mullany, L.C.; Hurley, K.M.; Katz, J.; Black, R.E. Nutrition and Maternal, Neonatal, and Child Health. Semin. Perinatol. 2015, 39, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Picciano, M.F. Pregnancy and Lactation: Physiological Adjustments, Nutritional Requirements and the Role of Dietary Supplements. J. Nutr. 2003, 133, 1997S–2002S. [Google Scholar] [CrossRef]
- Margalit, I.; Cohen, E.; Goldberg, E.; Krause, I. Vitamin B12 Deficiency and the Role of Gender: A Cross-Sectional Study of a Large Cohort. Ann. Nutr. Metab. 2018, 72, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Atan, D. Challenges and Opportunities in the Diagnosis of Nutritional Optic Neuropathy. Expert Rev. Ophthalmol. 2020, 15, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Chiu, M.; Dillon, A.; Watson, S. Vitamin A Deficiency and Xerophthalmia in Children of a Developed Country. J. Paediatr. Child Health 2016, 52, 699–703. [Google Scholar] [CrossRef]
- Zayed, M.G.; Hickman, S.J.; Batty, R.; McCloskey, E.V.; Pepper, I.M. Unilateral Compressive Optic Neuropathy Due to Skull Hyperostosis Secondary to Nutritional Vitamin A Deficiency. Clin. Cases Miner. Bone Metab. 2015, 12, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.D.; Cook, C.C.H.; Guerrini, I.; Sheedy, D.; Harper, C.; Marshall, E.J. Review Wernicke’s Encephalopathy Revisited Translation of the Case History Section of the Original Manuscript by Carl Wernicke ‘Lehrbuch Der Gehirnkrankheiten Fur Aerzte and Studirende’ (1881) with a Commentary. Alcohol 2008, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wardener, H.D.; Lennox, B. Cerebral beriberi (wernicke’s encephalopathy): Review of 52 cases in a singapore prisoner-of-war hospital. Lancet 1947, 249, 11–17. [Google Scholar] [CrossRef]
- Pacei, F.; Tesone, A.; Laudi, N.; Laudi, E.; Cretti, A.; Pnini, S.; Varesco, F.; Colombo, C. The Relevance of Thiamine Evaluation in a Practical Setting. Nutrients 2020, 12, 2810. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.; Postma, A. Wernicke-Korsakoff Syndrome despite No Alcohol Abuse: A Summary of Systematic Reports. J. Neurol. Sci. 2021, 426, 117482. [Google Scholar] [CrossRef]
- Whitfield, K.C.; Bourassa, M.W.; Adamolekun, B.; Bergeron, G.; Bettendorff, L.; Brown, K.H.; Cox, L.; Fattal-Valevski, A.; Fischer, P.R.; Frank, E.L.; et al. Thiamine Deficiency Disorders: Diagnosis, Prevalence, and a Roadmap for Global Control Programs. Ann. N. Y. Acad. Sci. 2018, 1430, 3–43. [Google Scholar] [CrossRef]
- Zuccoli, G.; Cruz, D.S.; Bertolini, M.; Rovira, A.; Gallucci, M.; Carollo, C.; Pipitone, N. MR Imaging Findings in 56 Patients with Wernicke Encephalopathy: Nonalcoholics May Differ from Alcoholics. Am. J. Neuroradiol. 2009, 30, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Podell, E.R.; Marcell, P.D.; Stabler, S.P.; Allen, R.H. Neuropsychiatric Disorders Caused by Cobalamin Deficiency in the Absence of Anemia or Macrocytosis. N. Engl. J. Med. 1988, 318, 1720–1728. [Google Scholar] [CrossRef]
- Roman, G.C. An Epidemic in Cuba of Optic Neuropathy, Sensorineural Deafness, Peripheral Sensory Neuropathy and Dorsolateral Myeloneuropathy. J. Neurol. Sci. 1994, 127, 11–28. [Google Scholar] [CrossRef]
- Ott, M.; Werneke, U. Wernicke’s Encephalopathy—From Basic Science to Clinical Practice. Part 1: Understanding the Role of Thiamine. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320978106. [Google Scholar] [CrossRef]
| Exogenous factors | Antibiotics: tetracyclines and derivatives, quinolones, e.g., nalidixic acid Vitamin A and derivatives: isotretinoin, all-transretinoic acid Hormonal agents: corticosteroid withdrawal, oral combined contraceptive pill, levonorgestrel implant, levothyroxine, tamoxifen, growth hormone, danazol, testosterone Other: ciclosporin, lithium, indomethacin, cimetidine |
| Endogenous factors | Haematological: anaemia, polycythaemia, thrombocythaemia Venous outflow obstruction: cerebral venous sinus thrombosis, superior vena cava obstruction, increased right-sided heart pressure Renal: chronic kidney disease/renal failure Endocrine: obesity, Addison’s disease/adrenal insufficiency, Cushing’s syndrome, hypoparathyroidism, hypothyroidism, hyperthyroidism, polycystic ovary syndrome Respiratory: obstructive sleep apnoea syndrome, chronic obstructive pulmonary disease, psittacosis Autoimmune: systemic lupus erythematosus Nutritional: hypervitaminosis A, iron deficiency |
| Vitamin A (n = 12) | Vitamin B1 (n = 40) | Vitamin B12 (n = 11) | Presumed (n = 2) | Whole series (n = 65) | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| (i) Sex | |||||
| Male (M) (n, %) | 9M (75%) | 4M (10.0%) | 9M (81.8%) | 0M (0%) | 22M (33.9%) |
| Female (F) (n, %) | 3F (25%) | 36F (90.0%) | 2F (18.2%) | 2F (100%) | 43F (66.1%) |
| Pregnant females (n, %) | 0/3F (0%) | 15/36F (41.7%) | 0/2F (0%) | 0/2F (0%) | 15/43 (34.8%) |
| (ii) Mean age (range) | 10.2 (3–27 years) | 28.1 (11–56 years) | 23.0 (15 months–47 years) | 14.0 (14 years) | 23.4 (15 months–56 years) |
| Cause of deficiency | |||||
| (i) Malabsorption (n, %) | 4/12 (33.3%) | 15/40 (37.5%) | 0/11 (0%) | 2/2 (100%) | 21/65 (32.3%) |
| Post-surgical (n, %) | 1/12 (8.3%) | 11/15 (73.3%) | 0 (0%) | 0/2 (0%) | 12/65 (18.5%) |
| Gastro-intestinal pathology (n, %) | 3/12 (25.0%) | 4/15 (26.7%) | 0 (0%) | 2/2 (100%) | 9/65 (13.8%) |
| (ii) Dietary (%) | 8/12 (66.6%) | 25/40 (62.5%) | 9/11 (81.8%) | 0/2 (0%) | 42/65 (64.6%) |
| Hyperemesis gravidarum (n, %) | 0/8 (0%) | 15/25 (60.0%) | 0/10 (0%) | 0 (0%) | 15/65 (23.1%) |
| Nausea and vomiting (n, %) | 0/8 (0%) | 5/25 (20.0%) | 0/10 (0%) | 0 (0%) | 5/65 (7.7%) |
| Reduced intake (n, %) | 8/8 (100%) | 5/25 (20.0%) | 9/9 (100%) | 0 (0%) | 22/65 (33.8%) |
| (iii) Undetermined (%) | 0/12 (0%) | 0/12 (0.0%) | 2/11 (18.2%) | 0 (0%) | 2/65 (3.0%) |
| IIH Diagnostic Criteria | Vitamin A (n = 12) | Vitamin B1 (n = 40) | Vitamin B12 (n = 11) | Presumed (n = 2) | Whole Series (n = 65) |
|---|---|---|---|---|---|
| (i) Papilledema (n, %) | 12/12 (100%) | 40/40 (100%) | 11/11 (100%) | 2/2 (100%) | 65/65 (100%) |
| (ii) Normal neurological examination * (n, %) | 11/12 (91.7%) | 3/40 (7.5%) | 11/11 (100%) | 2/2 (100%) | 30/65 (46.2%) |
| (iii) Reported Lumbar puncture results (n, %) | 10/12 (83.3%) | 11/40 (27.5%) | 04/11 (36.4%) | 2/2 (100%) | 25/65 (38.5%) |
| Raised opening pressure (n, %) | 7/10 (70.0%) | 0/11 (0%) | 3/4 (75.0%) | 2/2 (100%) | 12/27 (44.4%) |
| Normal opening pressure (n, %) | 3/10 (30.0%) | 11/11 (100%) | 1/4 (25.0%) | 0/0 (0%) | 15/27 (55.6%) |
| Normal CSF constituents (n, %) | 10/10 (100%) | 11/11 (100%) | 4/4 (100%) | 2/2(100%) | 25/25 (100%) |
| (iv) Reported neuro-imaging results (n, %) | 11/12 (91.7%) | 32/40 (80.0%) | 10/11 (90.9%) | 2/2 (100%) | 55/65 (84.8%) |
| No abnormality (n, %) | 9/11 (81.8%) | 7/32 (21.9%) | 8/10 (80.0%) | 2/2 (100%) | 26/55 (47.3%) |
| Other pathological features (n, %) | 2/11 (18.2%) | 25/32 (78.1%) | 2/10 (20.0%) | 0/2 (0%) | 29/55 (52.7.2%) |
| Cases meeting IIH diagnostic criteria of definite or probable IIH (n, %) | 11/12 (91.7%) | 3/40 (7.5%) | 11/11 (100%) | 2/2 (100%) | 27/65 (41.5%) |
| Vitamin A (n = 12) | Vitamin B1 (n = 40) | Vitamin B12 (n = 11) | Presumed (n = 2) | Whole Series (n = 65) | |
|---|---|---|---|---|---|
| Nutritional supplementation (n, %) | 12/12 (100%) | 40/40 (100%) | 11/11 (100%) | 2/2 (100%) | 65/65 (100%) |
| Oral (n, %) | 12/12 (100%) | 0/40 (0%) | 0/11 (0%) | 2/2 (100%) | 14/65 (21.5%) |
| Parenteral (n, %) | 0/12 (0%) | 39/40 (97.5%) | 11/11 (100%) | 0/2 (0%) | 50/65 (76.9%) |
| Not reported (n, %) | 0/12 (0%) | 1/40 (2.5%) | 0/11 (0%) | 0/2 (0%) | 1/65 (1.5%) |
| Other interventions | |||||
| Acetazolamide (n, %) | 2/12 (16.7%) | 0/40(0%) | 1/11 (9.1%) | 2/2 (100%) | 4/65(6.2%) |
| Clinical outcomes | |||||
| Full recovery (n, %) | 6/12 (50.0%) | 31/40 (77.5%) | 7/11 (63.6%) | 2/2 (100%) | 46/65 (70.8%) |
| Residual visual defects (n, %) | 6/12 (50.0%) | 4/40 (10.0%) | 4/11 (36.4%) | 0/2 (0%) | 14/65 (21.5%) |
| Residual neurological defects (n, %) | 0/12 (0%) | 5/40 (12.5%) | 0/11 (0%) | 0/2 (0%) | 5/65 (7.7%) |
| Death (n, %) | 0/12 (0%) | 4/40 (10.0%) | 0/11 (0%) | 0/2 (0%) | 5/65 (7.7%) |
| Maternal death (n, %) | - | 2/15 pregnancies (13.3%) | - | - | - |
| Fetal death (n, %) | - | 5/15 pregnancies (33.3%) | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, G.; Epps, S.; Huntley, A.; Atan, D. Micronutrient Deficiencies Presenting with Optic Disc Swelling Associated with or without Intracranial Hypertension: A Systematic Review. Nutrients 2022, 14, 3068. https://doi.org/10.3390/nu14153068
Reynolds G, Epps S, Huntley A, Atan D. Micronutrient Deficiencies Presenting with Optic Disc Swelling Associated with or without Intracranial Hypertension: A Systematic Review. Nutrients. 2022; 14(15):3068. https://doi.org/10.3390/nu14153068
Chicago/Turabian StyleReynolds, Gavin, Simon Epps, Alyson Huntley, and Denize Atan. 2022. "Micronutrient Deficiencies Presenting with Optic Disc Swelling Associated with or without Intracranial Hypertension: A Systematic Review" Nutrients 14, no. 15: 3068. https://doi.org/10.3390/nu14153068
APA StyleReynolds, G., Epps, S., Huntley, A., & Atan, D. (2022). Micronutrient Deficiencies Presenting with Optic Disc Swelling Associated with or without Intracranial Hypertension: A Systematic Review. Nutrients, 14(15), 3068. https://doi.org/10.3390/nu14153068






