Moringa Oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Moringa oleifera (MO) Extraction and Treatment
2.3. Behavioral Tests
2.3.1. Morris Water Maze (MWM) Test
2.3.2. Open Field Test (OFT)
2.3.3. Novel Objective Recognition Test (NOR)
2.3.4. Fear Conditioning Tests
2.4. Golgi Staining
2.5. Nissl Staining
2.6. Thioflavin S Staining
2.7. Western Blot Analysis
2.8. PP2B Activity Assay
2.9. ELISA Assay for Aβ40/42, IL-1β and TNF-α
2.10. Statistical Analysis
3. Results
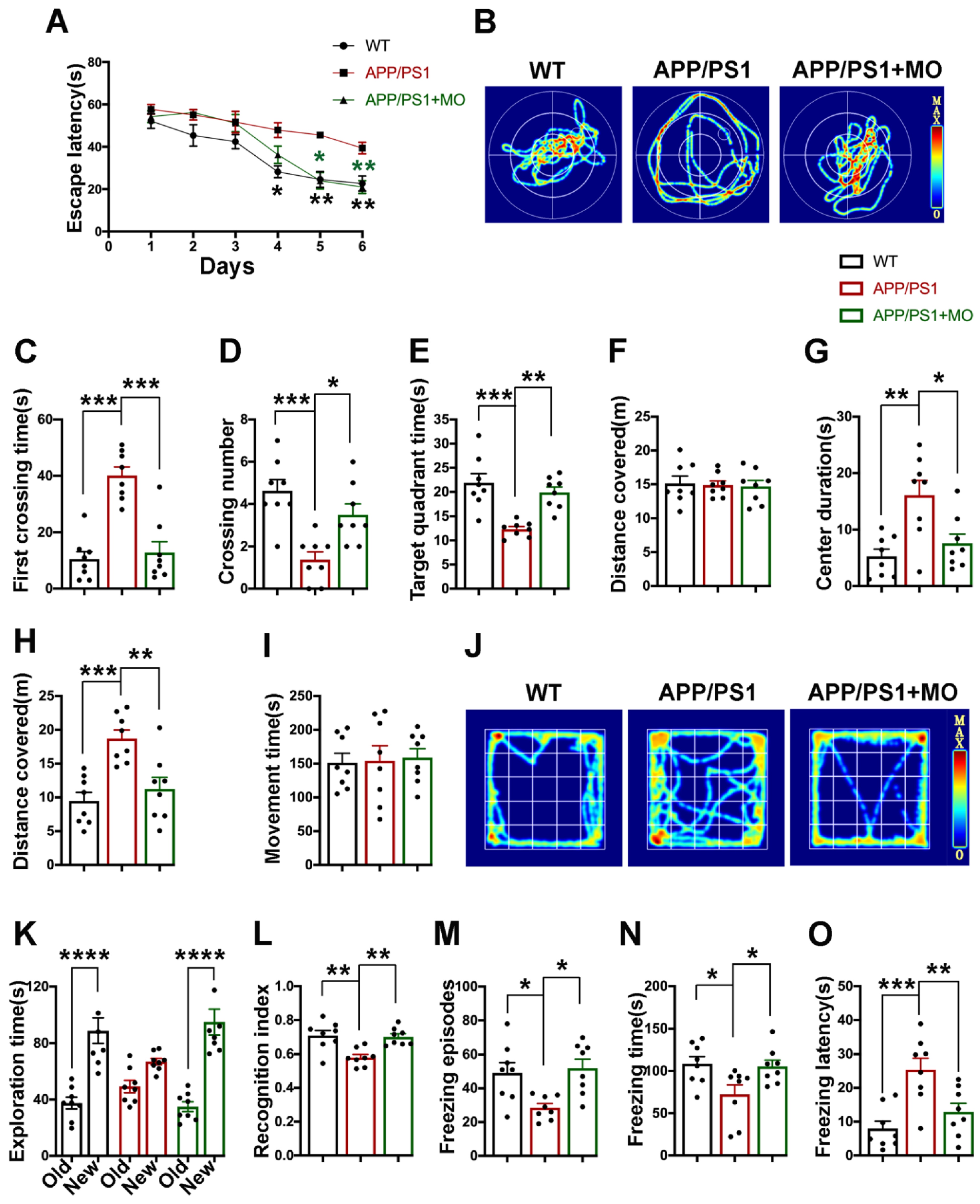
3.1. Moringa oleifera Improves Behavioral and Cognitive Alterations in APP/PS1 Mice
3.2. Moringa oleifera Alleviates Aβ Level and Plaques Burdens in APP/PS1 Mice
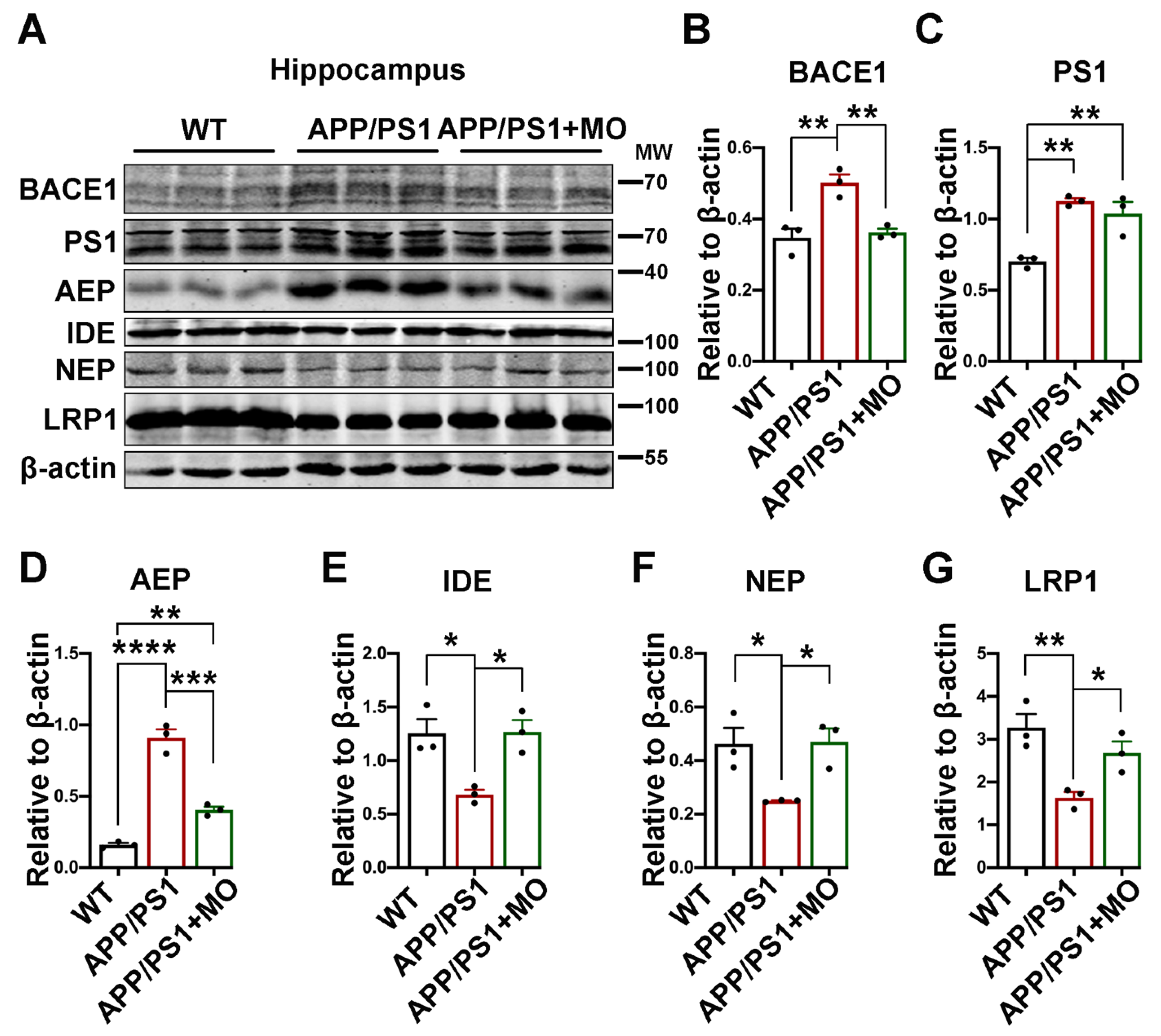
3.3. Moringa oleifera Modulates Both Production and Clearance Pathways of Aβ in APP/PS1 Mice
3.4. Moringa oleifera Improves p-Y1472 GluN2B by Decreasing STEP in APP/PS1 Mice
3.5. Moringa oleifera Modulates the PP2B/DARPP-32/PP1 Axis to Decrease STEP in APP/PS1 Mice
3.6. Moringa oleifera Improves Synaptic Loss and Neurodegeneration in APP/PS1 Mice
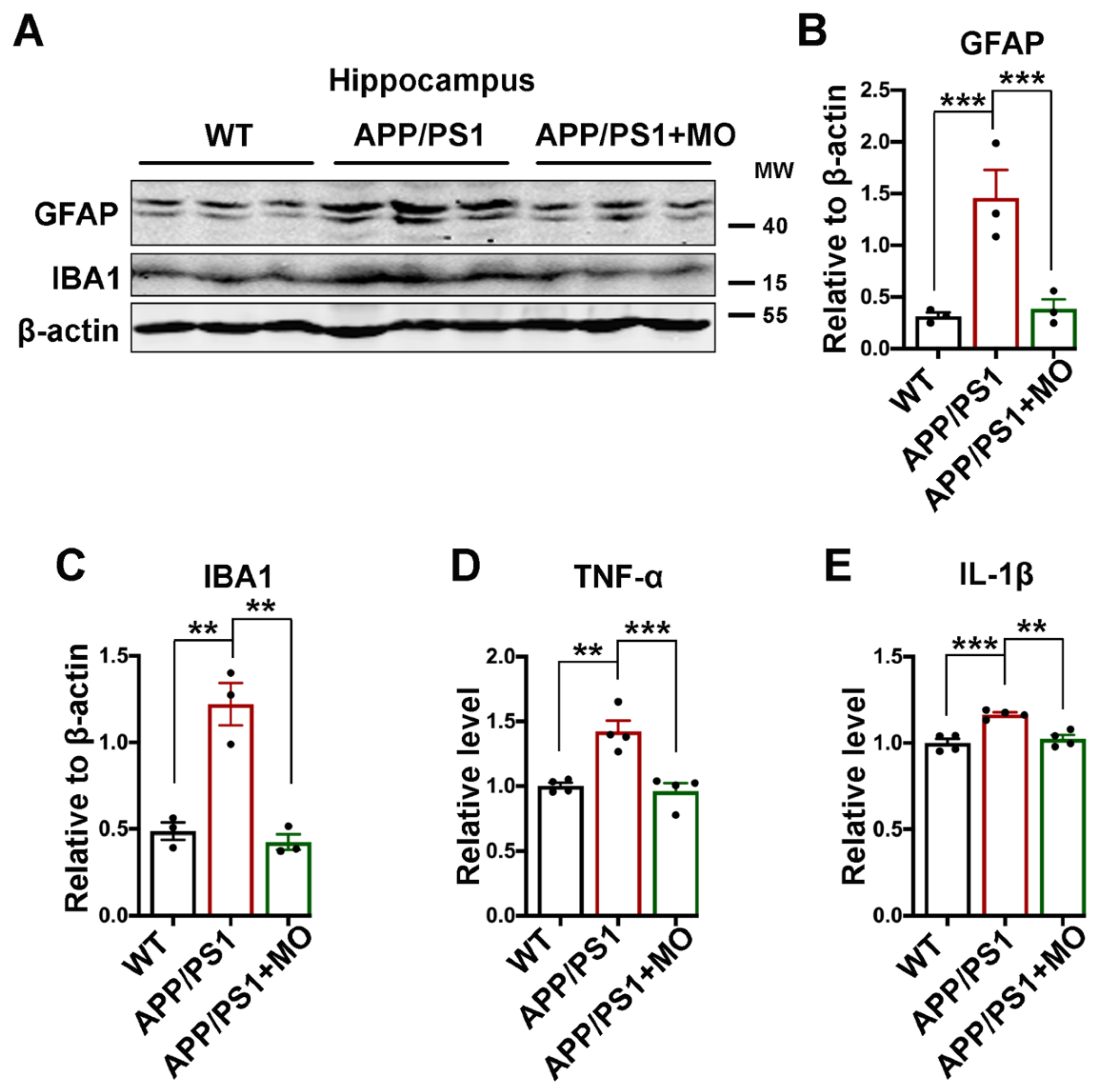
3.7. Moringa oleifera Improves Neuroinflammation in APP/PS1 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Rosenberg, R.N.; Lambracht-Washington, D.; Yu, G.; Xia, W. Genomics of Alzheimer Disease: A Review. JAMA Neurol. 2016, 73, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Xu, M.; Lai, M.T.; Huang, Q.; Castro, J.L.; DiMuzio-Mower, J.; Harrison, T.; Lellis, C.; Nadin, A.; Neduvelil, J.G.; et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 2000, 405, 689–694. [Google Scholar] [CrossRef]
- Wang, J.Z.; Wang, Z.H.; Tian, Q. Tau hyperphosphorylation induces apoptotic escape and triggers neurodegeneration in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 359–366. [Google Scholar] [CrossRef]
- Nativio, R.; Donahue, G.; Berson, A.; Lan, Y.; Amlie-Wolf, A.; Tuzer, F.; Toledo, J.B.; Gosai, S.J.; Gregory, B.D.; Torres, C.; et al. Dysregulation of the epigenetic landscape of normal aging in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 497–505. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Delatour, B.; Potier, M.C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef]
- Chami, L.; Checler, F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef]
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS 2022, 19, 21. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Qosa, H.; Kaddoumi, A. Age-Related Decline in Brain and Hepatic Clearance of Amyloid-Beta is Rectified by the Cholinesterase Inhibitors Donepezil and Rivastigmine in Rats. ACS Chem. Neurosci. 2015, 6, 725–736. [Google Scholar] [CrossRef]
- Prasad, H.; Rao, R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc. Natl. Acad. Sci. USA 2018, 115, E6640–E6649. [Google Scholar] [CrossRef]
- Tian, D.-Y.; Cheng, Y.; Zhuang, Z.-Q.; He, C.-Y.; Pan, Q.-G.; Tang, M.-Z.; Hu, X.-L.; Shen, Y.-Y.; Wang, Y.-R.; Chen, S.-H.; et al. Physiological clearance of amyloid-beta by the kidney and its therapeutic potential for Alzheimer’s disease. Mol. Psychiatry 2021, 26, 6074–6082. [Google Scholar] [CrossRef]
- Extance, A. Alzheimer’s failure raises questions about disease-modifying strategies. Nat. Rev. Drug Discov. 2010, 9, 749–751. [Google Scholar] [CrossRef]
- Rinne, J.O.; Brooks, D.J.; Rossor, M.N.; Fox, N.C.; Bullock, R.; Klunk, W.E.; Mathis, C.A.; Blennow, K.; Barakos, J.; Okello, A.A.; et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010, 9, 363–372. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer disease and aducanumab: Adjusting our approach. Nat. Rev. Neurol. 2019, 15, 365–366. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Huang, F.; Wu, M.; Wang, Y.; Wei, Z.; Bao, J.; Salissou, M.T.M.; Ke, D.; Wang, Q.; Liu, R.; et al. Moringa Oleifera Alleviates Homocysteine-Induced Alzheimer’s Disease-Like Pathology and Cognitive Impairments. J. Alzheimer’s Dis. 2018, 63, 1141–1159. [Google Scholar] [CrossRef]
- Salissou, M.T.M.; Mahaman, Y.A.R.; Zhu, F.; Huang, F.; Wang, Y.; Xu, Z.; Ke, D.; Wang, Q.; Liu, R.; Wang, J.Z.; et al. Methanolic extract of Tamarix Gallica attenuates hyperhomocysteinemia induced AD-like pathology and cognitive impairments in rats. Aging 2018, 10, 3229–3248. [Google Scholar] [CrossRef]
- Zeng, K.; Li, M.; Hu, J.; Mahaman, Y.A.R.; Bao, J.; Huang, F.; Xia, Y.; Liu, X.; Wang, Q.; Wang, J.Z.; et al. Ginkgo biloba Extract EGb761 Attenuates Hyperhomocysteinemia-induced AD Like Tau Hyperphosphorylation and Cognitive Impairment in Rats. Curr. Alzheimer Res. 2018, 15, 89–99. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, Y.; Luo, H.; Huang, S.; Wang, Y.; Shentu, Y.; Mahaman, Y.A.R.; Huang, F.; Ke, D.; Wang, Q.; et al. Codonopsis pilosula Polysaccharide Attenuates Tau Hyperphosphorylation and Cognitive Impairments in hTau Infected Mice. Front. Mol. Neurosci. 2018, 11, 437. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, Q.; Luo, H.; Xu, Z.; Huang, S.; Yang, F.; Liu, Y.; Mahaman, Y.A.R.; Ke, D.; Wang, Q.; et al. Codonopsis pilosula polysaccharide attenuates Aβ toxicity and cognitive defects in APP/PS1 mice. Aging 2020, 12, 13422–13436. [Google Scholar] [CrossRef]
- Meng, G.; Meng, X.; Ma, X.; Zhang, G.; Hu, X.; Jin, A.; Zhao, Y.; Liu, X. Application of Ferulic Acid for Alzheimer’s Disease: Combination of Text Mining and Experimental Validation. Front. Neuroinforma. 2018, 12, 31. [Google Scholar] [CrossRef]
- Abdelsayed, E.M.; Medhat, D.; Mandour, Y.M.; Hanafi, R.S.; Motaal, A.A. Niazimicin: A thiocarbamate glycoside from Moringa oleifera Lam. seeds with a novel neuroprotective activity. J. Food Biochem. 2021, 45, e13992. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Knobloch, M.; Mansuy, I.M. Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Mol. Neurobiol. 2008, 37, 73–82. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Dewachter, I.; Filipkowski, R.K.; Priller, C.; Ris, L.; Neyton, J.; Croes, S.; Terwel, D.; Gysemans, M.; Devijver, H.; Borghgraef, P.; et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol. Aging 2009, 30, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Nong, Y.; Almeida, C.G.; Paul, S.; Moran, T.; Choi, E.Y.; Nairn, A.C.; Salter, M.W.; Lombroso, P.J.; Gouras, G.K.; et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005, 8, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kurup, P.; Xu, J.; Carty, N.; Fernandez, S.M.; Nygaard, H.B.; Pittenger, C.; Greengard, P.; Strittmatter, S.M.; Nairn, A.C.; et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA 2010, 107, 19014–19019. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Kwon, J.; Benedict, J.; Kamceva, M.; Kurup, P.; Lombroso, P.J. STEP inhibition prevents Aβ-mediated damage in dendritic complexity and spine density in Alzheimer’s disease. Exp. Brain Res. 2021, 239, 881–890. [Google Scholar] [CrossRef]
- Xu, J.; Chatterjee, M.; Baguley, T.D.; Brouillette, J.; Kurup, P.; Ghosh, D.; Kanyo, J.; Zhang, Y.; Seyb, K.; Ononenyi, C.; et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer’s disease. PLoS Biol. 2014, 12, e1001923. [Google Scholar] [CrossRef]
- Nakazawa, T.; Komai, S.; Tezuka, T.; Hisatsune, C.; Umemori, H.; Semba, K.; Mishina, M.; Manabe, T.; Yamamoto, T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2001, 276, 693–699. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Huang, F.; Embaye, K.S.; Wang, X.; Zhu, F. The Implication of STEP in Synaptic Plasticity and Cognitive Impairments in Alzheimer’s Disease and Other Neurological Disorders. Front. Cell Dev. Biol. 2021, 9, 680118. [Google Scholar] [CrossRef]
- Trepanier, C.H.; Jackson, M.F.; MacDonald, J.F. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012, 279, 12–19. [Google Scholar] [CrossRef]
- Chin, J.; Palop, J.J.; Puoliväli, J.; Massaro, C.; Bien-Ly, N.; Gerstein, H.; Scearce-Levie, K.; Masliah, E.; Mucke, L. Fyn Kinase Induces Synaptic and Cognitive Impairments in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 9694–9703. [Google Scholar] [CrossRef]
- Dineley, K.T.; Westerman, M.; Bui, D.; Bell, K.; Ashe, K.H.; Sweatt, J.D. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J. Neurosci. 2001, 21, 4125–4133. [Google Scholar] [CrossRef]
- Stevens, T.R.; Krueger, S.R.; Fitzsimonds, R.M.; Picciotto, M.R. Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation. J. Neurosci. 2003, 23, 10093–10099. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Chang, L.; Fernandez, S.J.; Gong, Y.; Viola, K.L.; Lambert, M.P.; Velasco, P.T.; Bigio, E.H.; Finch, C.E.; et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J. Neurosci. 2004, 24, 10191–10200. [Google Scholar] [CrossRef]
- Dohadwala, M.; da Cruz e Silva, E.F.; Hall, F.L.; Williams, R.T.; Carbonaro-Hall, D.A.; Nairn, A.C.; Greengard, P.; Berndt, N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 1994, 91, 6408–6412. [Google Scholar] [CrossRef]
- Hou, H.; Sun, L.; Siddoway, B.A.; Petralia, R.S.; Yang, H.; Gu, H.; Nairn, A.C.; Xia, H. Synaptic NMDA receptor stimulation activates PP1 by inhibiting its phosphorylation by Cdk5. J. Cell Biol. 2013, 203, 521–535. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Tan, J.; Wang, H.; Wang, Z.; Li, P.; Zhao, C.; Liu, J. Comparative Analysis of Chemical Constituents of Moringa oleifera Leaves from China and India by Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-Of-Flight Mass Spectrometry. Molecules 2019, 24, 942. [Google Scholar] [CrossRef]
- Fernandes, Â.; Bancessi, A.; Pinela, J.; Dias, M.I.; Liberal, Â.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.; et al. Nutritional and phytochemical profiles and biological activities of Moringa oleifera Lam. edible parts from Guinea-Bissau (West Africa). Food Chem. 2021, 341, 128229. [Google Scholar] [CrossRef]
- Saa, R.W.; Fombang, E.N.; Ndjantou, E.B.; Njintang, N.Y. Treatments and uses of Moringa oleifera seeds in human nutrition: A review. Food Sci. Nutr. 2019, 7, 1911–1919. [Google Scholar] [CrossRef]
- Ahmad, J.; Khan, I.; Blundell, R. Moringa oleifera and glycemic control: A review of current evidence and possible mechanisms. Phytother. Res. 2019, 33, 2841–2848. [Google Scholar] [CrossRef]
- Ajagun-Ogunleye, M.O.; Ebuehi, O.A.T. Evaluation of the anti-aging and antioxidant action of Ananas sativa and Moringa oleifera in a fruit fly model organism. J. Food Biochem. 2020, 44, e13426. [Google Scholar] [CrossRef]
- Akinduti, P.A.; Emoh-Robinson, V.; Obamoh-Triumphant, H.F.; Obafemi, Y.D.; Banjo, T.T. Antibacterial activities of plant leaf extracts against multi-antibiotic resistant Staphylococcus aureus associated with skin and soft tissue infections. BMC Complement. Med. Ther. 2022, 22, 47. [Google Scholar] [CrossRef]
- Aldakheel, R.K.; Rehman, S.; Almessiere, M.A.; Khan, F.A.; Gondal, M.A.; Mostafa, A.; Baykal, A. Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy. Pharmaceuticals 2020, 13, 193. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, N. Antimicrobial Potential of Fungal Endophytes from Moringa oleifera. Appl. Biochem. Biotechnol. 2019, 187, 628–648. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S. Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef]
- Bhalla, N.; Ingle, N.; Patri, S.V.; Haranath, D. Phytochemical analysis of Moringa Oleifera leaves extracts by GC-MS and free radical scavenging potency for industrial applications. Saudi. J. Biol. Sci. 2021, 28, 6915–6928. [Google Scholar] [CrossRef]
- Siddiqui, S.; Upadhyay, S.; Ahmad, I.; Hussain, A.; Ahamed, M. Cytotoxicity of Moringa oleifera fruits on human liver cancer and molecular docking analysis of bioactive constituents against caspase-3 enzyme. J. Food Biochem. 2021, 45, e13720. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Elmorsy, E.; Aprioku, J.S.; Siminialayi, I.; Carter, W.G. In Vitro Anti-Cholinesterase and Antioxidant Activity of Extracts of Moringa oleifera Plants from Rivers State, Niger Delta, Nigeria. Medicines 2018, 5, 71. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, W.S.; Suo, D.Q.; Li, Y.; Peng, L.; Xu, L.X.; Zeng, K.Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharm. 2018, 9, 389. [Google Scholar] [CrossRef]
- Oboh, G.; Oyeleye, S.I.; Akintemi, O.A.; Olasehinde, T.A. Moringa oleifera supplemented diet modulates nootropic-related biomolecules in the brain of STZ-induced diabetic rats treated with acarbose. Metab. Brain Dis. 2018, 33, 457–466. [Google Scholar] [CrossRef]
- Asare, G.A.; Gyan, B.; Bugyei, K.; Adjei, S.; Mahama, R.; Addo, P.; Otu-Nyarko, L.; Wiredu, E.K.; Nyarko, A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012, 139, 265–272. [Google Scholar] [CrossRef]
- Bakre, A.G.; Aderibigbe, A.O.; Ademowo, O.G. Studies on neuropharmacological profile of ethanol extract of Moringa oleifera leaves in mice. J. Ethnopharmacol. 2013, 149, 783–789. [Google Scholar] [CrossRef]
- Asiedu-Gyekye, I.J.; Frimpong-Manso, S.; Awortwe, C.; Antwi, D.A.; Nyarko, A.K. Micro- and Macroelemental Composition and Safety Evaluation of the Nutraceutical Moringa oleifera Leaves. J. Toxicol. 2014, 2014, 786979. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Zhang, H.; Mahaman, Y.A.R.; Huang, F.; Meng, D.; Zhou, Y.; Wang, S.; Jiang, N.; Xiong, J.; et al. Chk1 Inhibition Ameliorates Alzheimer’s Disease Pathogenesis and Cognitive Dysfunction Through CIP2A/PP2A Signaling. Neurotherapeutics 2022, 19, 570–591. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Okamoto, S.; Lipton, S.A.; Xu, H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, Z.H.; Zhang, Z.; Liu, X.; Yu, S.P.; Wang, J.Z.; Wang, X.C.; Ye, K. Delta- and beta- secretases crosstalk amplifies the amyloidogenic pathway in Alzheimer’s disease. Prog. Neurobiol. 2021, 204, 102113. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.-G.; Wang, Z.-H.; Song, M.; Yu, S.P.; Kang, S.S.; Liu, X.; Zhang, Z.; Xie, M.; Liu, G.-P.; et al. δ-Secretase-cleaved Tau stimulates Aβ production via upregulating STAT1-BACE1 signaling in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 586–603. [Google Scholar] [CrossRef]
- Yao, Y.; Kang, S.S.; Xia, Y.; Wang, Z.H.; Liu, X.; Muller, T.; Sun, Y.E.; Ye, K. A delta-secretase-truncated APP fragment activates CEBPB, mediating Alzheimer’s disease pathologies. Brain 2021, 144, 1833–1852. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Liu, X.; Su Kang, S.; Duong, D.M.; Seyfried, N.T.; Cao, X.; Cheng, L.; Sun, Y.E.; Ping Yu, S.; et al. Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat. Commun. 2015, 6, 8762. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.H.; Liu, X.; Zhang, Z.; Gu, X.; Yu, S.P.; Keene, C.D.; Cheng, L.; Ye, K. Traumatic brain injury triggers APP and Tau cleavage by delta-secretase, mediating Alzheimer’s disease pathology. Prog. Neurobiol. 2020, 185, 101730. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Liu, X.; Kang, S.S.; Kwon, I.S.; Duong, D.M.; Seyfried, N.T.; Hu, W.T.; Liu, Z.; Wang, J.Z.; et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat. Med. 2014, 20, 1254–1262. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.Y.; Hyman, B.T.; Bacskai, B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B.J. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef]
- Koffie, R.M.; Meyer-Luehmann, M.; Hashimoto, T.; Adams, K.W.; Mielke, M.L.; Garcia-Alloza, M.; Micheva, K.D.; Smith, S.J.; Kim, M.L.; Lee, V.M.; et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 2009, 106, 4012–4017. [Google Scholar] [CrossRef]
- Stern, E.A.; Bacskai, B.J.; Hickey, G.A.; Attenello, F.J.; Lombardo, J.A.; Hyman, B.T. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J. Neurosci. 2004, 24, 4535–4540. [Google Scholar] [CrossRef]
- Sattler, R.; Xiong, Z.; Lu, W.Y.; MacDonald, J.F.; Tymianski, M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J. Neurosci. 2000, 20, 22–33. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Alvarez, A.; Cacabelos, R.; Sanpedro, C.; Garcia-Fantini, M.; Aleixandre, M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol. Aging 2007, 28, 533–536. [Google Scholar] [CrossRef]
- Pickering, M.; Cumiskey, D.; O’Connor, J.J. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005, 90, 663–670. [Google Scholar] [CrossRef]
- Frankola, K.A.; Greig, N.H.; Luo, W.; Tweedie, D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2011, 10, 391–403. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Yamazaki, Y.; Ren, Y.; Davis, M.D.; Liu, C.-C.; Lu, W.; Wang, X.; Chen, K.; Cherukuri, Y.; et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020, 11, 5540. [Google Scholar] [CrossRef]
- Salimi, A.; Li, H.; Lee, J.Y. Molecular insight into the early stage of amyloid-β(1-42) Homodimers aggregation influenced by histidine tautomerism. Int. J. Biol. Macromol. 2021, 184, 887–897. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Embaye, K.S.; Huang, F.; Li, L.; Zhu, F.; Wang, J.Z.; Liu, R.; Feng, J.; Wang, X. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing Res. Rev. 2022, 74, 101544. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef]
- Mahaman, Y.A.R.; Huang, F.; Kessete Afewerky, H.; Maibouge, T.M.S.; Ghose, B.; Wang, X. Involvement of calpain in the neuropathogenesis of Alzheimer’s disease. Med. Res. Rev. 2019, 39, 608–630. [Google Scholar] [CrossRef]
- Kang, S.S.; Liu, X.; Ahn, E.H.; Xiang, J.; Manfredsson, F.P.; Yang, X.; Luo, H.R.; Liles, L.C.; Weinshenker, D.; Ye, K. Norepinephrine metabolite DOPEGAL activates AEP and pathological Tau aggregation in locus coeruleus. J. Clin. Investig. 2020, 130, 422–437. [Google Scholar] [CrossRef]
- Kang, S.S.; Ahn, E.H.; Zhang, Z.; Liu, X.; Manfredsson, F.P.; Sandoval, I.M.; Dhakal, S.; Iuvone, P.M.; Cao, X.; Ye, K. α-Synuclein stimulation of monoamine oxidase-B and legumain protease mediates the pathology of Parkinson’s disease. EMBO J. 2018, 37, e98878. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Zhang, J.H.; Han, K.; Zhang, X.; Bai, X.; You, L.H.; Yu, P.; Shi, Z.; Chang, Y.Z.; et al. Astrocyte hepcidin ameliorates neuronal loss through attenuating brain iron deposition and oxidative stress in APP/PS1 mice. Free Radic. Biol. Med. 2020, 158, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, X.; Zheng, S.; Cao, P.; Chen, Y.; Yu, M.; Liao, C.; Zhang, Z.; Han, J.; Duan, Y.; et al. LongShengZhi Capsule Attenuates Alzheimer-Like Pathology in APP/PS1 Double Transgenic Mice by Reducing Neuronal Oxidative Stress and Inflammation. Front. Aging Neurosci. 2020, 12, 582455. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Watanabe, K.; Sekiguchi, M.; Hosoki, E.; Kawashima-Morishima, M.; Lee, H.J.; Hama, E.; Sekine-Aizawa, Y.; et al. Identification of the major Aβ1-42-degrading catabolic pathway in brain parenchyma: Suppression leads to biochemical and pathological deposition. Nat. Med. 2000, 6, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.P.; Lete, M.G.; Fowler, S.B.; Gimeno, A.; Rocha, J.F.; Sousa, S.F.; Webster, C.I.; Jiménez-Bar Bero, J.J.; Gales, L. Aβ(31-35) Decreases Neprilysin-Mediated Alzheimer’s Amyloid-β Peptide Degradation. ACS Chem. Neurosci. 2021, 12, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Stargardt, A.; Gillis, J.; Kamphuis, W.; Wiemhoefer, A.; Kooijman, L.; Raspe, M.; Benckhuijsen, W.; Drijfhout, J.W.; Hol, E.M.; Reits, E. Reduced amyloid-β degradation in early Alzheimer’s disease but not in the APPswePS1dE9 and 3xTg-AD mouse models. Aging Cell 2013, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Kurochkin, I.V.; Guarnera, E.; Berezovsky, I.N. Insulin-Degrading Enzyme in the Fight against Alzheimer’s Disease. Trends Pharm. Sci. 2018, 39, 49–58. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Helisalmi, S.; Mannermaa, A.; Pirttilä, T.; Soininen, H.; Hiltunen, M. Combined risk effects of IDE and NEP gene variants on Alzheimer disease. J. Neurol. Neurosurg Psychiatry 2009, 80, 1268–1270. [Google Scholar] [CrossRef]
- Chen, S.; Mima, D.; Jin, H.; Dan, Q.; Wang, F.; Cai, J.; Shi, L.; Wang, H.; Du, A.; Tang, Y.; et al. The Association between Neprilysin gene polymorphisms and Alzheimer’s disease in Tibetan population. Brain Behav. 2021, 11, e02002. [Google Scholar] [CrossRef]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J.; et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Investig. 2000, 106, 1489–1499. [Google Scholar] [CrossRef]
- Kang, D.E.; Pietrzik, C.U.; Baum, L.; Chevallier, N.; Merriam, D.E.; Kounnas, M.Z.; Wagner, S.L.; Troncoso, J.C.; Kawas, C.H.; Katzman, R.; et al. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Investig. 2000, 106, 1159–1166. [Google Scholar] [CrossRef]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef]
- Storck, S.E.; Meister, S.; Nahrath, J.; Meißner, J.N.; Schubert, N.; Di Spiezio, A.; Baches, S.; Vandenbroucke, R.E.; Bouter, Y.; Prikulis, I.; et al. Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. J. Clin. Investig. 2016, 126, 123–136. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018, 13, 57. [Google Scholar] [CrossRef]
- Moon, I.S.; Apperson, M.L.; Kennedy, M.B. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc. Natl. Acad. Sci. USA 1994, 91, 3954–3958. [Google Scholar] [CrossRef]
- Lussier, M.P.; Sanz-Clemente, A.; Roche, K.W. Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications. J. Biol. Chem. 2015, 290, 28596–28603. [Google Scholar] [CrossRef]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef]
- Barria, A.; Malinow, R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 2002, 35, 345–353. [Google Scholar] [CrossRef]
- Chatterjee, M.; Kurup, P.K.; Lundbye, C.J.; Hugger Toft, A.K.; Kwon, J.; Benedict, J.; Kamceva, M.; Banke, T.G.; Lombroso, P.J. STEP inhibition reverses behavioral, electrophysiologic, and synaptic abnormalities in Fmr1 KO mice. Neuropharmacology 2018, 128, 43–53. [Google Scholar] [CrossRef]
- Kurup, P.; Zhang, Y.; Xu, J.; Venkitaramani, D.V.; Haroutunian, V.; Greengard, P.; Nairn, A.C.; Lombroso, P.J. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J. Neurosci. 2010, 30, 5948–5957. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.W.; Yang, J.; Cao, Y.P. Tyrosine phosphatase STEP61 negatively regulates amyloid β-mediated ERK/CREB signaling pathways via α7 nicotinic acetylcholine receptors. J. Neurosci. Res. 2013, 91, 1581–1590. [Google Scholar] [CrossRef]
- Liu, B.; Kou, J.; Li, F.; Huo, D.; Xu, J.; Zhou, X.; Meng, D.; Ghulam, M.; Artyom, B.; Gao, X.; et al. Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging 2020, 12, 8622–8639. [Google Scholar] [CrossRef]
- Szögi, T.; Schuster, I.; Borbély, E.; Gyebrovszki, A.; Bozsó, Z.; Gera, J.; Rajkó, R.; Sántha, M.; Penke, B.; Fülöp, L. Effects of the Pentapeptide P33 on Memory and Synaptic Plasticity in APP/PS1 Transgenic Mice: A Novel Mechanism Presenting the Protein Fe65 as a Target. Int. J. Mol. Sci. 2019, 20, 3050. [Google Scholar] [CrossRef]
- Zhu, J.; Shang, Y.; Zhang, M. Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci. 2016, 17, 209–223. [Google Scholar] [CrossRef]
- Wang, W.; Weng, J.; Zhang, X.; Liu, M.; Zhang, M. Creating conformational entropy by increasing interdomain mobility in ligand binding regulation: A revisit to N-terminal tandem PDZ domains of PSD-95. J. Am. Chem. Soc. 2009, 131, 787–796. [Google Scholar] [CrossRef]
- Sheng, N.; Bemben, M.A.; Díaz-Alonso, J.; Tao, W.; Shi, Y.S.; Nicoll, R.A. LTP requires postsynaptic PDZ-domain interactions with glutamate receptor/auxiliary protein complexes. Proc. Natl. Acad. Sci. USA 2018, 115, 3948–3953. [Google Scholar] [CrossRef]
- Won, S.; Roche, K.W. Regulation of glutamate receptors by striatal-enriched tyrosine phosphatase 61 (STEP(61) ). J. Physiol. 2021, 599, 443–451. [Google Scholar] [CrossRef]
- Won, S.; Incontro, S.; Nicoll, R.A.; Roche, K.W. PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc. Natl. Acad. Sci. USA 2016, 113, E4736–E4744. [Google Scholar] [CrossRef]
- Dore, K.; Carrico, Z.; Alfonso, S.; Marino, M.; Koymans, K.; Kessels, H.W.; Malinow, R. PSD-95 protects synapses from β-amyloid. Cell Rep. 2021, 35, 109194. [Google Scholar] [CrossRef]
- Ugalde-Triviño, L.; Díaz-Guerra, M. PSD-95: An Effective Target for Stroke Therapy Using Neuroprotective Peptides. Int. J. Mol. Sci. 2021, 22, 12585. [Google Scholar] [CrossRef]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The synapsins: Key actors of synapse function and plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lu, B. Synapses and dendritic spines as pathogenic targets in Alzheimer’s disease. Neural Plast. 2012, 2012, 247150. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Synaptic Plasticity, Dementia and Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.F.; Tschida, K.A.; Klein, M.E.; Mooney, R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature 2010, 463, 948–952. [Google Scholar] [CrossRef]
- Cho, C.; MacDonald, R.; Shang, J.; Cho, M.J.; Chalifour, L.E.; Paudel, H.K. Early growth response-1-mediated down-regulation of drebrin correlates with loss of dendritic spines. J. Neurochem. 2017, 142, 56–73. [Google Scholar] [CrossRef]
- Omelchenko, A.; Menon, H.; Donofrio, S.G.; Kumar, G.; Chapman, H.M.; Roshal, J.; Martinez-Montes, E.R.; Wang, T.L.; Spaller, M.R.; Firestein, B.L. Interaction between CRIPT and PSD-95 Is Required for Proper Dendritic Arborization in Hippocampal Neurons. Mol. Neurobiol. 2020, 57, 2479–2493. [Google Scholar] [CrossRef]
- Helm, M.S.; Dankovich, T.M.; Mandad, S.; Rammner, B.; Jähne, S.; Salimi, V.; Koerbs, C.; Leibrandt, R.; Urlaub, H.; Schikorski, T.; et al. A large-scale nanoscopy and biochemistry analysis of postsynaptic dendritic spines. Nat. Neurosci. 2021, 24, 1151–1162. [Google Scholar] [CrossRef]
- Gardoni, F.; Bellone, C.; Viviani, B.; Marinovich, M.; Meli, E.; Pellegrini-Giampietro, D.E.; Cattabeni, F.; Di Luca, M. Lack of PSD-95 drives hippocampal neuronal cell death through activation of an alpha CaMKII transduction pathway. Eur. J. Neurosci. 2002, 16, 777–786. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T.X.; Hallett, P.J.; Watanabe, M.; Grant, S.G.; Isacson, O.; Yao, W.D. PSD-95 uncouples dopamine-glutamate interaction in the D1/PSD-95/NMDA receptor complex. J. Neurosci. 2009, 29, 2948–2960. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B.P. Moringa oleifera: A Tree of Life as a Promising Medicinal Plant for Neurodegenerative Diseases. J. Agric. Food Chem. 2021, 69, 14358–14371. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Castillo, E.; Leon, J.; Mazzei, G.; Abolhassani, N.; Haruyama, N.; Saito, T.; Saido, T.; Hokama, M.; Iwaki, T.; Ohara, T.; et al. Author Correction: Comparative profiling of cortical gene expression in Alzheimer’s disease patients and mouse models demonstrates a link between amyloidosis and neuroinflammation. Sci. Rep. 2021, 11, 18377. [Google Scholar] [CrossRef]
- Kou, J.-j.; Shi, J.-z.; He, Y.-y.; Hao, J.-j.; Zhang, H.-y.; Luo, D.-m.; Song, J.-k.; Yan, Y.; Xie, X.-m.; Du, G.-h.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2021, 43, 840–849. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Dzuvor, C.K.O.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications—A critical review. Crit. Rev. Biotechnol. 2022, 42, 271–293. [Google Scholar] [CrossRef]
- Hur, J.-Y.; Frost, G.R.; Wu, X.; Crump, C.; Pan, S.J.; Wong, E.; Barros, M.; Li, T.; Nie, P.; Zhai, Y.; et al. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature 2020, 586, 735–740. [Google Scholar] [CrossRef]
- Katsuki, H.; Nakai, S.; Hirai, Y.; Akaji, K.; Kiso, Y.; Satoh, M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur. J. Pharmacol. 1990, 181, 323–326. [Google Scholar] [CrossRef]
- Beattie, E.C.; Stellwagen, D.; Morishita, W.; Bresnahan, J.C.; Ha, B.K.; Von Zastrow, M.; Beattie, M.S.; Malenka, R.C. Control of synaptic strength by glial TNFalpha. Science 2002, 295, 2282–2285. [Google Scholar] [CrossRef]
- Bains, J.S.; Oliet, S.H. Glia: They make your memories stick! Trends Neurosci. 2007, 30, 417–424. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef]
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharm. 2001, 133, 89–96. [Google Scholar] [CrossRef]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef]




| Antibody | Specificity | Type | Species | Source (Catalog Number) |
|---|---|---|---|---|
| Anti-GluN1 | GluN1 | pAb | Rabbit | ABclonal (A7677) |
| Anti-GluN2A | GluN2A | mAb | Rabbit | ABclonal (A19089) |
| Anti-GluN2B | GluN2B C-terminus | pAb | Rabbit | ABclonal (A3056) |
| Anti-p-Y1472 | p-GluN2B (Y1472) | pAb | Rabbit | Abcam (ab3856) |
| Anti-STEP | STEP (23E5) | pAb | Mouse | Cell Signaling Technology (4396) |
| Anti-np-S221 | np-STEP (S221) | mAb | Rabbit | Cell Signaling Technology (5659) |
| Anti-FYN | FYN | mAb | Mouse | ABclonal (A0086) |
| Anti-p-Y416 | p-Sar family Y416 | pAb | Rabbit | ABclonal (RK06002) |
| Anti-PP1CA | PP1CA (a.a 1–330) | pAb | Rabbit | ABclonal (A12468) |
| Anti-p-T320 | p-PP1CA T320 | pAb | Rabbit | ABclonal (AP0786) |
| Anti-DARPP-32 | DARPP-32 | pAb | Rabbit | Abmart (Q9UD71) |
| Anti-p-T34 | p-T34 DARPP-32 | pAb | Rabbit | Abcam Ab254063 |
| Anti-APP | APP (APP695, APP770, APP751) | pAb | Rabbit | Cell Signaling Technology (2452) |
| Anti-APPβ | sAPPβ | pAb | Rabbit | IBL (18957) |
| Anti-BACE1 | BACE1 (D10E5) | mAb | Rabbit | Cell Signaling Technology (5606) |
| Anti-PS1 | PS1 | pAb | Rabbit | Cell Signaling Technology (3622) |
| Anti-AEP | Legumain (D6S4H) | mAb | Rabbit | Cell Signaling Technology (93627) |
| Anti-IDE | IDE | pAb | Rabbit | Abcam (ab32216) |
| Anti-NEP | CD10/MME | pAb | Rabbit | ABclonal (A5664) |
| Anti-LRP1 | LRP1 | pAb | Rabbit | ABclonal (A13509) |
| Anti-PSD95 | PSD95 N-terminal | mAb | Rabbit | Cell Signaling (2507) |
| Anti-Synapsin1 | Synapsin1 | pAb | Rabbit | Millipore (AB1543) |
| Anti-GFAP | GFAP C-terminus | pAb | Rabbit | ABclonal (A14673) |
| Anti-IBA1 | AIF1/IBA1 | mAb | Rabbit | ABclonal (A19776) |
| Anti-β-actin | β-actin | pAb | Rabbit | ABclonal, China (AC026) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahaman, Y.A.R.; Feng, J.; Huang, F.; Salissou, M.T.M.; Wang, J.; Liu, R.; Zhang, B.; Li, H.; Zhu, F.; Wang, X. Moringa Oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice. Nutrients 2022, 14, 4284. https://doi.org/10.3390/nu14204284
Mahaman YAR, Feng J, Huang F, Salissou MTM, Wang J, Liu R, Zhang B, Li H, Zhu F, Wang X. Moringa Oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice. Nutrients. 2022; 14(20):4284. https://doi.org/10.3390/nu14204284
Chicago/Turabian StyleMahaman, Yacoubou Abdoul Razak, Jun Feng, Fang Huang, Maibouge Tanko Mahamane Salissou, Jianzhi Wang, Rong Liu, Bin Zhang, Honglian Li, Feiqi Zhu, and Xiaochuan Wang. 2022. "Moringa Oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice" Nutrients 14, no. 20: 4284. https://doi.org/10.3390/nu14204284
APA StyleMahaman, Y. A. R., Feng, J., Huang, F., Salissou, M. T. M., Wang, J., Liu, R., Zhang, B., Li, H., Zhu, F., & Wang, X. (2022). Moringa Oleifera Alleviates Aβ Burden and Improves Synaptic Plasticity and Cognitive Impairments in APP/PS1 Mice. Nutrients, 14(20), 4284. https://doi.org/10.3390/nu14204284










