The Role of Diet and Dietary Patterns in Parkinson’s Disease
Abstract
:1. Introduction
2. General Dietary Patterns
2.1. Defining a Healthy Dietary Pattern
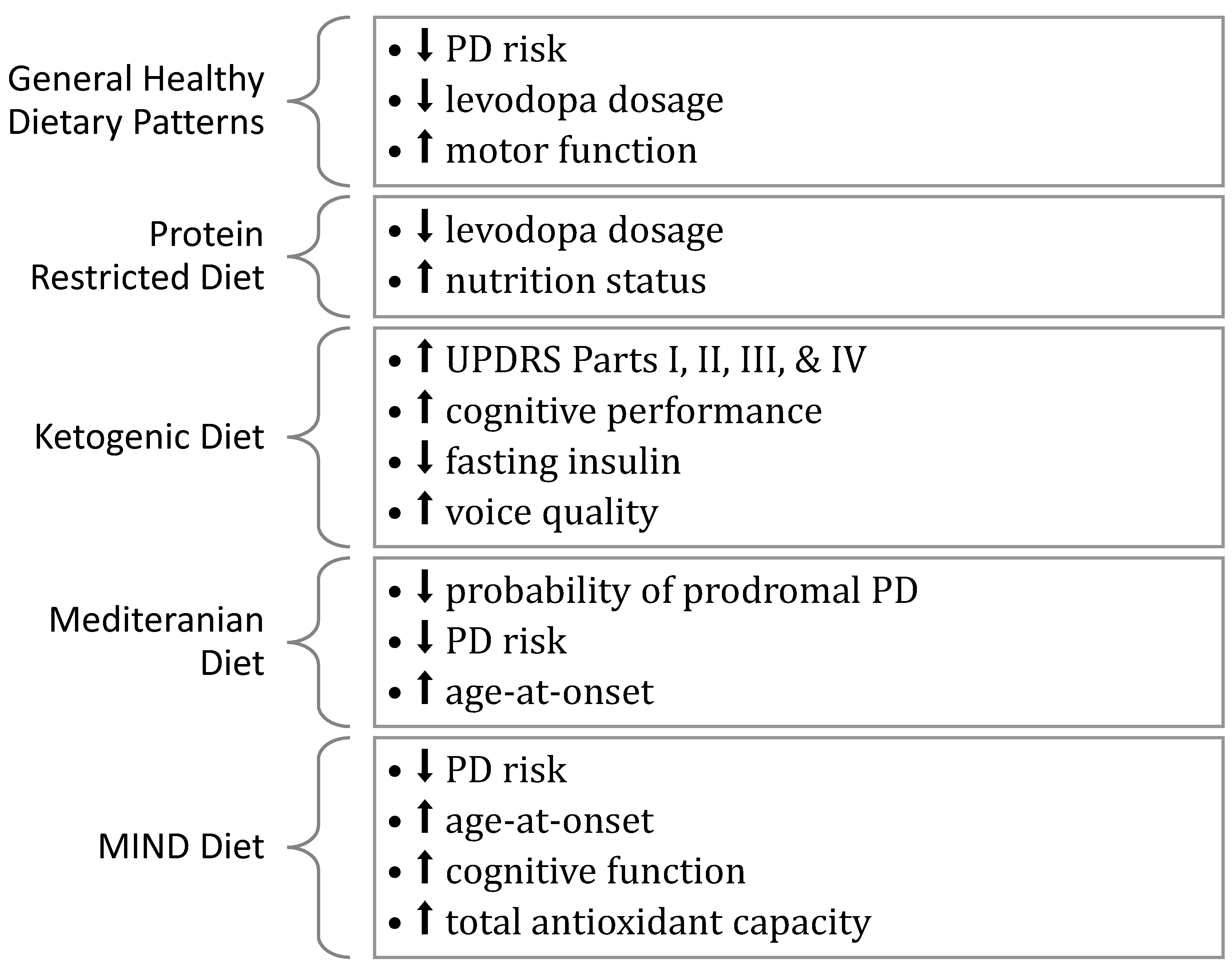
2.2. The Impact of General Dietary Patterns on PD
3. Protein-Restricted Diet
3.1. Defining a Protein-Restricted Diet
3.2. The Impact of a Protein-Restricted Diet on PD
4. Ketogenic Diet
4.1. Defining the Ketogenic Diet
4.2. The Impact of the Ketogenic Diet on PD
5. Mediterranean Diet
5.1. Defining the Mediterranean Diet
5.2. Impact of the Mediterranean Diet on PD
6. MIND Diet
6.1. Defining the MIND Diet
6.2. Impact of the MIND Diet on PD
7. Summary
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. Npj Parkinson’s Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J. An essay on the shaking palsy. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 2018, 46 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef] [Green Version]
- Goetz, C.G. The history of Parkinson’s disease: Early clinical descriptions and neurological therapies. Cold Spring Harb. Perspect. Med. 2011, 1, a008862. [Google Scholar] [CrossRef] [Green Version]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease-lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Zhang, M.; Yang, Y.; Wong, G. PDmethDB. A curated Parkinson’s disease associated methylation information database. Comput. Struct. Biotechnol. J. 2020, 18, 3745–3749. [Google Scholar] [CrossRef] [PubMed]
- De Rui, M.; Inelmen, E.M.; Trevisan, C.; Pigozzo, S.; Manzato, E.; Sergi, G. Parkinson’s disease and the non-motor symptoms: Hyposmia, weight loss, osteosarcopenia. Aging Clin. Exp. Res. 2020, 32, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Roheger, M.; Kalbe, E.; Liepelt-Scarfone, I. Progression of Cognitive Decline in Parkinson’s Disease. J. Parkinsons Dis. 2018, 8, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 1464–1470. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Seppi, K.; Poewe, W. The Concept of Prodromal Parkinson’s Disease. J. Parkinsons Dis. 2015, 5, 681–697. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martín, P.; Rodríguez-Blázquez, C.; Mario, A.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Rodríguez-Violante, M.; et al. Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.B.; Woo, H.; Lee, S.Y.; Cheon, S.M.; Kim, J.W. The burden of care and the understanding of disease in Parkinson’s disease. PLoS ONE 2019, 14, e0217581. [Google Scholar] [CrossRef]
- Trang, I.; Katz, M.; Galifianakis, N.; Fairclough, D.; Sillau, S.H.; Miyasaki, J.; Kluger, B.M. Predictors of general and health-related quality of life in Parkinson’s disease and related disorders including caregiver perspectives. Parkinsonism Relat. Disord. 2020, 77, 5–10. [Google Scholar] [CrossRef]
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016, a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Shalash, A.; Roushdy, T.; Essam, M.; Fathy, M.; Dawood, N.L.; Abushady, E.M.; Elrassas, H.; Helmi, A.; Hamid, E. Mental Health, Physical Activity, and Quality of Life in Parkinson’s Disease During COVID-19 Pandemic. Mov. Disord. 2020, 35, 1097–1099. [Google Scholar] [CrossRef]
- Brown, E.G.; Chahine, L.M.; Goldman, S.M.; Korell, M.; Mann, E.; Kinel, D.R.; Arnedo, V.; Marek, K.L.; Tanner, C.M. The Effect of the COVID-19 Pandemic on People with Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 1365–1377. [Google Scholar] [CrossRef]
- Brooks, S.K.; Weston, D.; Greenberg, N. Social and psychological impact of the COVID-19 pandemic on people with Parkinson’s disease: A scoping review. Public Health 2021, 199, 77–86. [Google Scholar] [CrossRef]
- Ongun, N. Does nutritional status affect Parkinson’s Disease features and quality of life? PLoS ONE 2018, 13, e0205100. [Google Scholar] [CrossRef]
- Gruber, M.T.; Witte, O.W.; Grosskreutz, J.; Prell, T. Association between malnutrition, clinical parameters and health-related quality of life in elderly hospitalized patients with Parkinson’s disease: A cross-sectional study. PLoS ONE 2020, 15, e0232764. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Ghazi, L.; Shafieesabet, M.; Shahidi, G.A.; Delbari, A.; Lökk, J. Motor, psychiatric and fatigue features associated with nutritional status and its effects on quality of life in Parkinson’s disease patients. PLoS ONE 2014, 9, e91153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enteral. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Aziz, N.A.S.; Teng, N.; Abdul Hamid, M.R.; Ismail, N.H. Assessing the nutritional status of hospitalized elderly. Clin. Interv. Aging 2017, 12, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, A.J.; Buitrago, G.; Rodríguez, N.; Gómez, G.; Sulo, S.; Gómez, C.; Partridge, J.; Misas, J.; Dennis, R.; Alba, M.J.; et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin. Nutr. 2019, 38, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Hudson, L.; Chittams, J.; Griffith, C.; Compher, C. Malnutrition Identified by Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition Is Associated with More 30-Day Readmissions, Greater Hospital Mortality, and Longer Hospital Stays: A Retrospective Analysis of Nutrition Assessment Data in a Major Medical Center. JPEN J. Parenter. Enteral. Nutr. 2018, 42, 892–897. [Google Scholar] [PubMed]
- Paul, B.S.; Singh, T.; Paul, G.; Jain, D.; Singh, G.; Kaushal, S.; Chhina, R.S. Prevalence of Malnutrition in Parkinson’s Disease and Correlation with Gastrointestinal Symptoms. Ann. Indian. Acad. Neurol. 2019, 22, 447–452. [Google Scholar] [CrossRef]
- Ma, K.; Xiong, N.; Shen, Y.; Han, C.; Liu, L.; Zhang, G.; Wang, L.; Guo, S.; Guo, X.; Xia, Y.; et al. Weight Loss and Malnutrition in Patients with Parkinson’s Disease: Current Knowledge and Future Prospects. Front. Aging Neurosci. 2018, 10, 1. [Google Scholar] [CrossRef]
- Yong, V.W.; Tan, Y.J.; Ng, Y.D.; Choo, X.Y.; Sugumaran, K.; Chinna, K.; Md Shah, M.N.; Raja Aman, R.R.A.; Moy, F.M.; Mohd Ramli, N.; et al. Progressive and accelerated weight and body fat loss in Parkinson’s disease: A three-year prospective longitudinal study. Parkinsonism Relat. Disord. 2020, 77, 28–35. [Google Scholar] [CrossRef]
- Bazán-Rodríguez, L.; Cruz-Vicioso, R.; Cervantes-Arriaga, A.; Alcocer-Salas, A.; Pinto-Solís, D.; Rodríguez-Violante, M. Malnutrition and Associated Motor and Non-motor Factors in People with Parkinson’s Disease. Rev. Invest. Clin. 2020, 72, 293–299. [Google Scholar] [CrossRef]
- Yang, T.; Zhan, Z.; Zhang, L.; Zhu, J.; Liu, Y.; Zhang, L.; Ge, J.; Zhao, Y.; Zhang, L.; Dong, J. Prevalence and Risk Factors for Malnutrition in Patients with Parkinson’s Disease. Front. Neurol. 2020, 11, 533731. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Coffin, B.; Gourcerol, G. Gastroparesis in Parkinson Disease: Pathophysiology, and Clinical Management. Brain Sci. 2021, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, G.; Furuya, H. Management of Dysphagia in Patients with Parkinson’s Disease and Related Disorders. Intern Med. 2020, 59, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Liria, R.; Parra-Egeda, J.; Vega-Ramírez, F.A.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Morales-Gázquez, M.J.; Rocamora-Pérez, P. Treatment of Dysphagia in Parkinson’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4140. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.S.; Oranje, O.J.M.; Freriksen, A.F.D.; Berendse, H.W.; Boesveldt, S. Flavor perception and the risk of malnutrition in patients with Parkinson’s disease. J. Neural Transm. 2018, 125, 925–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Bandres-Ciga, S.; Diez-Fairen, M.; Quinn, J.P.; Billingsley, K.J. Genetic Risk Profiling in Parkinson’s Disease and Utilizing Genetics to Gain Insight into Disease-Related Biological Pathways. Int. J. Mol. Sci. 2020, 21, 7332. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinsons Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Belvisi, D.; Pellicciari, R.; Fabbrini, G.; Tinazzi, M.; Berardelli, A.; Defazio, G. Modifiable risk and protective factors in disease development, progression and clinical subtypes of Parkinson’s disease: What do prospective studies suggest? Neurobiol. Dis. 2020, 134, 104671. [Google Scholar] [CrossRef]
- Chromiec, P.A.; Urbaś, Z.K.; Jacko, M.; Kaczor, J.J. The Proper Diet and Regular Physical Activity Slow Down the Development of Parkinson Disease. Aging Dis. 2021, 12, 1605. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Bai, C.H. The Effect of Caffeine on the Risk and Progression of Parkinson’s Disease: A Meta-Analysis. Nutrients 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wolk, A.; Håkansson, N.; Pedersen, N.L.; Wirdefeldt, K. Dietary antioxidants and risk of Parkinson’s disease in two population-based cohorts. Mov. Disord. 2017, 32, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-de-Mesquita, B.; Gallo, V. Vitamin A and carotenoids and the risk of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 2014, 42, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.; Wong, A.S.Y.; Tan, E.K.; Yuan, J.M.; Koh, W.P.; Tan, L.C.S. Dietary Antioxidants and Risk of Parkinson’s Disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Rimm, E.B.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of antioxidant vitamins and risk of Parkinson’s disease. Mov. Disord. 2016, 31, 1909–1914. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, Y.; Zhang, H.; Wang, L.; Wang, T.; Han, Z.; Wu, L.; Liu, G. Effect of plasma vitamin C levels on Parkinson’s disease and age at onset: A Mendelian randomization study. J. Transl. Med. 2021, 19, 221. [Google Scholar] [CrossRef]
- Park, H.A.; Ellis, A.C. Dietary Antioxidants and Parkinson’s Disease. Antioxidants 2020, 9, 570. [Google Scholar] [CrossRef]
- Dong, J.; Beard, J.D.; Umbach, D.M.; Park, Y.; Huang, X.; Blair, A.; Kamel, F.; Chen, H. Dietary fat intake and risk for Parkinson’s disease. Mov. Disord. 2014, 29, 1623–1630. [Google Scholar] [CrossRef] [Green Version]
- Newby, P.K.; Muller, D.; Tucker, K.L. Associations of empirically derived eating patterns with plasma lipid biomarkers: A comparison of factor and cluster analysis methods. Am. J. Clin. Nutr. 2004, 80, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Understanding meal patterns: Definitions, methodology and impact on nutrient intake and diet quality. Nutr. Res. Rev. 2015, 28, 1–21. [Google Scholar] [CrossRef]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Morze, J.; Danielewicz, A.; Hoffmann, G.; Schwingshackl, L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2020, 120, 1998–2031.e1915. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Juraschek, S.P.; Miller, E.R., 3rd; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef]
- Jafari Nasab, S.; Ghanavati, M.; Rafiee, P.; Bahrami, A.; Majidi, N.; Clark, C.C.T.; Sadeghi, A.; Houshyari, M.; Hejazi, E. A case-control study of Dietary Approaches to Stop Hypertension (DASH) diets, colorectal cancer and adenomas among Iranian population. BMC Cancer 2021, 21, 1050. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- McCarty, M.F.; Lerner, A. Perspective: Low Risk of Parkinson’s Disease in Quasi-Vegan Cultures May Reflect GCN2-Mediated Upregulation of Parkin. Adv. Nutr. 2021, 12, 355–362. [Google Scholar] [CrossRef]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid Med. Cell Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [Green Version]
- Olsson, E.; Byberg, L.; Höijer, J.; Kilander, L.; Larsson, S.C. Milk and Fermented Milk Intake and Parkinson’s Disease: Cohort Study. Nutrients 2020, 12, 2763. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of dairy foods and risk of Parkinson disease. Neurology 2017, 89, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.; Fossati, C.; Casali, M.; De Pandis, M.F.; Grassini, P.; Radicati, F.G.; Stirpe, P.; Vacca, L.; Iavicoli, I.; Leso, V.; et al. Effect of family history, occupation and diet on the risk of Parkinson disease: A case-control study. PLoS ONE 2020, 15, e0243612. [Google Scholar] [CrossRef]
- Okubo, H.; Miyake, Y.; Sasaki, S.; Murakami, K.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T. Dietary patterns and risk of Parkinson’s disease: A case–control study in Japan. Eur. J. Neurol. 2012, 19, 681–688. [Google Scholar] [CrossRef]
- Molsberry, S.; Bjornevik, K.; Hughes, K.C.; Healy, B.; Schwarzschild, M.; Ascherio, A. Diet pattern and prodromal features of Parkinson disease. Neurology 2020, 95, e2095–e2108. [Google Scholar] [CrossRef]
- Sääksjärvi, K.; Knekt, P.; Lundqvist, A.; Männistö, S.; Heliövaara, M.; Rissanen, H.; Järvinen, R. A cohort study on diet and the risk of Parkinson’s disease: The role of food groups and diet quality. Br. J. Nutr. 2013, 109, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Gallo, V.; Vineis, P.; Middleton, L.T.; Forsgren, L.; Sacerdote, C.; Sieri, S.; Kyrozis, A.; Chirlaque, M.D.; Zamora-Ros, R.; et al. Alcohol Consumption and Risk of Parkinson’s Disease: Data from a Large Prospective European Cohort. Mov. Disord. 2020, 35, 1258–1263. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jensen, G.L.; Na, M.; Mitchell, D.C.; Wood, G.C.; Still, C.D.; Gao, X. Diet Quality and Risk of Parkinson’s Disease: A Prospective Study and Meta-Analysis. J. Parkinsons. Dis. 2021, 11, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND Diet Associated with Reduced Incidence and Delayed Progression of Parkinsonism in Old Age. J. Nutr. Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Hegelmaier, T.; Lebbing, M.; Duscha, A.; Tomaske, L.; Tönges, L.; Holm, J.B.; Bjørn Nielsen, H.; Gatermann, S.G.; Przuntek, H.; Haghikia, A. Interventional Influence of the Intestinal Microbiome Through Dietary Intervention and Bowel Cleansing Might Improve Motor Symptoms in Parkinson’s Disease. Cells 2020, 9, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.K.; Verma, S.; Jain, V.; Surapaneni, B.K.; Vinayek, R.; Phillips, L.; Nair, P.P. Parkinson’s Disease: The Emerging Role of Gut Dysbiosis, Antibiotics, Probiotics, and Fecal Microbiota Transplantation. J. Neurogastroenterol. Motil. 2019, 25, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, N.; Garcia, J.J.; Diez, M.J.; Sahagun, A.M.; Díez, R.; Sierra, M. Effects of dietary factors on levodopa pharmacokinetics. Expert. Opin. Drug Metab. Toxicol. 2010, 6, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Cereda, E.; Barichella, M.; Pezzoli, G. Controlled-protein dietary regimens for Parkinson’s disease. Nutr. Neurosci. 2010, 13, 29–32. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, N.; Huang, J.; Guo, S.; Liu, L.; Han, C.; Zhang, G.; Jiang, H.; Ma, K.; Xia, Y.; et al. Protein-Restricted Diets for Ameliorating Motor Fluctuations in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Boelens Keun, J.T.; Arnoldussen, I.A.; Vriend, C.; van de Rest, O. Dietary Approaches to Improve Efficacy and Control Side Effects of Levodopa Therapy in Parkinson’s Disease: A Systematic Review. Adv. Nutr. 2021, 12, 2265–2287. [Google Scholar] [CrossRef]
- Juncos, J.L.; Fabbrini, G.; Mouradian, M.M.; Serrati, C.; Chase, T.N. Dietary influences on the antiparkinsonian response to levodopa. Arch. Neurol. 1987, 44, 1003–1005. [Google Scholar] [CrossRef]
- Silva, Z.C.M.; Carol Fritzen, N.; de Oliveira, M.; Paes da Silva, M.; Rasmussen Petterle, R.; Teive, H.A.; de Mesquita Barros Almeida Leite, C.; Rabito, E.I.; Madalozzo Schieferdecker, M.E.; Carvalho, M. Protein intake, nitrogen balance and nutritional status in patients with Parkinson’s disease; time for a change? Nutr. Hosp. 2015, 31, 2764–2770. [Google Scholar]
- Barichella, M.; Cereda, E.; Cassani, E.; Pinelli, G.; Iorio, L.; Ferri, V.; Privitera, G.; Pasqua, M.; Valentino, A.; Monajemi, F.; et al. Dietary habits and neurological features of Parkinson’s disease patients: Implications for practice. Clin. Nutr. 2017, 36, 1054–1061. [Google Scholar] [CrossRef]
- Cucca, A.; Mazzucco, S.; Bursomanno, A.; Antonutti, L.; Di Girolamo, F.G.; Pizzolato, G.; Koscica, N.; Gigli, G.L.; Catalan, M.; Biolo, G. Amino acid supplementation in l-dopa treated Parkinson’s disease patients. Clin. Nutr. 2015, 34, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Tazan, S.; Mazzoni, P.; Ford, B.; Greene, P.E. Motor fluctuations due to interaction between dietary protein and levodopa in Parkinson’s disease. J. Clin. Mov. Disord. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49 (Suppl. S8), 3–5. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM. a systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef] [Green Version]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [Green Version]
- Lilamand, M.; Porte, B.; Cognat, E.; Hugon, J.; Mouton-Liger, F.; Paquet, C. Are ketogenic diets promising for Alzheimer’s disease? A translational review. Alzheimers Res. Ther. 2020, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Avgerinos, K.I.; Egan, J.M.; Mattson, M.P.; Kapogiannis, D. Medium Chain Triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res. Rev. 2020, 58, 101001. [Google Scholar] [CrossRef]
- Roehl, K.; Sewak, S.L. Practice Paper of the Academy of Nutrition and Dietetics: Classic and Modified Ketogenic Diets for Treatment of Epilepsy. J. Acad. Nutr. Diet. 2017, 117, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossoff, E.H.; Dorward, J.L. The modified Atkins diet. Epilepsia 2008, 49 (Suppl. S8), 37–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Wang, H.S. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed. J. 2013, 36, 9–15. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef] [Green Version]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin. Parkinsonism Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef]
- Koyuncu, H.; Fidan, V.; Toktas, H.; Binay, O.; Celik, H. Effect of ketogenic diet versus regular diet on voice quality of patients with Parkinson’s disease. Acta Neurol. Belg. 2021, 121, 1729–1732. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Varela, L.M.; Martin, M.E.; Bermudez, B.; Montserrat-de la Paz, S. High-Density Lipoproteins and Mediterranean Diet: A Systematic Review. Nutrients 2021, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Ghasemi-Nasab, M.; Riasatian, M. Mediterranean diet for patients with non-alcoholic fatty liver disease, a systematic review and meta-analysis of observational and clinical investigations. J. Diabetes Metab. Disord. 2020, 19, 575–584. [Google Scholar] [CrossRef]
- Gołąbek, K.D.; Regulska-Ilow, B. Dietary support in insulin resistance: An overview of current scientific reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef]
- Fresán, U.; Bes-Rastrollo, M.; Segovia-Siapco, G.; Sanchez-Villegas, A.; Lahortiga, F.; de la Rosa, P.-A.; Martínez-Gonzalez, M.-A. Does the MIND diet decrease depression risk? A comparison with Mediterranean diet in the SUN cohort. Eur. J. Nutr. 2019, 58, 1271–1282. [Google Scholar] [CrossRef]
- Sparling, P.B. Legacy of Nutritionist Ancel Keys. Mayo Clin. Proc. 2020, 95, 615–617. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, A.; Jennings, A.; Hayhoe, R.P.G.; Awuzudike, V.E.; Welch, A.A. High variability of food and nutrient intake exists across the Mediterranean Dietary Pattern-A systematic review. Food Sci. Nutr. 2020, 8, 4907–4918. [Google Scholar] [CrossRef]
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaragoza-Martí, A.; Cabañero-Martínez, M.J.; Hurtado-Sánchez, J.A.; Laguna-Pérez, A.; Ferrer-Cascales, R. Evaluation of Mediterranean diet adherence scores: A systematic review. BMJ Open 2018, 8, e019033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Moreno-Franco, B.; Ordovás, J.M.; León, M.; Casasnovas, J.A.; Peñalvo, J.L. Design and development of an instrument to measure overall lifestyle habits for epidemiological research: The Mediterranean Lifestyle (MEDLIFE) index. Public Health Nutr. 2015, 18, 959–967. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Gu, Y.; Mejia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The association between Mediterranean diet adherence and Parkinson’s disease. Mov. Disord. 2012, 27, 771–774. [Google Scholar] [CrossRef] [Green Version]
- Cassani, E.; Barichella, M.; Ferri, V.; Pinelli, G.; Iorio, L.; Bolliri, C.; Caronni, S.; Faierman, S.A.; Mottolese, A.; Pusani, C.; et al. Dietary habits in Parkinson’s disease: Adherence to Mediterranean diet. Parkinsonism Relat. Disord. 2017, 42, 40–46. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Yu, A.C.; Golz, E.; Cirstea, M.; Sundvick, K.; Kliger, D.; Foulger, L.H.; Mackenzie, M.; Finlay, B.B.; Appel-Cresswell, S. MIND and Mediterranean Diets Associated with Later Onset of Parkinson’s Disease. Mov. Disord. 2021, 36, 977–984. [Google Scholar] [CrossRef]
- Yin, W.; Löf, M.; Pedersen, N.L.; Sandin, S.; Fang, F. Mediterranean Dietary Pattern at Middle Age and Risk of Parkinson’s Disease: A Swedish Cohort Study. Mov. Disord. 2021, 36, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D.N. Adherence to a Mediterranean diet and survival in a Greek population. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Hashemian, M.; Murphy, G.; Etemadi, A.; Liao, L.M.; Dawsey, S.M.; Malekzadeh, R.; Abnet, C.C. Potato consumption and the risk of overall and cause specific mortality in the NIH-AARP study. PLoS ONE 2019, 14, e0216348. [Google Scholar] [CrossRef] [Green Version]
- Paknahad, Z.; Sheklabadi, E.; Derakhshan, Y.; Bagherniya, M.; Chitsaz, A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement. Ther. Med. 2020, 50, 102366. [Google Scholar] [CrossRef]
- Paknahad, Z.; Sheklabadi, E.; Moravejolahkami, A.R.; Chitsaz, A.; Hassanzadeh, A. The effects of Mediterranean diet on severity of disease and serum Total Antioxidant Capacity (TAC) in patients with Parkinson’s disease: A single center, randomized controlled trial. Nutr. Neurosci. 2020, 25, 313–320. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Corley, J. Adherence to the MIND diet is associated with 12-year all-cause mortality in older adults. Public Health Nutr. 2020, 25, 358–367. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Keshteli, A.H.; Mousavi, S.M.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to the MIND diet and prevalence of psychological disorders in adults. J. Affect. Disord. 2019, 256, 96–102. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Holland, T.; Agarwal, P.; Aggarwal, N.; Morris, M.C. DASH and Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Fewer Depressive Symptoms Over Time. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kheirouri, S.; Alizadeh, M. MIND diet and cognitive performance in older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talegawkar, S.A.; Jin, Y.; Simonsick, E.M.; Tucker, K.L.; Ferrucci, L.; Tanaka, T. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet is associated with physical function and grip strength in older men and women. Am. J. Clin. Nutr. 2021, 115, 625–632. [Google Scholar] [CrossRef] [PubMed]
| Citation | Year Published | Study Type | Diet Type | n | Location | Key Results |
|---|---|---|---|---|---|---|
| Alcalay et al. [128] | 2012 | Case-Control | MD | 257 cases 198 controls | United States | Greater adherence to the MD was associated with a reduced risk of PD and later age-at-onset of PD. |
| Okubo et al. [77] | 2012 | Case-Control | Healthy Western Light Meal | 249 cases 368 controls | Japan | The healthy dietary pattern was associated with a reduced risk of PD, but not statistically significant (p = 0.06). The light meal and Western dietary patterns were not associated with PD risk. |
| Sääksjärvi et al. [79] | 2012 | Cohort | AHEI | 4524 (85 cases) | Finland | Adherence to the AHEI was not associated with PD risk. Greater intake of berries was associated with a reduced risk of PD in women but was associated with an increased risk among men. |
| Virmani et al. [93] | 2016 | Cohort | PRD | 1037 (1037 cases) | United States | Only 5.9% of participants on levodopa reported PIL. Only 20 participants reported following a PRD. |
| Barichella et al. [91] | 2017 | Case-Control | PRD | 600 cases 600 controls | Italy | Adherence to a PRD was associated with a lower levodopa dosage. Protein intake was not associated with levodopa-related motor complications. An intake of 10 g protein over 0.8 g/kg/day was associated with an increase in levodopa dosage by 0.7 mg/kg. |
| Cassani et al. [129] | 2017 | Case-Control | MD | 600 cases 600 controls | Italy | No difference in adherence to the MD existed between cases and controls. Adherence to the MD was not associated with disease duration, or age-at-onset. |
| Mischley et al. [73] | 2017 | Cross-Sectional | General | 1053 (1053 cases) | United States | A plant/fish based dietary pattern was associated with a reduced rate of PD progression. Foods associated with a reduced rate: fresh vegetables, fresh fruit, nuts, seeds, fish, olive oil, coconut oil, and wine. Foods associated with an increased rate: canned vegetables, canned fruit, beef, fried food, cheese, yogurt, ice cream, and soda. |
| Agarwal et al. [82] | 2018 | Cohort | DASH MD MIND | 706 (302 cases) | United States | The DASH Diet was not associated with PD risk. Both the MIND diet and the MD were associated with a reduced risk of PD, with the MIND diet having the strongest relationship to PD risk. Each unit increase in the MIND diet score was associated with a 13% reduction in PD risk. |
| Maraki et al. [133] | 2018 | Cohort | MD | 1765 (34 cases) | Greece | Adherence to the MD was associated with a lower probability of prodromal PD. The study’s results remained unchanged after excluding constipation as a feature of prodromal PD. |
| Liu et al. [81] | 2020 | Cohort | DST | 3653 (47 cases) | United States | Greater diet quality was associated with a significantly reduced risk of PD. |
| Molsberry et al. [78] | 2020 | Cohort | MD AHEI | 17,400 | United States | Greater adherence to both the MD and AHEI was associated with a reduced risk of developing features of prodromal PD. |
| Metcalfe-Roach et al. [130] | 2021 | Case-Control | MD MIND | 167 cases 119 controls | Canada | Greater adherence to the MIND diet or the Greek MD was associated with later age of onset of PD. The relationship was stronger for the MIND diet than the MD. The relationship between the MIND diet and age of onset was strongest among women, while the relationship between the MD (Panagiotakos et al. [134]) was strongest among men. |
| Yin et al. [131] | 2021 | Cohort | MD | 41,715 (101 cases) | Sweden | Greater adherence to the MD was associated with a reduced risk of PD. Each unit increase in MD score was associated with an 11% reduction in PD risk. |
| Citation | Year Published | Diet Manipulation | Length | Location | Key Results |
|---|---|---|---|---|---|
| General Dietary Patterns | |||||
| Hegelmaier et al. [83] | 2020 | Individuals with PD were randomized to receive either an enema for 8 days and an ovo-lacto-vegetarian diet (n = 10) or diet only (n = 6). | 2 weeks | Germany | Compared to baseline:
|
| PRD | |||||
| Cucca et al. [92] | 2015 | Individuals with PD on a PRD were randomized to consume either 16 g amino acid supplement (n = 12) or a placebo (n = 10) daily. | 6 months | Italy | Compared to baseline:
|
| KD | |||||
| Phillips et al. [105] | 2018 | Individuals with PD were randomized to follow either a low-fat diet (n = 23) or a KD (n = 24). | 8 weeks | New Zealand | Compared to baseline:
|
| Krikorian et al. [106] | 2019 | Individuals with PD randomized to follow either a low carbohydrate (n = 10) or a high carbohydrate (n = 8) diet. | 8 weeks | United States | Compared to baseline:
|
| Koyuncu et al. [107] | 2020 | Individuals with PD were randomized to follow either a KD (n = 37) or their regular diet (n = 37). | 3 months | Turkey | Compared to baseline:
|
| MD | |||||
| Paknabad et al. [137] | 2020 | Individuals with PD were randomized to follow either a MD (n = 40) or the traditional Iranian diet (n = 40). | 10 weeks | Iran | Compared to baseline:
|
| Paknahad et al. [136] | 2020 | Individuals with PD were randomized to follow either a MD (n = 40) or their regular diet (n = 40). | 10 weeks | Iran | Compared to baseline:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knight, E.; Geetha, T.; Burnett, D.; Babu, J.R. The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients 2022, 14, 4472. https://doi.org/10.3390/nu14214472
Knight E, Geetha T, Burnett D, Babu JR. The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients. 2022; 14(21):4472. https://doi.org/10.3390/nu14214472
Chicago/Turabian StyleKnight, Emily, Thangiah Geetha, Donna Burnett, and Jeganathan Ramesh Babu. 2022. "The Role of Diet and Dietary Patterns in Parkinson’s Disease" Nutrients 14, no. 21: 4472. https://doi.org/10.3390/nu14214472
APA StyleKnight, E., Geetha, T., Burnett, D., & Babu, J. R. (2022). The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients, 14(21), 4472. https://doi.org/10.3390/nu14214472