Eating Speed Is Associated with the Presence of Sarcopenia in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Assessments of Eating Speed and Habitual Dietary Intakes
2.4. Assessment of Sarcopenia
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, D. Type 2 Diabetes in the Elderly: Challenges in a Unique Patient Population. J. Geriatr. Med. Gerontol. 2016, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S139–S147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.C.; Drefahl, S.; Ahlbom, A.; Lambe, M.; Modig, K. Trends in life expectancy: Did the gap between the healthy and the ill widen or close? BMC Med. 2020, 18, 41. [Google Scholar] [CrossRef]
- Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liccini, A.; Malmstrom, T.K. Frailty and Sarcopenia as Predictors of Adverse Health Outcomes in Persons with Diabetes Mellitus. J. Am. Med. Dir. Assoc. 2016, 17, 846–851. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; Senmaru, T.; et al. Sarcopenia Is Associated with a Risk of Mortality in People with Type 2 Diabetes Mellitus. Front. Endocrinol. (Lausanne) 2021, 12, 783363. [Google Scholar] [CrossRef]
- Miyake, H.; Kanazawa, I.; Tanaka, K.I.; Sugimoto, T. Low skeletal muscle mass is associated with the risk of all-cause mortality in patients with type 2 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819842971. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Xu, R.; Liu, L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, Q.; Hu, K.; Wu, M.; Wang, Z.; Chen, F.; Mei, F.; Zhao, L.; Ma, B. Prevalence and risk factors of sarcopenia in patients with diabetes: A meta-analysis. J. Clin. Endocrinol. Metab. 2021, in press. [CrossRef]
- Yoshimura, Y.; Kamada, C.; Takahashi, K.; Kaimoto, T.; Iimuro, S.; Ohashi, Y.; Araki, A.; Umegaki, H.; Sakurai, T.; Ito, H.; et al. Relations of nutritional intake to age, sex and body mass index in Japanese elderly patients with type 2 diabetes: The Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2012, 12 (Suppl. S1), 29–40. [Google Scholar] [CrossRef] [PubMed]
- Rahi, B.; Morais, J.A.; Gaudreau, P.; Payette, H.; Shatenstein, B. Energy and protein intakes and their association with a decline in functional capacity among diabetic older adults from the NuAge cohort. Eur. J. Nutr. 2016, 55, 1729–1739. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kondo, Y.; Fukuda, T.; Kitagawa, N.; Okada, H.; et al. Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients 2021, 13, 2335. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Takahashi, F.; Hashimoto, Y.; Okamura, T.; Miki, A.; Kaji, A.; Sakai, R.; Kitagawa, N.; Senmaru, T.; Majima, S.; et al. Short energy intake is associated with muscle mass loss in older patients with type 2 diabetes: A prospective study of the KAMOGAWA-DM cohort. Clin. Nutr. 2021, 40, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of KAMOGAWA-DM cohort study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.S.; van Dam, R.M.; Whitton, C.; Tan, L.W.L.; Forde, C.G. Association Between Self-Reported Eating Rate, Energy Intake, and Cardiovascular Risk Factors in a Multi-Ethnic Asian Population. Nutrients 2020, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Kolay, E.; Bykowska-Derda, A.; Abdulsamad, S.; Kaluzna, M.; Samarzewska, K.; Ruchala, M.; Czlapka-Matyasik, M. Self-Reported Eating Speed Is Associated with Indicators of Obesity in Adults: A Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1559. [Google Scholar] [CrossRef]
- Sakai, R.; Hashimoto, Y.; Ushigome, E.; Miki, A.; Okamura, T.; Matsugasumi, M.; Fukuda, T.; Majima, S.; Matsumoto, S.; Senmaru, T.; et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr. J. 2018, 65, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Hashimoto, Y.; Kawano, R.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Ushigome, E.; Kitagawa, N.; Majima, S.; et al. Eating Fast Is Associated with Nonalcoholic Fatty Liver Disease in Men But Not in Women with Type 2 Diabetes: A Cross-Sectional Study. Nutrients 2020, 12, 2174. [Google Scholar] [CrossRef]
- Kim, M.; Shinkai, S.; Murayama, H.; Mori, S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr. Gerontol. Int. 2015, 15, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Miyaji, N.; Kochi, T.; Eguchi, M.; Kabe, I.; Mizoue, T. Eating speed and risk of metabolic syndrome among Japanese workers: The Furukawa Nutrition and Health Study. Nutrition 2020, 78, 110962. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Asahi, K.; Satoh, H.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Fujimoto, S.; Narita, I.; Konta, T.; et al. Fast eating is a strong risk factor for new-onset diabetes among the Japanese general population. Sci. Rep. 2019, 9, 8210. [Google Scholar] [CrossRef]
- Cao, X.; Gu, Y.; Bian, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Zhang, S.; Wang, Y.; Zhang, T.; et al. Association between eating speed and newly diagnosed nonalcoholic fatty liver disease among the general population. Nutr. Res. 2020, 80, 78–88. [Google Scholar] [CrossRef]
- Saito, Y.; Kajiyama, S.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Fukui, M.; Imai, S.; et al. Eating Fast Has a Significant Impact on Glycemic Excursion in Healthy Women: Randomized Controlled Cross-Over Trial. Nutrients 2020, 12, 2767. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakamura, Y.; Takashima, N.; Kadota, A.; Miura, K.; Ueshima, H.; Kita, Y. Eating Slowly Is Associated with Undernutrition among Community-Dwelling Adult Men and Older Adult Women. Nutrients 2021, 14, 54. [Google Scholar] [CrossRef]
- Otsuka, R.; Tamakoshi, K.; Yatsuya, H.; Wada, K.; Matsushita, K.; OuYang, P.; Hotta, Y.; Takefuji, S.; Mitsuhashi, H.; Sugiura, K.; et al. Eating fast leads to insulin resistance: Findings in middle-aged Japanese men and women. Prev. Med. 2008, 46, 154–159. [Google Scholar] [PubMed]
- Robinson, E.; Almiron-Roig, E.; Rutters, F.; de Graaf, C.; Forde, C.G.; Tudur Smith, C.; Nolan, S.J.; Jebb, S.A. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am. J. Clin. Nutr. 2014, 100, 123–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakata, T.; Yoshimatsu, H.; Masaki, T.; Tsuda, K. Anti-obesity actions of mastication driven by histamine neurons in rats. Exp. Biol. Med. (Maywood) 2003, 228, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, A.; le Roux, C.W.; Alexiadou, K.; Tentolouris, N.; Vincent, R.P.; Kyriaki, D.; Perrea, D.; Ghatei, M.A.; Bloom, S.R.; Katsilambros, N.; et al. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. J. Clin. Endocrinol. Metab. 2010, 95, 333–337. [Google Scholar] [CrossRef]
- Miyazawa, I.; Morino, K.; Fuse, K.; Kondo, K.; Ohi, A.; Nishida, K.; Kurihara, M.; Yasuhara, S.; Nakanishi, N.; Nishida, Y.; et al. Impact of obesity on underreporting of energy intake in type 2 diabetic patients: Clinical Evaluation of Energy Requirements in Patients with Diabetes Mellitus (CLEVER-DM) study. Clin. Nutr. ESPEN 2020, 39, 251–254. [Google Scholar] [CrossRef]
- Shiraishi, A.; Wakabayashi, H.; Yoshimura, Y. Oral Management in Rehabilitation Medicine: Oral Frailty, Oral Sarcopenia, and Hospital-Associated Oral Problems. J. Nutr. Health Aging 2020, 24, 1094–1099. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K.; et al. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar]
- Kaji, A.; Hashimoto, Y.; Kobayashi, Y.; Sakai, R.; Okamura, T.; Miki, A.; Hamaguchi, M.; Kuwahata, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with tongue pressure in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2019, 19, 153–158. [Google Scholar] [CrossRef]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Obuchi, S.; Kawai, H.; Fujiwara, Y.; et al. Relationship between oral hypofunction and sarcopenia in community-dwelling older adults: The otassha study. Int. J. Environ. Res. Public Health 2021, 18, 6666. [Google Scholar] [CrossRef]
- Hatta, K.; Ikebe, K. Association between oral health and sarcopenia: A literature review. J. Prosthodont. Res. 2021, 65, 131–136. [Google Scholar] [CrossRef]
- Chung, S.M.; Moon, J.S.; Chang, M.C. Prevalence of Sarcopenia and Its Association With Diabetes: A Meta-Analysis of Community-Dwelling Asian Population. Front. Med. 2021, 8, 681232. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Okamura, T.; Iwai, K.; Hashimoto, Y.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; Asano, M.; Yamazaki, M.; Fukui, M. Japanese radio calisthenics prevents the reduction of skeletal muscle mass volume in people with type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition management in older adults with diabetes: A review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef] [PubMed]

| All n = 239 | Fast, n = 113 | Normal, n = 77 | Slow, n = 49 | p | |
|---|---|---|---|---|---|
| Age, years | 71.6 (6.2) | 70.7 (6.2) | 72.1 (6.2) | 73.0 (6.0) | 0.075 |
| Men, % (n) | 58.6% (140) | 60.2% (68) | 62.3% (48) | 49.0% (24) | 0.297 |
| Duration of diabetes, years | 18.4 (11.5) | 17.9 (10.8) | 18.8 (13.1) | 19.1 (10.7) | 0.776 |
| Family history of diabetes, % (n) | 41.8% (100) | 46.0% (52) | 28.6% (22) | 53.1% (26) | 0.012 |
| Height, cm | 160.7 (8.6) | 161.4 (8.6) | 161.3 (8.1) | 158.0 (9.1) | 0.048 |
| Body weight, kg | 61.1 (10.8) | 63.3 (10.7) | 61.5 (10.6) | 55.2 (9.7) †‡ | <0.001 |
| Body mass index, kg/m2 | 23.6 (3.5) | 24.2 (3.1) | 23.6 (3.8) | 22.2 (3.8) †‡ | 0.004 |
| Appendicular muscle mass, kg | 17.8 (4.0) | 18.5 (4.1) | 18.1 (3.8) | 15.8 (3.7) †‡ | 0.001 |
| Skeletal muscle mass index, kg/m2 | 6.8 (1.0) | 7.0 (1.0) | 6.9 (1.1) | 6.2 (0.9) †‡ | <0.001 |
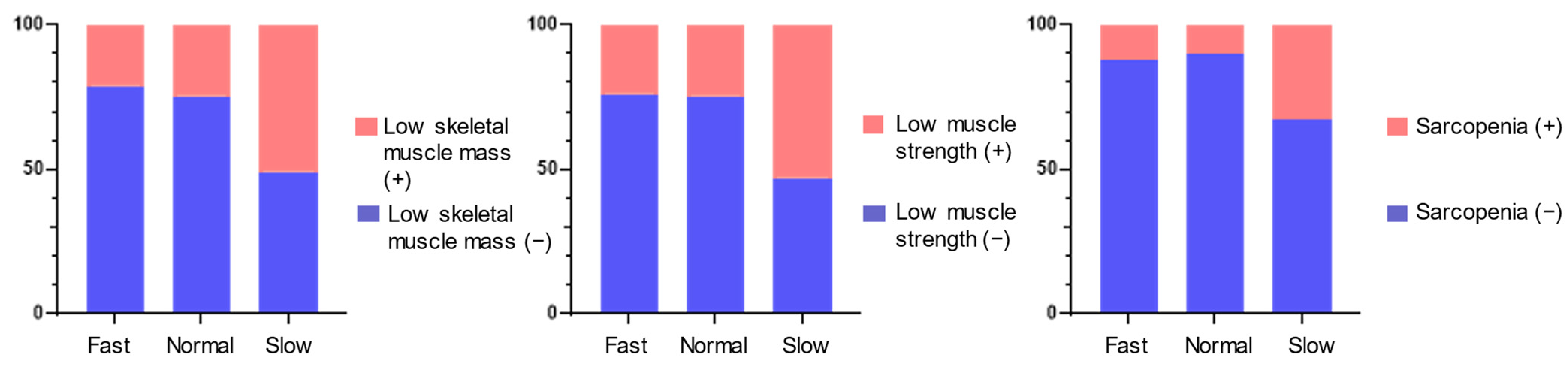
| Low skeletal muscle mass, % (n) | 28.4% (68) | 21.2% (24) | 24.7% (19) | 51.0% (25) | <0.001 |
| Handgrip strength, kg | 26.5 (8.3) | 27.9 (8.7) | 27.1 (7.2) | 22.4 (7.7) †‡ | <0.001 |
| Low muscle strength, % (n) | 29.3% (70) | 22.1% (25) | 24.7% (19) | 53.1% (26) | <0.001 |
| Presence of sarcopenia, % (n) | 15.9% (38) | 12.4% (14) | 10.4% (8) | 32.7% (16) | 0.001 |
| Insulin, % (n) | 23.5% (56) | 23.2% (26) | 23.4% (18) | 24.5% (12) | 0.984 |
| GLP-1 antagonist, % (n) | 8.4% (20) | 12.5% (14) | 5.2% (4) | 4.1% (2) | 0.097 |
| SGLT2 inhibitor, % (n) | 16.8% (40) | 22.3% (25) | 11.7% (9) | 12.2% (6) | 0.100 |
| Smoker, % (n) | 13.4% (32) | 15.9% (18) | 10.4% (8) | 12.2% (6) | 0.527 |
| Exerciser, % (n) | 50.6% (121) | 51.3% (58) | 52.0% (40) | 46.9% (23) | 0.843 |
| History of cancer, % (n) | 22.2% (53) | 24.8% (28) | 36.4 (28) | 30.6 (15) | 0.227 |
| History of heart diseases, % (n) | 29.7% (71) | 21.2 (24) | 23.4% (18) | 22.5% (11) | 0.940 |
| CKD stage ≥4, % (n) | 4.6% (11) | 0.9% (1) | 9.1% (7) | 6.3% (3) | 0.025 |
| HbA1c, mmol/mol | 54.3 (8.4) | 54.9 (8.6) | 53.8 (8.7) | 53.9 (7.4) | 0.683 |
| HbA1c, % | 7.1 (0.8) | 7.2 (0.8) | 7.1 (0.8) | 7.1 (0.7) | 0.683 |
| Plasma glucose, mmol/L | 8.1 (2.3) | 8.0 (2.1) | 8.1 (2.4) | 8.3 (2.6) | 0.678 |
| Total energy intake, kcal/day | 1778 (666) | 1765 (649) | 1849 (714) | 1699 (627) | 0.453 |
| Total energy intake, kcal/kg IBW/day | 31.3 (11.7) | 30.7 (10.9) | 32.6 (13.0) | 30.8 (11.6) | 0.523 |
| Protein intake, g/day | 75.9 (34.7) | 74.8 (30.1) | 81.4 (37.0) | 69.8 (29.5) | 0.130 |
| Protein intake, % Energy | 17.1 (3.5) | 17.1 (3.5) | 17.5 (3.6) | 16.4 (3.1) | 0.173 |
| Fat intake, g/day | 57.6 (25.3) | 56.7 (24.1) | 59.4 (26.2) | 56.6 (27.1) | 0.739 |
| Fat intake, % Energy | 29.2 (6.3) | 29.0 (6.5) | 29.1 (6.4) | 29.6 (5.7) | 0.862 |
| Carbohydrate intake, g/day | 221.7 (86.5) | 223.3 (90.5) | 229.6 (86.9) | 205.5 (75.2) | 0.303 |
| Carbohydrate intake, % Energy | 50.3 (8.7) | 50.7 (9.1) | 50.4 (8.2) | 49.3 (8.6) | 0.623 |
| Alcohol consumption, g/day | 0 (0–2.4) | 0 (0–2.9) | 0 (0–0.2) | 0.1 (0–9.9) ‡ | 0.033 |
| Dietary fiber intake, g/day | 12.6 (5.4) | 12.4 (5.4) | 13.3 (5.6) | 12.2 (5.2) | 0.416 |
| The presence of low muscle mass | Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Age (year) | ― | ― | 1.06 (1.01–1.12) | 0.011 | 1.07 (1.02–1.12) | 0.009 | 1.07 (1.02–1.13) | 0.009 |
| Men | ― | ― | 1.09 (0.60–2.01) | 0.770 | 1.22 (0.65–2.28) | 0.537 | 1.23 (0.65–2.32) | 0.528 |
| Insulin usage | ― | ― | ― | ― | 1.43 (0.72–2.87) | 0.303 | 1.49 (0.75–2.98) | 0.259 |
| Smoking | ― | ― | ― | ― | 0.82 (0.31–2.14) | 0.681 | 0.77 (0.29–2.03) | 0.593 |
| Exercise | ― | ― | ― | ― | 1.66 (0.91–3.05) | 0.106 | 1.64 (0.89–3.03) | 0.116 |
| Total energy intake (kcal/kg IBW/day) | ― | ― | ― | ― | 0.97 (0.94–1.01) | 0.092 | 0.97 (0.94–1.01) | 0.089 |
| CKD stage ≥4 | ― | ― | ― | ― | ― | ― | 0.68 (0.16–2.88) | 0.600 |
| History of cancer | ― | ― | ― | ― | ― | ― | 0.73 (0.34–1.56) | 0.415 |
| History of heart diseases | ― | ― | ― | ― | ― | ― | 0.84 (0.42–1.68) | 0.625 |
| Eating speed | ||||||||
| Fast | 0.26 (0.13–0.53) | <0.001 | 0.28 (0.14–0.59) | <0.001 | 0.27 (0.13–0.56) | <0.001 | 0.27 (0.12–0.57) | <0.001 |
| Normal | 0.31 (0.15–0.67) | 0.003 | 0.32 (0.15–0.69) | 0.004 | 0.31 (0.14–0.68) | 0.004 | 0.32 (0.14–0.71) | 0.005 |
| Slow | Reference | ― | Reference | ― | Reference | ― | Reference | ― |
| The presence of low handgrip strength | Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Age (year) | ― | ― | 1.18 (1.12–1.25) | <0.001 | 1.19 (1.12–1.26) | <0.001 | 1.19 (1.12–1.27) | <0.001 |
| Men | ― | ― | 0.58 (0.30–1.11) | 0.099 | 0.64 (0.33–1.25) | 0.191 | 0.59 (0.29–1.18) | 0.138 |
| Insulin usage | ― | ― | ― | ― | 1.85 (0.87–3.95) | 0.111 | 1.88 (0.86–4.09) | 0.112 |
| Smoking | ― | ― | ― | ― | 0.70 (0.22–2.19) | 0.535 | 0.65 (0.20–2.15) | 0.482 |
| Exercise | ― | ― | ― | ― | 1.58 (0.82–3.06) | 0.175 | 1.70 (0.86–3.37) | 0.129 |
| Total energy intake (kcal/kg IBW/day) | ― | ― | ― | ― | 1.00 (0.97–1.03) | 0.761 | 1.00 (0.97–1.03) | 0.923 |
| CKD stage ≥4 | ― | ― | ― | ― | ― | ― | 4.37 (1.00–19.1) | 0.005 |
| History of cancer | ― | ― | ― | ― | ― | ― | 0.37 (0.15–0.92) | 0.033 |
| History of heart diseases | ― | ― | ― | ― | ― | ― | 1.40 (0.67–2.91) | 0.372 |
| Eating speed | ||||||||
| Fast | 0.25 (0.12–0.51) | <0.001 | 0.28 (0.13–0.62) | 0.002 | 0.27 (0.12–0.61) | 0.002 | 0.28 (0.12–0.65) | 0.003 |
| Normal | 0.29 (0.14–0.62) | 0.002 | 0.28 (0.12–0.67) | 0.004 | 0.27 (0.11–0.64) | 0.003 | 0.22 (0.09–0.57) | 0.002 |
| Slow | Reference | ― | Reference | ― | Reference | ― | Reference | ― |
| The presence of sarcopenia | Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Age (year) | ― | ― | 1.16 (1.09–1.24) | <0.001 | 1.18 (1.10–1.26) | <0.001 | 1.17 (1.09–1.26) | <0.001 |
| Men | ― | ― | 1.11 (0.51–2.43) | 0.796 | 1.29 (0.57–2.90) | 0.539 | 1.19 (0.52–2.73) | 0.681 |
| Insulin usage | ― | ― | ― | ― | 1.53 (0.63–3.75) | 0.351 | 1.63 (0.65–4.04) | 0.295 |
| Smoking | ― | ― | ― | ― | 0.76 (0.19–2.97) | 0.693 | 0.67 (0.17–2.72) | 0.579 |
| Exercise | ― | ― | ― | ― | 2.35 (1.04–5.31) | 0.040 | 2.54 (1.09–5.94) | 0.032 |
| Total energy intake (kcal/kg IBW/day) | ― | ― | ― | ― | 0.99 (0.95–1.03) | 0.564 | 0.99 (0.95–1.03) | 0.700 |
| CKD stage ≥4 | ― | ― | ― | ― | ― | ― | 2.33 (0.47–11.6) | 0.299 |
| History of cancer | ― | ― | ― | ― | ― | ― | 0.36 (0.12–1.10) | 0.072 |
| History of heart diseases | ― | ― | ― | ― | ― | ― | 1.29 (0.56–3.01) | 0.550 |
| Eating speed | ||||||||
| Fast | 0.29 (0.13–0.66) | 0.003 | 0.34 (0.14–0.81) | 0.015 | 0.31 (0.12–0.76) | 0.010 | 0.31 (0.12–0.80) | 0.016 |
| Normal | 0.24 (0.09–0.62) | 0.003 | 0.22 (0.08–0.60) | 0.003 | 0.19 (0.07–0.55) | 0.002 | 0.18 (0.06–0.53) | 0.002 |
| Slow | Reference | ― | Reference | ― | Reference | ― | Reference | ― |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, Y.; Takahashi, F.; Kaji, A.; Sakai, R.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; Senmaru, T.; et al. Eating Speed Is Associated with the Presence of Sarcopenia in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort. Nutrients 2022, 14, 759. https://doi.org/10.3390/nu14040759
Hashimoto Y, Takahashi F, Kaji A, Sakai R, Okamura T, Kitagawa N, Okada H, Nakanishi N, Majima S, Senmaru T, et al. Eating Speed Is Associated with the Presence of Sarcopenia in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort. Nutrients. 2022; 14(4):759. https://doi.org/10.3390/nu14040759
Chicago/Turabian StyleHashimoto, Yoshitaka, Fuyuko Takahashi, Ayumi Kaji, Ryosuke Sakai, Takuro Okamura, Noriyuki Kitagawa, Hiroshi Okada, Naoko Nakanishi, Saori Majima, Takafumi Senmaru, and et al. 2022. "Eating Speed Is Associated with the Presence of Sarcopenia in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort" Nutrients 14, no. 4: 759. https://doi.org/10.3390/nu14040759
APA StyleHashimoto, Y., Takahashi, F., Kaji, A., Sakai, R., Okamura, T., Kitagawa, N., Okada, H., Nakanishi, N., Majima, S., Senmaru, T., Ushigome, E., Asano, M., Hamaguchi, M., Yamazaki, M., & Fukui, M. (2022). Eating Speed Is Associated with the Presence of Sarcopenia in Older Patients with Type 2 Diabetes: A Cross-Sectional Study of the KAMOGAWA-DM Cohort. Nutrients, 14(4), 759. https://doi.org/10.3390/nu14040759











