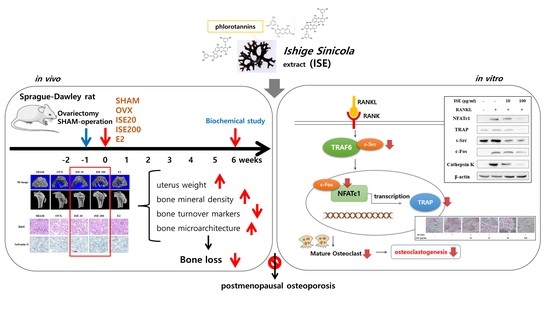
The Brown Algae Ishige sinicola Extract Ameliorates Ovariectomy-Induced Bone Loss in Rats and Suppresses Osteoclastogenesis through Downregulation of NFATc1/c-Fos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ISE
2.2. Animals and Treatment Regimen
2.3. Biochemical Parametric Analysis
2.4. Determination of the BMD and Biomechanical Properties
2.5. Tissue Histology
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. In Vitro Osteoclastogenesis Assay
2.9. Western Blotting
2.10. Statistical Analyses
3. Results
3.1. Effects of ISE on Body and Uterine Weight of Rats with OVX-Induced Osteoporosis
3.2. Effects of ISE on the Serum Levels of Biochemical Parameters in Rats with OVX-Induced Osteoporosis
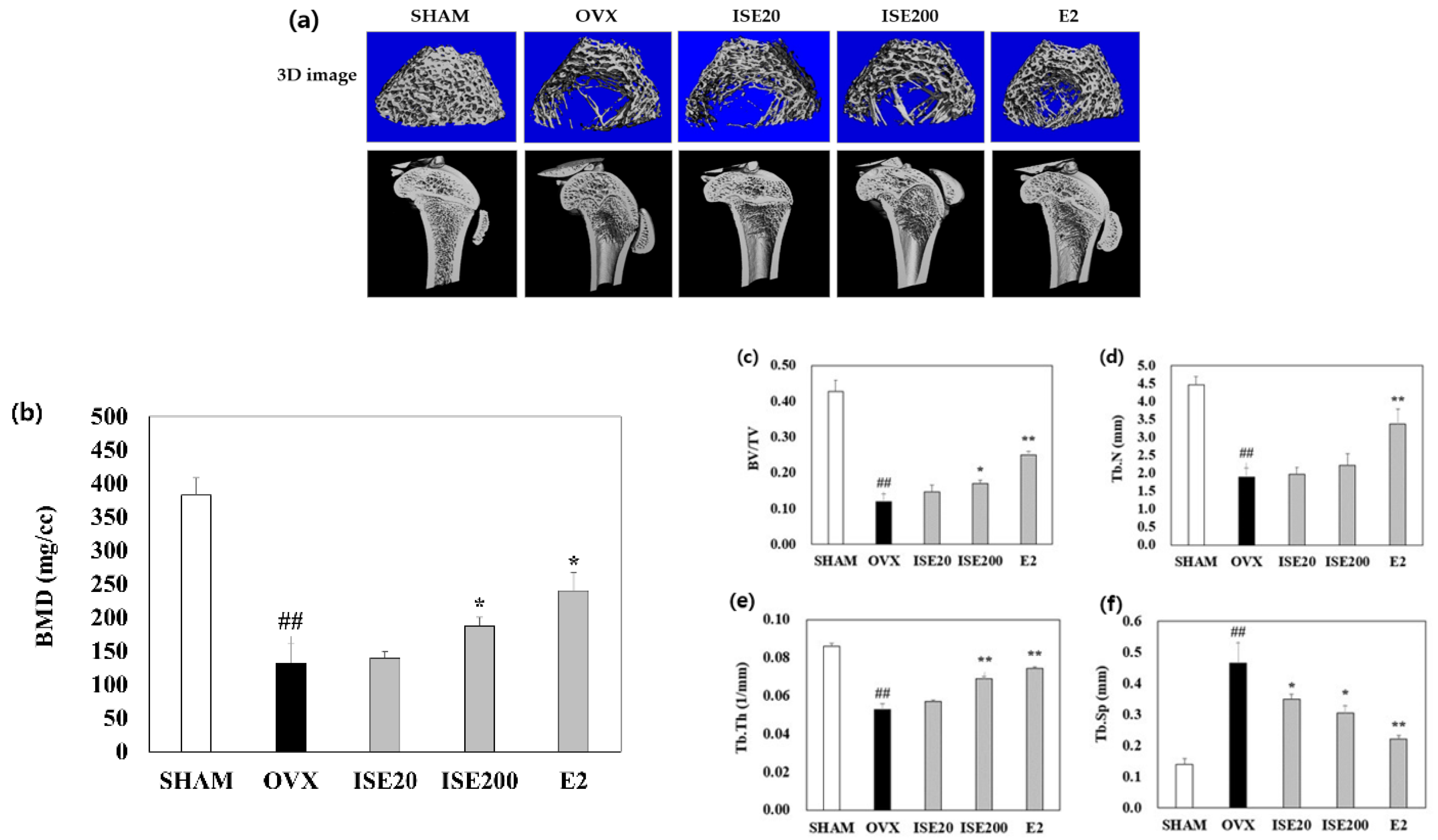
3.3. Effects of ISE on the Femoral BMD in Rats with OVX-Induced Osteoporosis
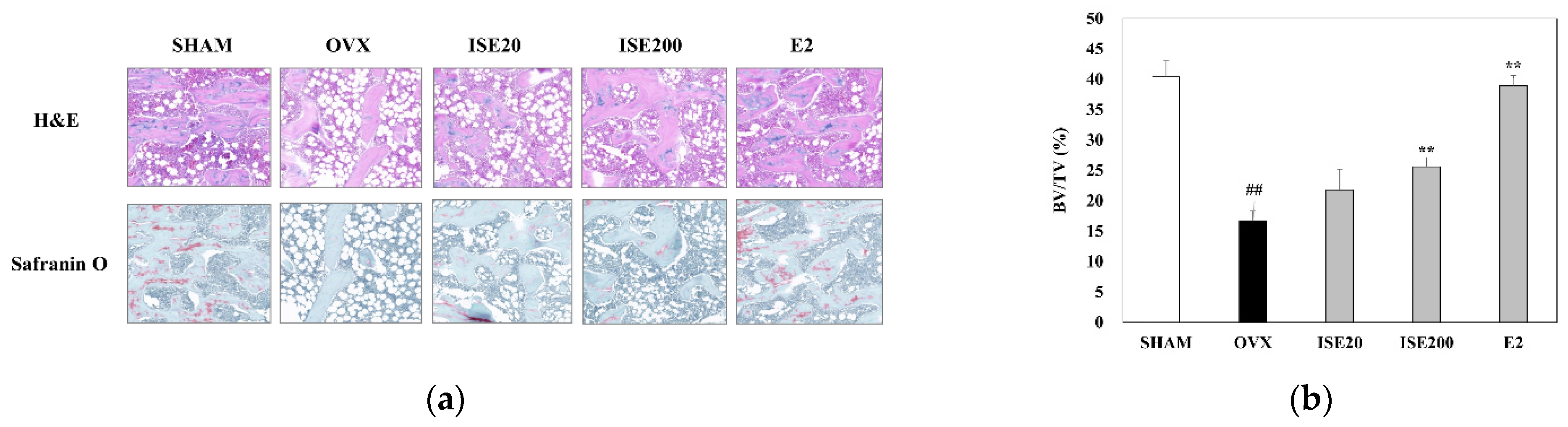
3.4. Effects of ISE on Morphological and Histomorphometrical Characteristics of Rats with OVX-Induced Osteoporosis
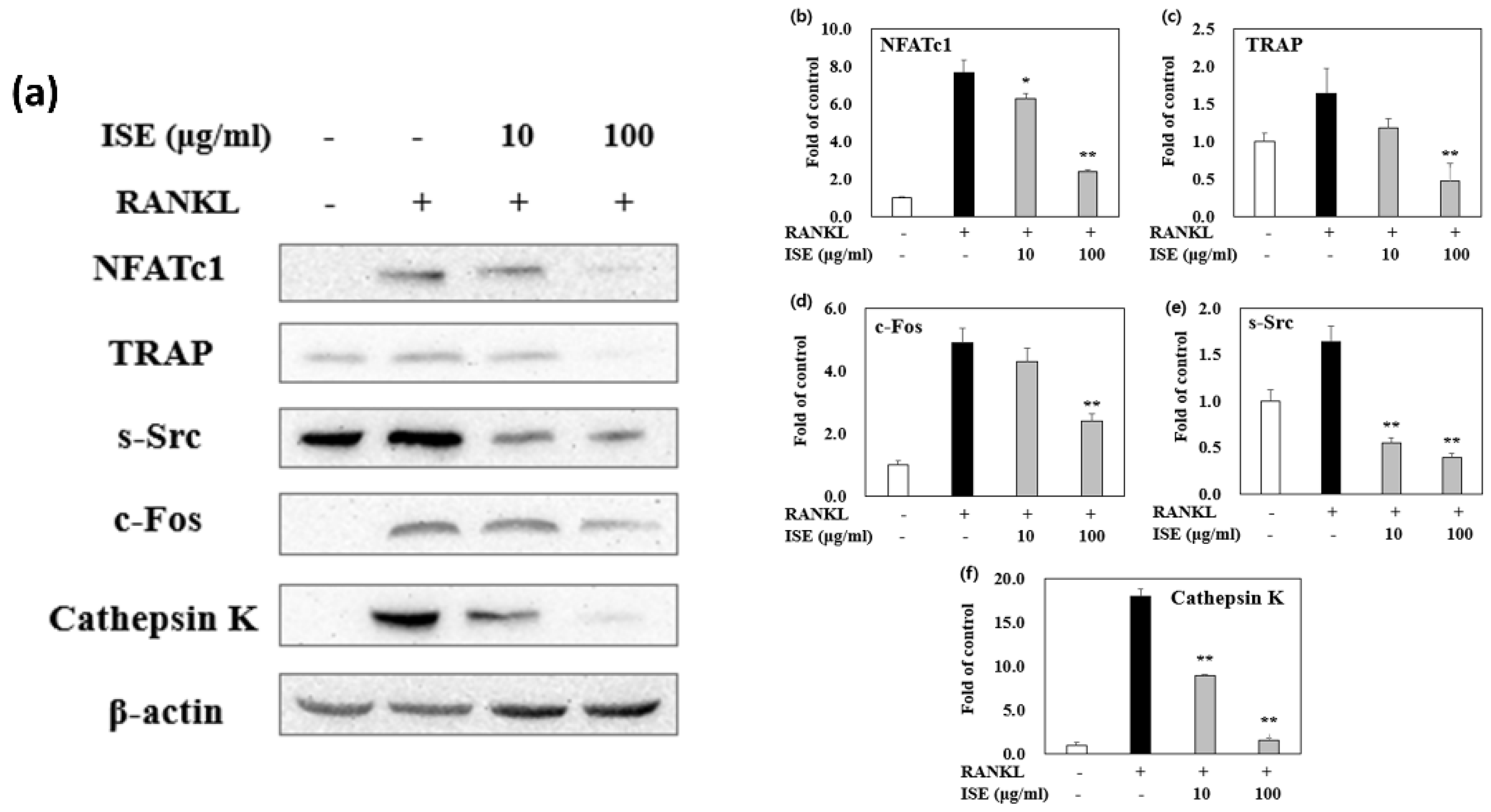
3.5. Effects of ISE on Osteoclast Differentiation
3.6. Effects of ISE on RANKL-Induced Expression of Osteoclastogenesis-Specific Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Ren, H.; Shen, G.; Qiu, T.; Liang, D.; Yang, Z.; Yao, Z.; Tang, J.; Jiang, X.; Wei, Q. Animal models for glucocorticoid-induced postmenopausal osteoporosis: An updated review. Biomed. Pharmacother. 2016, 84, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.A. Estrogen therapy for postmenopausal osteoporosis. Arq. Bras. Endocrinol. Metab. 2006, 50, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei-Malazy, O.; Salari, P.; Khashayar, P.; Larijani, B. New horizons in treatment of osteoporosis. Daru 2017, 25, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mørch, L.S.; Løkkegaard, E.; Andreasen, A.H.; Krüger-Kjaer, S.; Lidegaard, O. Hormone therapy and ovarian cancer. JAMA 2009, 302, 298–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, P.; Jørgensen, N.R.; Abrahamsen, B. Status of drug development for the prevention and treatment of osteoporosis. Expert. Opin. Drug Discov. 2014, 9, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tan, L.J.; Wang, P.; Chen, X.D.; Zhu, L.H.; Zeng, Q.; Hu, Y.; Deng, H.W. Functional relevance for associations between osteoporosis and genetic variants. PLoS ONE 2017, 12, e0174808. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, L.; Li, X.; Jia, J.; Zhang, Y.; Sun, X.; Ma, J.; Liu, Z.; Ma, X. Low-molecular weight fucoidan inhibits the differentiation of osteoclasts and reduces osteoporosis in ovariectomized rats. Mol. Med. Rep. 2017, 15, 890–898. [Google Scholar] [CrossRef]
- Xu, Z.; Greenblatt, M.B.; Yan, G.; Feng, H.; Sun, J.; Lotinun, S.; Brady, N.; Baron, R.; Glimcher, L.H.; Zou, W. SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts. Nat. Commun. 2017, 8, 14570. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R. Immune regulation of osteoclast function in postmenopausal osteoporosis: A critical interdisciplinary perspective. Int. J. Med. Sci. 2012, 9, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, K.S.; Yi, S.H.; Kook, S.H.; Lee, J.C. Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-κB pathway and attenuating ROS production. PLoS ONE 2013, 8, e80873. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.Q.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Kim, S.K. Neuropharmacological properties of marine plants. In Marine Pharmacognosy, Trends and Applications, 1st ed.; Kim, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2012; 454p. [Google Scholar]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2018, 120, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Chang, C.F. Chinese seaweeds in herbal medicine. Hydrobiologia 1984, 116-117, 152–154. [Google Scholar]
- Chandini, S.K.; Ganesan, P.; Bhaskar, N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008, 107, 707–713. [Google Scholar] [CrossRef]
- Kang, J.Y.; Khan, M.N.; Park, N.H.; Cho, J.Y.; Lee, M.C.; Fujii, H.; Hong, Y.K. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J. Ethnopharmacol. 2008, 116, 187–190. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef]
- Choi, J.S.; Bae, H.J.; Kim, S.J.; Choi, I.S. In vitro antibacterial and antiinflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar]
- Kang, J.I.; Kim, E.J.; Kim, M.K.; Jeon, Y.J.; Kang, S.M.; Koh, Y.S.; Yoo, E.S.; Kang, H.K. The promotion effect of Ishige sinicola on hair growth. Mar. Drugs 2013, 11, 1783–1799. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kang, J.H.; Oh, G.H.; Park, M.H. Ishige sinicola extract stimulates osteoblast proliferation and differentiation via the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. Z. Naturforsch. C J. Biosci. 2019, 74, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J. Minireview: The year in review of estrogen regulation of metabolism. Mol. Endocrinol. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [Green Version]
- Naaz, A.; Zakroczymski, M.; Heine, P.; Taylor, J.; Saunders, P.; Lubahn, D.; Cooke, P.S. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERα): A potential role for estrogen receptor beta (ERβ). Horm. Metab. Res. 2002, 34, 758–763. [Google Scholar] [CrossRef]
- Wegorzewska, I.N.; Walters, K.; Weiser, M.J.; Cruthirds, D.F.; Ewell, E.; Larco, D.O.; Handa, R.J.; Wu, T.J. Postovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist, propylpyrazoletriol. Am. J. Obstet. Gynecol. 2008, 199, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiang, Z.; Chen, F.; Yin, D.; Huang, Y.; Xu, J.; Hu, L.; Xu, H.; Wang, X.; Sheng, J. Theabrownin suppresses in vitro osteoclastogenesis and prevents bone loss in ovariectomized rats. Biomed. Pharmacother. 2018, 106, 1339–1347. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Khosla, S.; Atkinson, E.J.; O’Fallon, W.M.; Riggs, B.L. Relationship of bone turnover to bone density and fractures. J. Bone Miner. Res. 1997, 12, 1083–1091. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.W.; Wang, Z.L.; Qi, W.; Zhao, G.Y. The effects of Cordyceps sinensis phytoestrogen on estrogen deficiency-induced osteoporosis in ovariectomized rats. BMC Complement. Altern. Med. 2014, 14, 484. [Google Scholar] [CrossRef] [Green Version]
- Polak-Jonkisz, D.; Zwolinska, D. Osteocalcin as a biochemical marker of bone turnover. Nephrology 1998, 4, 339–346. [Google Scholar] [CrossRef]
- Vural, P.; Akgul, C.; Canbaz, M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol. Res. 2006, 54, 298–302. [Google Scholar] [CrossRef]
- Wahba, H.M.A.; Al-Zahrany, M.S.H. Effect of feeding on diets supplemented by some vegetable oils on blood lipids and bone mineral content in osteoporotic rats. Life Sci. 2013, 10, 1458–1465. [Google Scholar]
- Borje, E.; Christopher, N. Evolution of the calcium paradigm: The relation between vitamin D, serum calcium and calcium absorption. Nutrients 2010, 2, 997–1004. [Google Scholar]
- Park, J.A.; Ha, S.K.; Kang, T.H.; Oh, M.S.; Cho, M.H.; Lee, S.Y.; Park, J.H.; Kim, S.Y. Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci. 2008, 82, 1217–1223. [Google Scholar] [CrossRef]
- Ye, Q.; Ma, X.Q.; Hu, C.L.; Lin, B.; Xu, L.S.; Zheng, C.J.; Qin, L.P. Antiosteoporotic activity and constituents of Podocarpium podocarpum. Phytomedicine 2015, 22, 94–102. [Google Scholar] [CrossRef]
- Ryu, B.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Biol. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Baek, S.H.; Jeong, S.C.; Yoon, Y.D.; Kim, O.H.; Oh, B.C.; Jung, J.W.; Kim, J.H. Osteoprotective effects of polysaccharide-enriched Hizikia fusiforme processing by product in vitro and in vivo models. J. Med. Food 2016, 19, 805–814. [Google Scholar] [CrossRef]
- Wu, Y.X.; Sun, Y.; Ye, Y.P.; Zhang, P.; Guo, J.C.; Huang, J.M.; Jing, X.Z.; Xiang, W.; Yu, S.Y.; Guo, F.J. Iguratimod prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferator-activated receptor-γ. Mol. Med. Rep. 2017, 16, 8200–8208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Li, J.; Du, J.; Lv, X.; Weng, L.; Ling, C. Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB and MAPKs pathways. Food Chem. Toxicol. 2012, 50, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med. 2005, 83, 170–179. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | SHAM | OVX | ISE20 | ISE200 | E2 |
|---|---|---|---|---|---|
| CTx (ng/mL) | 1.02 ± 0.22 | 4.14 ± 0.19 ## | 1.93 ± 0.21 ** | 1.16 ± 0.18 ** | 1.94 ± 0.09 ** |
| NTx (ng/mL) | 31.48 ± 3.29 | 79.40 ± 8.99 ## | 35.92 ± 3.84 ** | 24.37 ± 2.98 ** | 33.42 ± 6.66 ** |
| TRAP (U/L) | 4.33 ± 0.42 | 16.58 ± 1.07 ## | 9.55 ± 1.43 ** | 4.16 ± 0.09 ** | 5.94 ± 0.48 ** |
| ALP (U/L) | 180.14 ± 24.85 | 629.44 ± 16.30 ## | 512.00 ± 21.21 ** | 223.00 ± 14.24 ** | 443.10 ± 18.4 |
| Osteocalcin (pg/mL) | 6.37 ± 0.28 | 10.21 ± 0.89 ## | 6.38 ± 0.77 ** | 5.49 ± 1.06 ** | 4.77 ± 0.91 ** |
| RANKL (pg/mL) | 301.08 ± 3.23 | 423.38 ± 4.40 ## | 351.85 ± 8.47 ** | 333.38 ± 9.02 ** | 341.46 ± 6.70 ** |
| Ca (mg/dL) | 13.23 ± 0.63 | 11.90 ± 0.29 # | 13.53 ± 0.84 | 13.78 ± 1.41 * | 13.10 ± 0.26 |
| P (mmol/L) | 5.26 ± 0.42 | 6.67 ± 0.70 # | 6.20 ± 0.38 | 5.23 ± 0.84 | 5.21 ± 0.31 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, M. The Brown Algae Ishige sinicola Extract Ameliorates Ovariectomy-Induced Bone Loss in Rats and Suppresses Osteoclastogenesis through Downregulation of NFATc1/c-Fos. Nutrients 2022, 14, 1683. https://doi.org/10.3390/nu14091683
Kim M, Park M. The Brown Algae Ishige sinicola Extract Ameliorates Ovariectomy-Induced Bone Loss in Rats and Suppresses Osteoclastogenesis through Downregulation of NFATc1/c-Fos. Nutrients. 2022; 14(9):1683. https://doi.org/10.3390/nu14091683
Chicago/Turabian StyleKim, Mihyang, and Mihwa Park. 2022. "The Brown Algae Ishige sinicola Extract Ameliorates Ovariectomy-Induced Bone Loss in Rats and Suppresses Osteoclastogenesis through Downregulation of NFATc1/c-Fos" Nutrients 14, no. 9: 1683. https://doi.org/10.3390/nu14091683
APA StyleKim, M., & Park, M. (2022). The Brown Algae Ishige sinicola Extract Ameliorates Ovariectomy-Induced Bone Loss in Rats and Suppresses Osteoclastogenesis through Downregulation of NFATc1/c-Fos. Nutrients, 14(9), 1683. https://doi.org/10.3390/nu14091683