How to Improve Health with Biological Agents—Narrative Review
Abstract
:1. Introduction
2. Probiotics
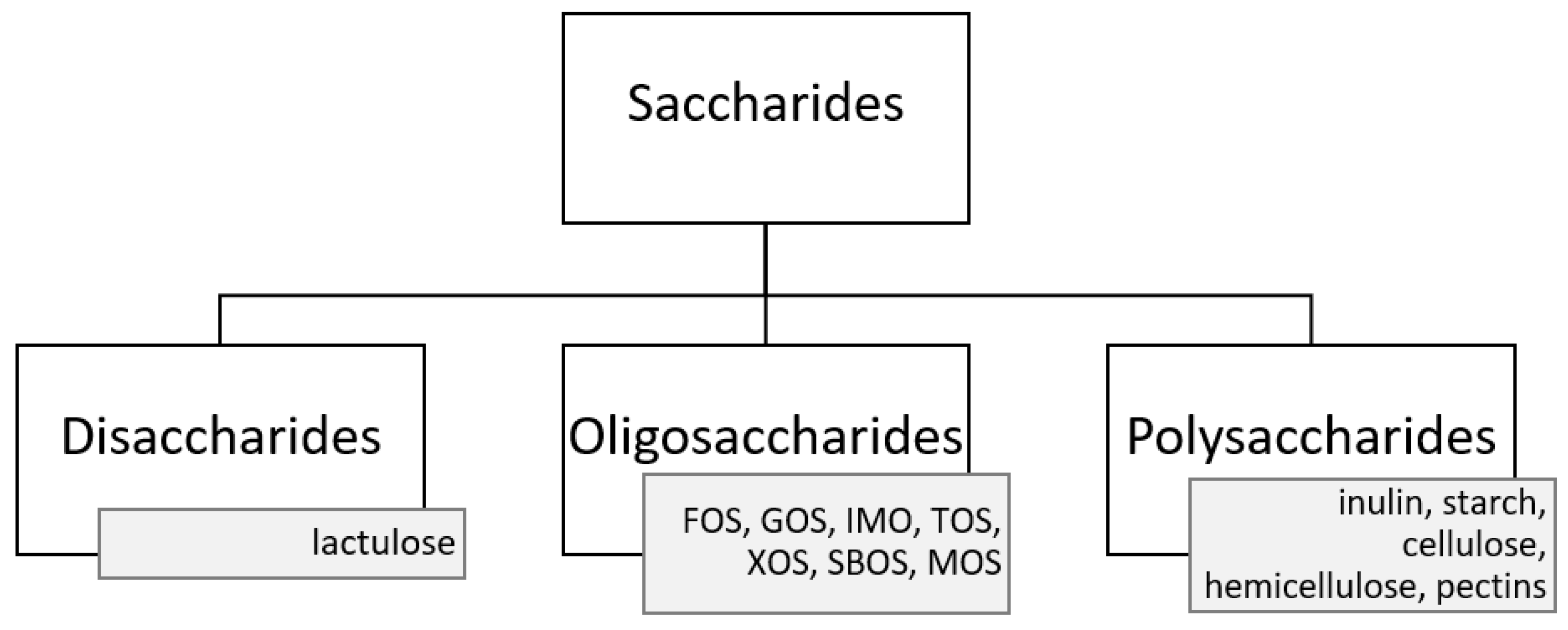
3. Prebiotics
4. Synbiotics
5. Postbiotics
6. Paraprobiotics
7. Psychobiotics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Cryan, J.F. Microbiota-targeted interventions for mental health. Curr. Opin. Psychiatry 2021, 35, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Ross, R.P.; Ryan, C.A.; Dempsey, E.M.; Stanton, C. Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Front. Nutr. 2021, 8, 667188. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota and epigenetics: Promising therapeutic approaches? Environ. Sci. Pollut. Res. 2021, 28, 49343–49361. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. 2001. Available online: https://www.fao.org/3/y6398e/y6398e.pdf (accessed on 19 July 2021).
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 19 July 2021).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Siciliano, R.; Reale, A.; Mazzeo, M.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Probiotics and Psychobiotics: The Role of Microbial Neurochemicals. Probiotics Antimicrob. Proteins 2019, 11, 1071–1085. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramírez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [Green Version]
- Perković, L.; Djedović, E.; Vujović, T.; Baković, M.; Paradžik, T.; Čož-Rakovac, R. Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend? Mar. Drugs 2022, 20, 142. [Google Scholar] [CrossRef]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus Sepsis Associated with Probiotic Therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Antibiotic resistance of probiotic organisms and safety of probiotic dairy products. Int. Food Res. J. 2011, 18, 837–853. [Google Scholar]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R. PROBIOTICS. Indian J. Med Microbiol. 2009, 27, 202–209. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 14: Suitability of taxonomic units notified to EFSA until March 2021. EFSA J. 2021, 19, e06689. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp.—Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Schillinger, U. Introduction to pre- and probiotics. Food Res. Int. 2002, 35, 109–116. [Google Scholar] [CrossRef]
- Shehata, H.R.; Ragupathy, S.; Shanmughanandhan, D.; Kesanakurti, P.; Ehlinger, T.M.; Newmaster, S.G. Guidelines for Validation of Qualitative Real-Time PCR Methods for Molecular Diagnostic Identification of Probiotics. J. AOAC Int. 2019, 102, 1774–1778. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- De Vrese, M.; Schrezenmeir, J. Probiotics, Prebiotics, and Synbiotics. Food Biotechnol. 2008, 111, 1–66. [Google Scholar] [CrossRef]
- Antoine, J.M. Probiotics: Beneficial factors of the defence system. Proc. Nutr. Soc. 2010, 69, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Chauviere, G.; Coconnier, M.H.; Kerneis, S.; Darfuille-Michaud, A.; Joly, B.; Servin, A.L. Competitive exclusion of diarrheagenic Escherichia coli (ETEC) from human enterocyte-like Caco-2 cells by heat-killed Lactobacillus. FEMS Microbiol. Lett. 1992, 91, 213–217. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. Int. Rev. J. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Słońska, A.; Klimuszko, D. Bacteriocins produced by probiotic rods of the genus Lactobacillus. Adv. Microbiol. 2010, 40, 87–96. [Google Scholar]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Hernández-González, J.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.; Castrejón-Jiménez, N. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Timothy, B.; Iliyasu, A.H.; Anvikar, A.R. Bacteriocins of Lactic Acid Bacteria and Their Industrial Application. Curr. Top. Lact. Acid Bact. Probiotics 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Cesa-Luna, C.; Alatorre-Cruz, J.-M.; Carreño-López, R.; Quintero-Hernández, V.; Baez, A. Emerging Applications of Bacteriocins as Antimicrobials, Anticancer Drugs, and Modulators of The Gastrointestinal Microbiota. Pol. J. Microbiol. 2021, 70, 143–159. [Google Scholar] [CrossRef]
- Divella, R.; DE Palma, G.; Tufaro, A.; Pelagio, G.; Gadaleta-Caldarola, G.; Bringiotti, R.; Paradiso, A. Diet, Probiotics and Physical Activity: The Right Allies for a Healthy Microbiota. Anticancer Res. 2021, 41, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Trindade, L.M.; Souza, A.; Torres, L.; Andrade, M.E.R.; Cardoso, V.N.; Generoso, S.V. Non-pharmacologic strategies for the management of intestinal inflammation. Biomed. Pharmacother. 2021, 145, 112414. [Google Scholar] [CrossRef] [PubMed]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Morita, H.; He, F.; Fuse, T.; Ouwehand, A.; Hashimoto, H.; Hosoda, M.; Mizumachi, K.; Kurisaki, J.-I. Adhesion of Lactic Acid Bacteria to Caco-2 Cells and Their Effect on Cytokine Secretion. Microbiol. Immunol. 2002, 46, 293–297. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [Green Version]
- Piątek, J.; Gibas-Dorna, M.; Olejnik, A.; Krauss, H.; Wierzbicki, K.; Żukiewicz-Sobczak, W.; Głowacki, M. The viability and intestinal epithelial cell adhesion of probiotic strain combination—In vitro study. Ann. Agric. Environ. Med. 2012, 19, 99–102. [Google Scholar]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef]
- Gao, X.W.; Mubasher, M.; Fang, C.Y.; Reifer, C.; Miller, L. Dose-Response Efficacy of a Proprietary Probiotic Formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for Antibiotic-Associated Diarrhea and Clostridium difficile-Associated Diarrhea Prophylaxis in Adult Patients. Am. J. Gastroenterol. 2010, 105, 1636–1641. [Google Scholar] [CrossRef]
- Zawistowska-Rojek, A.; Tyski, S. Are Probiotic Really Safe for Humans? Pol. J. Microbiol. 2018, 67, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Srivastava, S.; Leyer, G.J. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients 2020, 12, 363. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Ma, C.; Zhao, F.; Chen, P.; Liu, Y.; Sun, Z.; Cui, L.; Kwok, L.-Y.; Zhang, H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Z. Ernährungswissenschaft 2021, 60, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Sission, G.; Hayee, B. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Zhang, G.; Xie, H.; You, L.; Li, H.; Zhang, Y.; Du, C.; Xu, S.; Melsaether, C.; Yuan, S. Efficacy of Bifidobacterium animalis subsp. lactis, BB-12® on infant colic – a randomised, double-blinded, placebo-controlled study. Benef. Microbes 2021, 12, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.-H.; Zheng, W.-X.; Zhao, Z.-T.; Meng, N.; Zhang, Q.-R.; Zhu, W.-J.; Li, R.-D.; Liang, X.-L.; Li, Q.-Y. A Pilot Study of the Effect of Lactobacillus casei Obtained from Long-Lived Elderly on Blood Biochemical, Oxidative, and Inflammatory Markers, and on Gut Microbiota in Young Volunteers. Nutrients 2021, 13, 3891. [Google Scholar] [CrossRef]
- Yang, B.; Yue, Y.; Chen, Y.; Ding, M.; Li, B.; Wang, L.; Wang, Q.; Stanton, C.; Ross, R.P.; Zhao, J.; et al. Lactobacillus plantarum CCFM1143 Alleviates Chronic Diarrhea via Inflammation Regulation and Gut Microbiota Modulation: A Double-Blind, Randomized, Placebo-Controlled Study. Front. Immunol. 2021, 12, 746585. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Kottmann, T.; Alavi, M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J. Gastroenterol. 2014, 20, 15837–15844. [Google Scholar] [CrossRef]
- Cimperman, L.; Bayless, G.; Best, K.; Diligente, A.; Mordarski, B.; Oster, M.; Smith, M.; Vatakis, F.; Wiese, D.; Steiber, A.; et al. A Randomized, Double-blind, Placebo-controlled Pilot Study of Lactobacillus reuteri ATCC 55730 for the Prevention of Antibiotic-associated Diarrhea in Hospitalized Adults. J. Clin. Gastroenterol. 2011, 45, 785–789. [Google Scholar] [CrossRef]
- Shin, C.M.; Choi, Y.J.; Lee, D.H.; Moon, J.S.; Kim, T.-Y.; Kim, Y.-K.; Lee, W.-H.; Yoon, H.; Park, Y.S.; Kim, N. Validity and safety of ID-JPL934 in lower gastrointestinal symptom improvement. Sci. Rep. 2021, 11, 13046. [Google Scholar] [CrossRef] [PubMed]
- Prakoeswa, C.R.S.; Herwanto, N.; Prameswari, R.; Astari, L.; Sawitri, S.; Hidayati, A.N.; Indramaya, D.; Kusumowidagdo, E.; Surono, I. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef. Microbes 2017, 8, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, B.; Ban, J.; Lee, J.; Kim, B.J.; Choi, B.S.; Hwang, S.; Ahn, K.; Kim, J. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2012, 23, 667–673. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Bonita, L.; Karim, A.; Herwanto, N.; Umborowati, M.A.; Setyaningrum, T.; Hidayati, A.N.; Surono, I.S. Beneficial effect of Lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: A randomized controlled trial. J. Dermatol. Treat. 2020, 8, 1836310. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Eliakim, R.; Fedorak, R.; Gangl, A.; Garisch, J.; Kaufmann, P.; Karakan, T.; Khan, A.G.; Kim, N.; et al. WGO Practice Guideline—Probiotics and Prebiotics. 2017. Available online: https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf (accessed on 19 July 2021).
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowski, J.; Witkowski, J.M. Lactulose: Patient- and dose-dependent prebiotic properties in humans. Anaerobe 2019, 59, 100–106. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pak, D.; Crandall, P.G.; Lee, S.O.; Ricke, S.C. The role of prebiotics and probiotics in human health. J. Prob. Health 2013, 1, 108. [Google Scholar]
- Rahim, M.A.; Saeed, F.; Khalid, W.; Hussain, M.; Anjum, F.M. Functional and nutraceutical properties of fructo-oligosaccharides derivatives: A review. Int. J. Food Prop. 2021, 24, 1588–1602. [Google Scholar] [CrossRef]
- Costa, G.; Vasconcelos, Q.; Abreu, G.; Albuquerque, A.; Vilar, J.; Aragão, G. Systematic review of the ingestion of fructooligosaccharides on the absorption of minerals and trace elements versus control groups. Clin. Nutr. ESPEN 2021, 41, 68–76. [Google Scholar] [CrossRef]
- Lamsal, B.P. Production, health aspects and potential food uses of dairy prebiotic galactooligosaccharides. J. Sci. Food Agric. 2012, 92, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Fara, A.; Sabater, C.; Palacios, J.; Requena, T.; Montilla, A.; Zárate, G. Prebiotic galactooligosaccharides production from lactose and lactulose by Lactobacillus delbrueckii subsp. bulgaricus CRL450. Food Funct. 2020, 11, 5875–5886. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Taylor, G.R.J.; Clohessy, A.M.; Williams, C.M. The effect of the daily intake of inulin on fasting lipid, insulin and glucose concentrations in middle-aged men and women. Br. J. Nutr. 1999, 82, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Maldonado-Gomez, M.X.; Ramer-Tait, A.E.; Hutkins, R.W. Prebiotics and synbiotics: Dietary strategies for improving gut health. Curr. Opin. Gastroenterol. 2016, 32, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T. Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fernandez, B.; Subirade, M.; Fliss, I. Study and Understanding Behavior of Alginate-Inulin Synbiotics Beads for Protection and Delivery of Antimicrobial-Producing Probiotics in Colonic Simulated Conditions. Probiotics Antimicrob. Proteins 2018, 10, 157–167. [Google Scholar] [CrossRef]
- Manigandan, T.; Mangaiyarkarasi, S.; Hemalatha, R.; Hemalatha, V.; Murali, N. Probiotics, Prebiotics and Synbiotics—A Review. Biomed. Pharmacol. J. 2012, 5, 295–304. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Rufino, M.N.; da Costa, A.L.; Jorge, E.N.; Paiano, V.F.; Camparoto, M.L.; Keller, R.; Bremer-Neto, H. Synbiotics improve clinical indicators of ulcerative colitis: Systematic review with meta-analysis. Nutr. Rev. 2021, 80, 157–164. [Google Scholar] [CrossRef]
- Gurry, T. Synbiotic approaches to human health and well-being. Microb. Biotechnol. 2017, 10, 1070–1073. [Google Scholar] [CrossRef] [Green Version]
- Simon, E.; Călinoiu, L.; Mitrea, L.; Vodnar, D. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Cho, D.-Y.; Lee, S.-H.; Han, K.-S.; Yang, S.-W.; Kim, J.-H.; Lee, S.-H.; Kim, S.-M.; Kim, K.-N. A Randomized Clinical Trial of Synbiotics in Irritable Bowel Syndrome: Dose-Dependent Effects on Gastrointestinal Symptoms and Fatigue. Korean J. Fam. Med. 2019, 40, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Park, S.U.; Jang, Y.S.; Kim, Y.-H.; Rhee, P.-L.; Ko, S.H.; Joo, N.; Kim, S.I.; Kim, C.-H.; Chang, D.K. Effect of composite yogurt enriched with acacia fiber and Bifidobacterium lactis. World J. Gastroenterol. 2012, 18, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Šmid, A.; Strniša, L.; Bajc, K.; Vujić-Podlipec, D.; Matijašić, B.B.; Rogelj, I. Randomized clinical trial: The effect of fermented milk with the probiotic cultures Lactobacillus acidophilus La-5® and Bifidobacterium BB-12® and Beneo dietary fibres on health-related quality of life and the symptoms of irritable bowel syndrome in adults. J. Funct. Foods 2016, 24, 549–557. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Kondepudi, K.K.; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.; Vyas, B. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes 2013, 4, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.J.; Merrifield, C.A.; Younes, J.A.; Pekelharing, E.P. Pre-, pro- and synbiotics in cancer prevention and treatment—A review of basic and clinical research. Ecancermedicalscience 2018, 12, 869. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lim, Y.J. The role of microbiome in colorectal carcinogenesis and its clinical potential as a target for cancer treatment. Intest. Res. 2022, 20, 31–42. [Google Scholar] [CrossRef]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Flesch, A.T.; Tonial, S.T.; Contu, P.D.C.; Damin, D.C. Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: A randomized, double-blind clinical trial. Rev. Colégio Bras. Cir. 2017, 44, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Krebs, B. Prebiotic and Synbiotic Treatment before Colorectal Surgery—Randomised Double Blind Trial. Coll. Antropol. 2016, 40, 35–40. [Google Scholar] [PubMed]
- Olas, B. Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef]
- Karimi, E.; Heshmati, J.; Shirzad, N.; Vesali, S.; Hosseinzadeh-Attar, M.J.; Moini, A.; Sepidarkish, M. The effect of synbiotics supplementation on anthropometric indicators and lipid profiles in women with polycystic ovary syndrome: A randomized controlled trial. Lipids Health Dis. 2020, 19, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghizadeh, M.; Hashemi, T.; Shakeri, H.; Abedi, F.; Sabihi, S.-S.; Alizadeh, S.-A.; Asemi, Z. Synbiotic Food Consumption Reduces Levels of Triacylglycerols and VLDL, but not Cholesterol, LDL, or HDL in Plasma from Pregnant Women. Lipids 2014, 49, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of Synbiotic Bread Decreases Triacylglycerol and VLDL Levels While Increasing HDL Levels in Serum from Patients with Type-2 Diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Gilani, N.; Abbasi, A.; Khani, N. Postbiotics, as Dynamic Biomolecules, and Their Promising Role in Promoting Food Safety. Biointerface Res. Appl. Chem. 2021, 11, 14529–14544. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Amsco Healthcare. Available online: http://amscohealthcare.com/products/lacteol-fort-sachet/ (accessed on 16 December 2021).
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, M.; Jeon, S.A.; Kim, Y.; Lee, S. A randomized trial of Lactobacillus rhamnosus IDCC 3201 tyndallizate (RHT3201) for treating atopic dermatitis. Pediatr. Allergy Immunol. 2020, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Park, J.-H.; Kil Jung, H. Potential Health-Promoting Benefits of Paraprobiotics, Inactivated Probiotic Cells. J. Microbiol. Biotechnol. 2020, 30, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Maehata, H.; Arai, S.; Iwabuchi, N.; Abe, F. Immuno-modulation by heat-killed Lacticaseibacillus paracasei MCC1849 and its application to food products. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008291. [Google Scholar] [CrossRef]
- Sugawara, T.; Sawada, D.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb. Ecol. Health Dis. 2016, 27, 30259. [Google Scholar] [CrossRef]
- Nakamura, F.; Ishida, Y.; Aihara, K.; Sawada, D.; Ashida, N.; Sugawara, T.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of fragmented Lactobacillus amylovorus CP1563 on lipid metabolism in overweight and mildly obese individuals: A randomized controlled trial. Microb. Ecol. Health Dis. 2016, 27, 30312. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Sawada, D.; Yanagihara, S.; Aoki, Y.; Takehara, I.; Sugahara, H.; Hirota, T.; Nakamura, Y.; Ishikawa, S. Daily Intake of Paraprobiotic Lactobacillus amylovorus CP1563 Improves Pre-Obese Conditions and Affects the Gut Microbial Community in Healthy Pre-Obese Subjects: A Double-Blind, Randomized, Placebo-Controlled Study. Microorganisms 2020, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef]
- Pihurov, M.; Păcularu-Burada, B.; Cotârleţ, M.; Vasile, M.A.; Bahrim, G.E. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 2021, 9, 2184. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.; Harty, S.; Dinan, T.; Cryan, J.F.; Burnet, P.W. Psychobiotics and the Manipulation of Bacteria–Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Zou, R.; Tian, P.; Xu, M.; Zhu, H.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Psychobiotics as a novel strategy for alleviating anxiety and depression. J. Funct. Foods 2021, 86, 104718. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A.; Rogovsky, V.S. Role of Neurochemicals in the Interaction between the Microbiota and the Immune and the Nervous System of the Host Organism. Probiotics Antimicrob. Proteins 2017, 9, 215–234. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- Otaka, M.; Kikuchi-Hayakawa, H.; Ogura, J.; Ishikawa, H.; Yomogida, Y.; Ota, M.; Hidese, S.; Ishida, I.; Aida, M.; Matsuda, K.; et al. Effect of Lacticaseibacillus paracasei Strain Shirota on Improvement in Depressive Symptoms, and Its Association with Abundance of Actinobacteria in Gut Microbiota. Microorganisms 2021, 9, 1026. [Google Scholar] [CrossRef]
- Venkataraman, R.; Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Vinay, H.R.; Lal, A.; Thomas, G.; Stephen, S. Effect of Multi-strain Probiotic Formulation on Students Facing Examination Stress: A Double-Blind, Placebo-Controlled Study. Probiotics Antimicrob. Proteins 2021, 13, 12–18. [Google Scholar] [CrossRef]
- Dickerson, F.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; Goga, J.; et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disord. 2018, 20, 614–621. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [Green Version]
| Name | Definition | References |
|---|---|---|
| Probiotics | live microorganisms that, when administered in adequate amounts, confer a health benefit on the host | [7] |
| Prebiotics | a substrate that is selectively utilized by host microorganisms conferring a health benefit | [8] |
| Synbiotics | a mixture comprising of live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host | [9] |
| Postbiotics | preparation of inanimate microorganisms and/or their components that confers a health benefit on the host | [10] |
| Paraprobiotics | non-viable microbial cells (either intact or broken), or crude cell extracts, which, when administered (orally or topically) in adequate amounts, confer a benefit on the human or animal consumer | [11] |
| Psychobiotics | probiotics that confer mental health benefits to the host when consumed in a particular quantity through the interaction with commensal gut bacteria | [12,13,14,15] |
| Genus | Species |
|---|---|
| Lactobacillus | L. rhamnosus (Lacticaseibacillus rhamnosus *), L. acidophilus, L. plantarum (Lactiplantibacillus plantarum *), L. casei (Lacticaseibacillus casei *), L. delbrueckii subsp. bulgaricus, L. brevis (Levilactobacillus brevis *), L. johnsonii, L. fermentum (Limosilactobacillus fermentum *), L. reuteri (Limosilactobacillus reuteri *), L. gasseri, L. paracasei (Lacticaseibacillus paracasei *), L. salivarius (Ligilactobacillus salivarius *) |
| Bifidobacterium | B. infantis, B. animalis subsp. lactis, B. bifidum, B. longum, B. breve, B. animalis subsp. animalis, B. adolescentis |
| Enterococcus | E. durans, E. faecium, E. faecalis, E. lactis, E. hirae |
| Bacillus | B. coagulans, B. subtilis, B. cereus, B. clausii, B. pumilus, B. licheniformis |
| Other | Lactococcus lactis subsp. lactis, Streptococcus thermophilus, Pediococcus acidilactici, Leuconostoc mesenteroides, Escherichia coli Nissle 1917, Saccharomyces boulardii |
| Disease | Probiotic Strains/Duration of Treatment | Effects of Activity | References |
|---|---|---|---|
| Rheumatoid arthritis | L. acidophilus 2 × 109 CFU/g L. casei 2 × 109 CFU/g B. bifidum 2 × 109 CFU/g 8 weeks |
| [54] |
| Irritable bowel syndrome (IBS) | L. acidophilus DDS-1, 1 × 1010 CFU/day B. animalis subsp. lactis UABla-12, 1 × 1010 CFU/day 6 weeks |
| [55] |
| L. casei Zhang, 3 × 109 CFU/g B. animalis subsp. lactis V9, 4 × 109 CFU/g L. plantarum P-8, 3 × 109 CFU/g 28 days |
| [56] | |
| Ulcerative colitis | L. rhamnosus NCIMB 30174 L.plantarum NCIMB 30173 L. acidophilus NCIMB 30175 E. faecium NCIMB 30176 1 × 1010 CFU/dose; 4 weeks |
| [57] |
| Infant colic | B. animalis subsp. lactis BB-12® (BB-12), 1 × 109 CFU/day 21 days |
| [58] |
| Biochemical, oxidative and inflammatory markers | L. casei LTL 1879 1.41 ± 0.12 × 1011 CFU/g 3 weeks |
| [59] |
| Chronic diarrhea | L. plantarum CCFM1143 3.52 × 109 CFU/day 30 days |
| [60] |
| Antibiotic associated diarrhea | L. casei DN 114001 1 × 1010 CFU/dose 3 months |
| [61] |
| L. reuteri ATCC 55730 1 × 108 CFU/dose 2 × a day/28 days |
| [62] | |
| Gastrointestinal symptoms | L. johnsonii IDCC 9203 L. plantarum IDCC 3501 B. lactis IDCC 4301 1.0 × 1010 CFU/capsule 8 weeks |
| [63] |
| Atopic dermatitis | L. plantarum IS-10506 1010 CFU/day 12 weeks |
| [64] |
| L. plantarum CJLP133 0.5 × 1010 CFU/dose 2 × a day/12 weeks |
| [65] | |
| L. plantarum IS-10506 2 × 1010 CFU/day 8 weeks |
| [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawistowska-Rojek, A.; Tyski, S. How to Improve Health with Biological Agents—Narrative Review. Nutrients 2022, 14, 1700. https://doi.org/10.3390/nu14091700
Zawistowska-Rojek A, Tyski S. How to Improve Health with Biological Agents—Narrative Review. Nutrients. 2022; 14(9):1700. https://doi.org/10.3390/nu14091700
Chicago/Turabian StyleZawistowska-Rojek, Anna, and Stefan Tyski. 2022. "How to Improve Health with Biological Agents—Narrative Review" Nutrients 14, no. 9: 1700. https://doi.org/10.3390/nu14091700
APA StyleZawistowska-Rojek, A., & Tyski, S. (2022). How to Improve Health with Biological Agents—Narrative Review. Nutrients, 14(9), 1700. https://doi.org/10.3390/nu14091700






