Plasma Kynurenine to Tryptophan Ratio Is Not Associated with Undernutrition in Adults but Reduced after Nutrition Intervention: Results from a Community-Based Study in Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Population and Ethical Considerations
2.2. Data Collection and Biological Sample Collection and Storage
2.3. Assessment of TRP and KYN Concentrations Using LC-MS/MS and KT Ratio Determination
2.4. Plasma and Stool Biomarkers Analysis Using ELISA
2.5. Statistical Analyses
3. Results
3.1. Socio-Demographic and Clinical Characteristics
3.2. Plasma Concentrations of TRP and KYN and the KT Ratio in Study Participants
3.3. Correlation of TRP, KYN and KT Ratio with Fecal and Plasma Biomarkers in All Individuals
3.4. Association of the KT Ratio and Related Variables with BMI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letamo, G.; Navaneetham, K. Prevalence and determinants of adult under-nutrition in Botswana. PLoS ONE 2014, 9, e102675. [Google Scholar] [CrossRef] [PubMed]
- Nube, M.; Van Den Boom, G. Gender and adult undernutrition in developing countries. Ann. Hum. Biol. 2003, 30, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Campbell, R.K.; Schulze, K.J.; Shaikh, S.; Raqib, R.; Wu, L.S.F.; Ali, H.; Mehra, S.; West, K.P.; Christian, P. Environmental enteric dysfunction and systemic inflammation predict reduced weight but not length gain in rural Bangladeshi children. Br. J. Nutr. 2018, 119, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, R.J.; Jones, K.; Berkley, J. Environmental Enteric Dysfunction: An Overview. Food Nutr. Bull. 2015, 36, S76–S87. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Burr, A.H.; Overacre-Delgoffe, A.E.; Tometich, J.T.; Yang, D.; Huckestein, B.R.; Linehan, J.L.; Spencer, S.P.; Hall, J.A.; Harrison, O.J.; et al. Environmental enteric dysfunction induces regulatory T cells that inhibit local CD4+ T cell responses and impair oral vaccine efficacy. Immunity 2021, 54, 1745–1757.e7. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A.V. The requirements of protein & amino acid during acute & chronic infections. Indian J. Med. Res. 2006, 124, 129. [Google Scholar] [PubMed]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, nutrition, and health benefits of tryptophan. Int. J. Tryptophan. Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef] [Green Version]
- Pett, S.L.; Kunisaki, K.; Wentworth, D.; Griffin, T.; Kalomenidis, I.; Nahra, R.; Sanchez, R.M.; Hodgson, S.W.; Ruxrungtham, K.; Dwyer, D.; et al. Increased Indoleamine-2,3-dioxygenase Activity Is Associated with Poor Clinical Outcome in Adults Hospitalized with Influenza in the INSIGHT FLU003Plus Study; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Suzuki, Y.; Suda, T.; Furuhashi, K.; Suzuki, M.; Fujie, M.; Hahimoto, D.; Nakamura, Y.; Inui, N.; Nakamura, H.; Chida, K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010, 67, 361–365. [Google Scholar] [CrossRef]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated with Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Darcy, C.J.; Davis, J.S.; Woodberry, T.; McNeil, Y.R.; Stephens, D.P.; Yeo, T.W.; Anstey, N.M. An Observational Cohort Study of the Kynurenine to Tryptophan Ratio in Sepsis: Association with Impaired Immune and Microvascular Function. PLoS ONE 2011, 6, e21185. [Google Scholar] [CrossRef] [Green Version]
- Rebnord, E.W.; Strand, E.; Midttun, Ø.; Svingen, G.F.; Christensen, M.H.; Ueland, P.M.; Mellgren, G.; Njølstad, P.R.; Tell, G.S.; Nygård, O.K.; et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia 2017, 60, 1712–1721. [Google Scholar] [CrossRef]
- Mangge, H.; Summers, K.L.; Meinitzer, A.; Zelzer, S.; Almer, G.; Prassl, R.; Schnedl, W.J.; Reininghaus, E.; Paulmichl, K.; Weghuber, D.; et al. Obesity-related dysregulation of the Tryptophan–Kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity 2013, 22, 195–201. [Google Scholar] [CrossRef]
- Sekine, A.; Okamoto, M.; Kanatani, Y.; Sano, M.; Shibata, K.; Fukuwatari, T. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. SpringerPlus 2015, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Farup, P.G. The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front. Psychol. 2017, 8, 1561. [Google Scholar] [CrossRef] [Green Version]
- Kosek, M.; Mduma, E.; Kosek, P.S.; Lee, G.O.; Svensen, E.; Pan, W.K.Y.; Olortegui, M.P.; Bream, J.; Patil, C.; Asayag, C.R.; et al. Plasma Tryptophan and the Kynurenine–Tryptophan Ratio are Associated with the Acquisition of Statural Growth Deficits and Oral Vaccine Underperformance in Populations with Environmental Enteropathy. Am. J. Trop. Med. Hyg. 2016, 95, 928–937. [Google Scholar] [CrossRef]
- Semba, R.D.; Shardell, M.; Trehan, I.; Moaddel, R.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.; Ferrucci, L.; Manary, M.J. Metabolic alterations in children with environmental enteric dysfunction. Sci. Rep. 2016, 6, 28009. [Google Scholar] [CrossRef] [Green Version]
- Gazi, M.A.; Das, S.; Siddique, M.A.; Alam, M.A.; Fahim, S.M.; Hasan, M.M.; Hossaini, F.; Kabir, M.; Noor, Z.; Haque, R.; et al. Plasma kynurenine to tryptophan ratio is negatively associated with linear growth of children living in a slum of Bangladesh: Results from a community-based intervention study. Am. J. Trop. Med. Hyg. 2021, 104, 766. [Google Scholar] [CrossRef]
- Mahfuz, M.; Alam, M.A.; Das, S.; Fahim, S.M.; Hossain, M.S.; Petri, W.A., Jr.; Ashorn, P.; Ashorn, U.; Ahmed, T. Daily Supplementation with Egg, Cow Milk, and Multiple Micronutrients Increases Linear Growth of Young Children with Short Stature. J. Nutr. 2019, 150, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.; McNamara, L.; Maskew, M.; Selibas, K.; Van Amsterdam, D.; Baines, N.; Webster, T.; Sanne, I. Impact of nutritional supplementation on immune response, body mass index and bioelectrical impedance in HIV-positive patients starting antiretroviral therapy. Nutr. J. 2013, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- Mahfuz, M.; Das, S.; Mazumder, R.N.; Rahman, B.M.M.; Haque, R.; Bhuiyan, M.R.; Akhter, H.; Alam, S.M.S.; Mondal, D.; Muaz, S.S.A.; et al. Bangladesh Environmental Enteric Dysfunction (BEED) study: Protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. BMJ Open 2017, 7, e017768. [Google Scholar] [CrossRef] [Green Version]
- Fahim, S.M.; Das, S.; Gazi, A.; Alam, A.; Mahfuz, M.; Ahmed, T. Evidence of gut enteropathy and factors associated with undernutrition among slum-dwelling adults in Bangladesh. Am. J. Clin. Nutr. 2020, 111, 657–666. [Google Scholar] [CrossRef]
- Ball, H.J.; Yuasa, H.J.; Austin, C.J.; Weiser, S.; Hunt, N.H. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int. J. Biochem. Cell Biol. 2009, 41, 467–471. [Google Scholar] [CrossRef]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; De Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. Int. J. Tryptophan Res. 2009, 2, IJTR-S2097. [Google Scholar] [CrossRef] [Green Version]
- Adu-Gyamfi, C.; Savulescu, D.; Mikhathani, L.; Otwombe, K.; Salazar-Austin, N.; Chaisson, R.; Martinson, N.; George, J.; Suchard, M. Plasma Kynurenine-to-Tryptophan Ratio, a Highly Sensitive Blood-Based Diagnostic Tool for Tuberculosis in Pregnant Women Living with Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2021, 73, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, R.L.; Oriá, R.B.; Moore, S.R.; Oriá, M.O.; Lima, A.A. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr. Rev. 2008, 66, 487–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katona, P.; Katona-Apte, J. The Interaction between Nutrition and Infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Alqarni, M.; Awad, D.A.B.; Algammal, A.M.; Nyamota, R.; Wahed, M.I.I.; Shah, M.A.; Amin, M.N.; Adetuyi, B.O.; Hetta, H.F.; et al. Dairy-Derived and Egg White Proteins in Enhancing Immune System Against COVID-19. Front. Nutr. 2021, 8, 629440. [Google Scholar] [CrossRef]
- Maltos, A.L.; Portari, G.V.; Moraes, G.V.; Monteiro, M.C.R.; Vannucchi, H.; da Cunha, D.F. Niacin metabolism and indoleamine 2,3-dioxygenase activation in malnourished patients with flaky paint dermatosis. Nutrition 2015, 31, 890–892. [Google Scholar] [CrossRef]
- Zaric, B.L.; Radovanović, J.; Gluvic, Z.; Stewart, A.J.; Essack, M.; Motwalli, O.; Gojobori, T.; Isenovic, E.R. Atherosclerosis Linked to Aberrant Amino Acid Metabolism and Immunosuppressive Amino Acid Catabolizing Enzymes. Front. Immunol. 2020, 11, 551758. [Google Scholar] [CrossRef]
- Wongpraparut, N.; Pengchata, P.; Piyophirapong, S.; Panchavinnin, P.; Pongakasira, R.; Arechep, N.; Kasetsinsombat, K.; Maneechotesuwan, K. Indoleamine 2,3 dioxygenase (IDO) level as a marker for significant coronary artery disease. BMC Cardiovasc. Disord. 2021, 21, 353. [Google Scholar] [CrossRef]
- Schefold, J.C.; Zeden, J.-P.; Fotopoulou, C.; von Haehling, S.; Pschowski, R.; Hasper, D.; Volk, H.-D.; Schuett, C.; Reinke, P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 2009, 24, 1901–1908. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Fahim, S.M.; Das, S.; Gazi, A.; Mahfuz, M.; Ahmed, T. Association of plasma low-density lipoprotein receptor-related protein-1 (LRP1) with undernutrition: A case-control study in Bangladeshi adults. Biomarkers 2021, 26, 625–631. [Google Scholar] [CrossRef]
- Hasan, M.M.; Gazi, A.; Das, S.; Fahim, S.M.; Hossaini, F.; Alam, A.; Mahfuz, M.; Ahmed, T. Association of lipocalin-2 and low-density lipoprotein receptor-related protein-1 (LRP1) with biomarkers of environmental enteric dysfunction (EED) among under 2 children in Bangladesh: Results from a community-based intervention study. BMJ Paediatr. Open 2021, 5, e001138. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Rodriguez, R.A.; Humphreys, M.H. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 141–149. [Google Scholar] [CrossRef]
- Van Wissen, M.; Snoek, M.; Smids, B.; Jansen, H.M.; Lutter, R. IFN-γ amplifies IL-6 and IL-8 responses by airway epithelial-like cells via indoleamine 2,3-dioxygenase. J. Immunol. 2002, 169, 7039–7044. [Google Scholar] [CrossRef] [Green Version]
| Indicators | Baseline (n = 525) | Endline (n = 512) | Overall (n = 1037) | p-Value |
|---|---|---|---|---|
| Age in years, Mean (SD) | 23.8 (6.83) | - | - | |
| Female gender, n (%) | 382 (72.8) | - | - | |
| Weight in kg, Mean (SD) | 41.4 (4.79) | 42.3 (5.03) | 41.8 (4.93) | <0.001 |
| Height in cm, Mean (SD) | 155 (8.04) | 155 (8.13) | 155 (8.08) | 0.318 |
| BMI in kg/m2, Mean (SD) | 17.3 (0.83) | 17.6 (1.13) | 17.4 (1.01) | <0.001 |
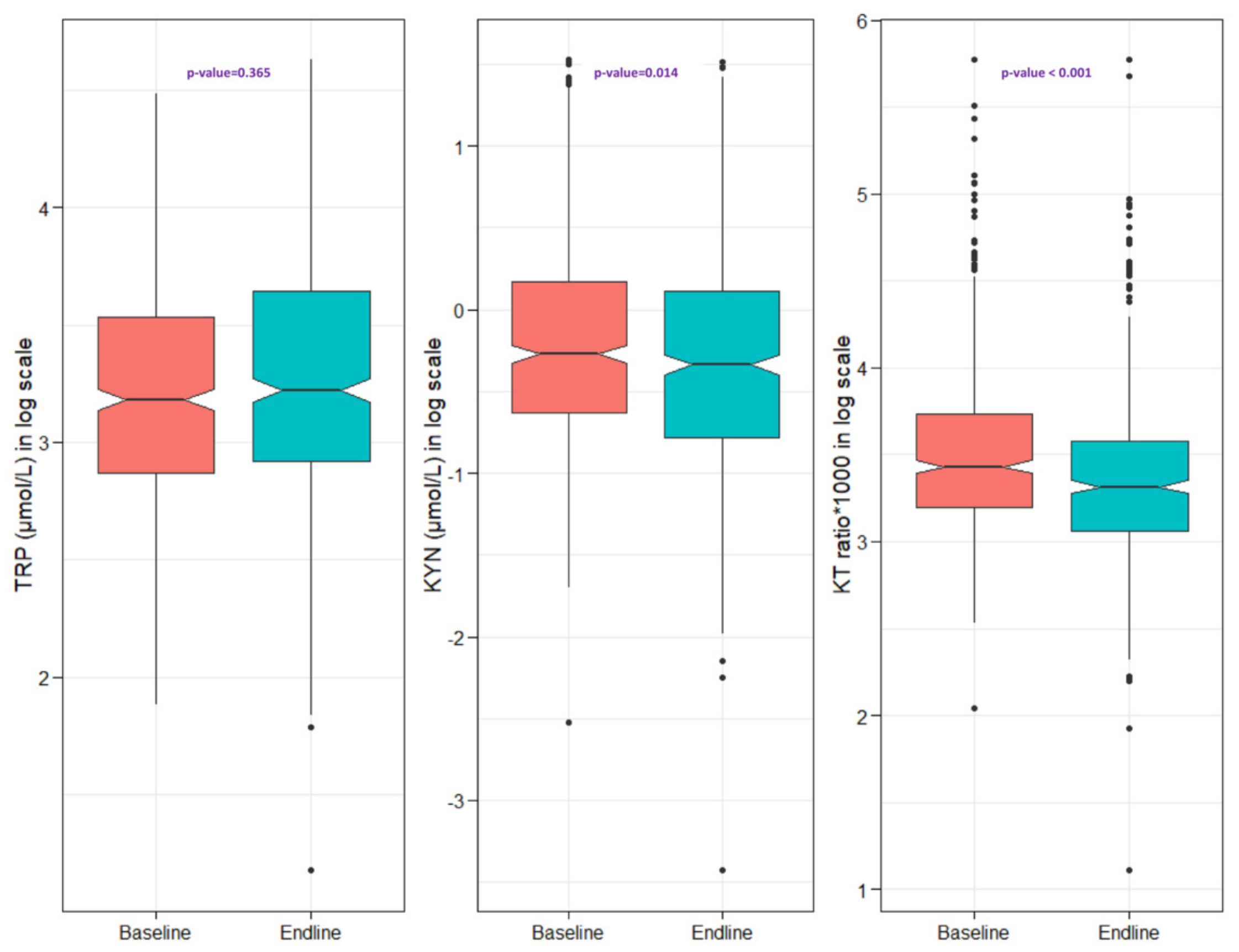
| TRP (µmol/L), Median (Q1, Q3) | 24.1 (17.6, 34.3) | 25.1 (18.5, 38.3) | 24.4 (18.1, 36.2) | 0.365 |
| KYN (µmol/L), Median (Q1, Q3) | 0.76 (0.53, 1.18) | 0.71 (0.46, 1.12) | 0.73 (0.49, 1.15) | 0.014 |
| KT ratio, Median (Q1, Q3) | 30.9 (24.5, 41.7) | 27.5 (21.3, 35.8) | 29.3 (22.7, 38.8) | <0.001 |
| Infection with H. pylori, n (%) | 421 (80.3) | 403 (80.8) | 824 (80.5) | 0.866 |
| NEO (nmol/L), Median (Q1, Q3) | 1010 (392, 1830) | 1070 (396, 1850) | 1040 (392, 1840) | 0.530 |
| MPO (ng/mL), Median (Q1, Q3) | 737 (355, 1460) | 664 (383, 1480) | 711 (366, 1460) | 0.369 |
| AAT (mg/g), Median (Q1, Q3) | 0.14 (0.06, 0.30) | 0.16 (0.07, 0.30) | 0.15 (0.06, 0.30) | 0.684 |
| CRP (mg/L), Median (Q1, Q3) | 0.65 (0.23, 1.57) | 0.63 (0.24, 1.8) | 0.64 (0.23, 1.66) | 0.719 |
| LRP1 (ng/mL), Median (Q1, Q3) | 807 (629, 1060) | 873 (619, 1200) | 832 (621, 1120) | 0.015 |
| Income in USD, Median (Q1, Q3) | 179 (122, 239) | - | - | |
| Improved sanitation, n (%) | 377 (71.8) | - | - |
| Variables | Unadjusted β (95% CI) | p-Value | Adjusted β (95% CI) | p-Value |
|---|---|---|---|---|
| Age in years | 0.01 (0, 0.02) | 0.15 | 0.01 (0, 0.02) | 0.04 |
| Sex (Female) | 0.09 (−0.09, 0.27) | 0.34 | 0.02 (−0.17, 0.22) | 0.80 |
| KT ratio | −0.11 (−0.21, −0.02) | 0.02 | −0.09 (−0.18, 0) | 0.06 |
| AAT | 0.00 (−0.04, 0.04) | 0.90 | ||
| MPO | −0.03 (−0.06, 0.01) | 0.12 | −0.02 (−0.06, 0.01) | 0.17 |
| NEO | 0.00 (−0.03, 0.04) | 0.83 | ||
| Calprotectin | −0.03 (−0.07, 0.01) | 0.17 | −0.01 (−0.05, 0.03) | 0.60 |
| Reg1B | −0.01 (−0.03, 0.01) | 0.48 | ||
| Infection with H. pylori | −0.07 (−0.22, 0.09) | 0.40 | ||
| AGP | 0.12 (0.01, 0.22) | 0.04 | 0.09 (−0.02, 0.2) | 0.09 |
| CRP | −0.01 (−0.04, 0.02) | 0.55 | ||
| Ferritin | −0.13 (−0.19, −0.07) | <0.001 | −0.13 (−0.19, −0.07) | <0.001 |
| RBP4 | 0.07 (−0.08, 0.21) | 0.38 | ||
| LRP1 | 0.38 (0.23, 0.52) | <0.001 | 0.34 (0.2, 0.49) | <0.001 |
| zinc | 0.15 (−0.15, 0.45) | 0.34 | ||
| Family income ($100 increase) | 0.00 (0.00, 0.00) | 0.20 | 0.00 (0.00, 0.00) | 0.09 |
| Improved sanitation | 0.17 (−0.01, 0.35) | 0.06 | 0.14 (−0.03, 0.32) | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazi, M.A.; Siddique, M.A.; Alam, M.A.; Hossaini, F.; Hasan, M.M.; Fahim, S.M.; Wahid, B.Z.; Kabir, M.M.; Das, S.; Mahfuz, M.; et al. Plasma Kynurenine to Tryptophan Ratio Is Not Associated with Undernutrition in Adults but Reduced after Nutrition Intervention: Results from a Community-Based Study in Bangladesh. Nutrients 2022, 14, 1708. https://doi.org/10.3390/nu14091708
Gazi MA, Siddique MA, Alam MA, Hossaini F, Hasan MM, Fahim SM, Wahid BZ, Kabir MM, Das S, Mahfuz M, et al. Plasma Kynurenine to Tryptophan Ratio Is Not Associated with Undernutrition in Adults but Reduced after Nutrition Intervention: Results from a Community-Based Study in Bangladesh. Nutrients. 2022; 14(9):1708. https://doi.org/10.3390/nu14091708
Chicago/Turabian StyleGazi, Md. Amran, Md. Abdullah Siddique, Md. Ashraful Alam, Farzana Hossaini, Md. Mehedi Hasan, Shah Mohammad Fahim, Barbie Zaman Wahid, Md. Mamun Kabir, Subhasish Das, Mustafa Mahfuz, and et al. 2022. "Plasma Kynurenine to Tryptophan Ratio Is Not Associated with Undernutrition in Adults but Reduced after Nutrition Intervention: Results from a Community-Based Study in Bangladesh" Nutrients 14, no. 9: 1708. https://doi.org/10.3390/nu14091708
APA StyleGazi, M. A., Siddique, M. A., Alam, M. A., Hossaini, F., Hasan, M. M., Fahim, S. M., Wahid, B. Z., Kabir, M. M., Das, S., Mahfuz, M., & Ahmed, T. (2022). Plasma Kynurenine to Tryptophan Ratio Is Not Associated with Undernutrition in Adults but Reduced after Nutrition Intervention: Results from a Community-Based Study in Bangladesh. Nutrients, 14(9), 1708. https://doi.org/10.3390/nu14091708






