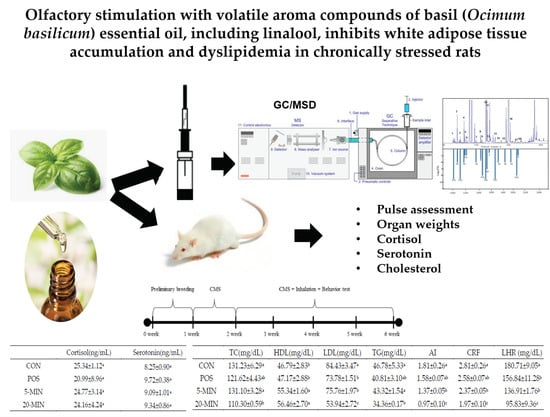
Olfactory Stimulation with Volatile Aroma Compounds of Basil (Ocimum basilicum L.) Essential Oil and Linalool Ameliorates White Fat Accumulation and Dyslipidemia in Chronically Stressed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Odor-Active Aroma Compounds
2.3. Animal Care and Experimental Design
2.4. Forced Swimming Test
2.5. Pulse
2.6. Stress Hormones
2.7. Analyses of Serum Biomarkers and Hepatotoxicity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Odor-Active Aroma Compounds
3.2. Total Food Intake and Total Weight Gain
3.3. Forced Swimming Test
3.4. Pulse
3.5. Organ Weights
3.6. Stress Hormones
3.7. Serum Biomarkers and Hepatotoxicity Indicators
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ochoa-Amaya, J.E.; Malucelli, B.E.; Cruz-Casallas, P.E.; Nasello, A.G.; Felicio, L.F.; Carvalho-Freitas, M.I.R. Acute and chronic stress and the inflammatory response in hyperprolactinemic rats. Neuroimmunomodulation 2010, 17, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Piskunov, A.; Stepanichev, M.; Tishkina, A.; Novikova, M.; Levshina, I.; Gulyaeva, N. Chronic combined stress induces selective and long-lasting inflammatory response evoked by changes in corticosterone accumulation and signaling in rat hippocampus. Metab. Brain Dis. 2016, 31, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Pacák, K.; Palkovits, M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr. Rev. 2001, 22, 502–548. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cariol. 2012, 9, 360–370. [Google Scholar] [CrossRef]
- Hong, S.J.; Cho, J.; Boo, C.G.; Youn, M.Y.; Pan, J.H.; Kim, J.K.; Shin, E.C. Inhalation of patchouli (Pogostemon Cablin Benth.) essential oil improved metabolic parameters in obesity-induced sprague dawley rats. Nutrients 2020, 12, 2077. [Google Scholar] [CrossRef]
- Baek, H.; Kim, S.; Lee, I.; Kang, S.; Yoo, J.; Yoon, W.; Kim, Y.; Kim, H.; Kim, J. Anti-obesity and anti-lipidemic effects of linalool in high-fat diet-induced obese mice. J. Biomed. Res. 2012, 13, 229–235. [Google Scholar] [CrossRef]
- Harnafi, H.; Aziz, M.; Amrani, S. Sweet basil (Ocimum basilicum L.) improves lipid metabolism in hypercholesterolemic rats. e-SPEN Eur. J. Clin. Nutr. 2009, 4, e181–e186. [Google Scholar] [CrossRef] [Green Version]
- de Moura Linck, V.; da Silva, A.L.; Figueiró, M.; Piato, A.L.; Herrmann, A.P.; Birck, F.D.; Caramão, E.B.; Nunes, D.S.; Moreno, P.R.H.; Elisabetsky, E. Inhaled linalool-induced sedation in mice. Phytomedicine 2009, 16, 303–307. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T. Geographical impact on essential oil composition of endemic Kundmannia anatolica Hub.-Mor.(Apiaceae). Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 131–137. [Google Scholar] [CrossRef]
- Maria, G.A.; Riccardo, N. Citrus bergamia, Risso: The peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emir. J. Food Agric. 2020, 32, 522–532. [Google Scholar] [CrossRef]
- Gioffrè, G.; Ursino, D.; Labate, M.L.C.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A review. Emir. J. Food Agric. 2020, 32, 835–845. [Google Scholar] [CrossRef]
- Inan, M.; Kirpik, M.; Kaya, D.A.; Kirici, S. Effect of harvest time on essential oil composition of Thymbra spicata L. growing in flora of Adıyaman. Adv. Environ. Biol. 2011, 5, 356–358. [Google Scholar]
- Chatterjee, S.; Gupta, S.; Variyar, P.S. Comparison of essential oils obtained from different extraction techniques as an aid in identifying aroma significant compounds of nutmeg (Myristica fragrans). Nat. Prod. Commun. 2015, 10, 1443–1446. [Google Scholar] [CrossRef] [Green Version]
- Türkmen, M. The effect of different Phenological periods and harvest times on the essential oil ratio and components of basil genotypes. J. Essent. Oil-Bear. Plants. 2021, 24, 94–109. [Google Scholar] [CrossRef]
- Coelho, J.; Veiga, J.; Karmali, A.; Nicolai, M.; Pinto Reis, C.; Nobre, B.; Palavra, A. Supercritical CO2 extracts and volatile oil of basil (Ocimum basilicum L.) comparison with conventional methods. Separations 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The sense of smell impacts metabolic health and obesity. Cell Metab. 2017, 26, 198–211. [Google Scholar] [CrossRef] [Green Version]
- da Silva Haeser, A.; Sitta, A.; Barschak, A.G.; Deon, M.; Barden, A.T.; Schmitt, G.O.; Landgraff, S.; Gomez, R.; Barros, H.M.T.; Vargas, C.R. Oxidative stress parameters in diabetic rats submitted to forced swimming test: The clonazepam effect. Brain Res. 2007, 1154, 137–143. [Google Scholar] [CrossRef]
- Atescelik, M.; Yilmaz, M.; Korkmaz, S.; Goktekin, M.C.; Gurger, M.; Ilhan, N. The relationship between ghrelin and copeptin levels, and anxiety and depression levels in suicide attempts. Clin. Psychopharmacol. Neurosci. 2017, 15, 256. [Google Scholar] [CrossRef] [Green Version]
- Dora, M.F.; Taha, N.M.; Lebda, M.A.; Hashem, A.E.; Elfeky, M.S.; El-Sayed, Y.S.; Jaouni, S.A.; El-Far, A.H. Quercetin attenuates brain oxidative alterations induced by iron oxide nanoparticles in rats. Int. J. Mol. Sci. 2021, 22, 3829. [Google Scholar] [CrossRef]
- Jun, H.; Lee, J.H.; Kim, J.; Jia, Y.; Kim, K.H.; Hwang, K.Y.; Yun, E.J.; Do, K.; Lee, S. Linalool is a PPARα ligand that reduces plasma TG levels and rewires the hepatic transcriptome and plasma metabolome. J. Lipid Res. 2014, 55, 1098–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto-Maior, F.N.; de Carvalho, F.L.; de Morais, L.C.S.L.; Netto, S.M.; de Sousa, D.P.; de Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggersdorfer, M. Terpenes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 29–45. [Google Scholar] [CrossRef]
- Da Fonsêca, D.V.; da Silva Maia Bezerra Filho, C.; Lima, T.C.; de Almeida, R.N.; de Sousa, D.P. Anticonvulsant essential oils and their relationship with oxidative stress in epilepsy. Biomolecules 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padalia, R.C.; Verma, R.S. Comparative volatile oil composition of four Ocimum species from northern India. Nat. Prod. Res. 2011, 25, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Riyazi, S.R.; Ebrahimnezhad, Y.; Hosseini, S.A.; Meimandipour, A.; Ghorbani, A. Comparison of the effects of basil (Ocimum basilicum) essential oil, avilamycin and protexin on broiler performance, blood biochemistry and carcass characteristics. Arch. Anim. Breed. 2015, 58, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Sentari, M.; Harahap, U.; Sapiie, T.W.A.; Ritarwan, K. Blood cortisol level and blood serotonin level in depression mice with basil leaf essential oil treatment. Open Access Maced. J. Med. Sci. 2019, 7, 2652. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Niijima, A.; Tanida, M.; Horii, Y.; Maeda, K.; Nagai, K. Olfactory stimulation with scent of lavender oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005, 383, 188–193. [Google Scholar] [CrossRef]
- Aschbacher, K.; Kornfeld, S.; Picard, M.; Puterman, E.; Havel, P.J.; Stanhope, K.; Lustig, R.H.; Epel, E. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology 2014, 46, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Fujiwara, S.; Matsumoto, I.; Abe, K. Stress repression in restrained rats by (R)-(−)-linalool inhalation and gene expression profiling of their whole blood cells. J. Agric. Food Chem. 2009, 57, 5480–5485. [Google Scholar] [CrossRef]
- Gökçe, Y.; Kanmaz, H.; Er, B.; Sahin, K.; Hayaloglu, A.A. Influence of purple basil (Ocimum basilicum L.) extract and essential oil on hyperlipidemia and oxidative stress in rats fed high-cholesterol diet. Food Biosci. 2021, 43, 101228. [Google Scholar] [CrossRef]
- Umezu, T. Evaluation of central nervous system acting effects of plant-derived essential oils using ambulatory activity in mice. J. Pharm. Pharmacol. 2013, 4, 29704. [Google Scholar] [CrossRef] [Green Version]
- Tanida, M.; Niijima, A.; Shen, J.; Nakamura, T.; Nagai, K. Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neurosci. Lett. 2006, 398, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ch, M.A.; Naz, S.B.; Sharif, A.; Akram, M.; Saeed, M.A. Biological and pharmacological properties of the sweet basil (Ocimum basilicum). J. Pharm. Res. Int. 2015, 7, 330–339. [Google Scholar] [CrossRef]
- Cheng, B.H.; Sheen, L.Y.; Chang, S.T. Hypolipidemic effects of S-(+)-linalool and essential oil from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2018, 8, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hirano, N.; Sakamoto, K. Linalool odor stimulation improves heat stress tolerance and decreases fat accumulation in nematodes. Biosci. Biotechnol. Biochem. 2019, 83, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manting, L.; Haihong, Z.; Jing, L.; Shaodong, C.; Yihua, L. The model of rat lipid metabolism disorder induced by chronic stress accompanying high-fat-diet. Lipids Health Dis. 2011, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Zhao, H.L.; Cho, K.H.; Ha, Y.W.; Jeong, T.S.; Lee, W.S.; Kim, Y.S. Cholesterol-lowering effect of platycodin D in hypercholesterolemic ICR mice. Eur. J. Pharmacol. 2006, 537, 166–173. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [Green Version]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Ahn, H.; Go, G.W. Pinus densiflora bark extract (PineXol) decreases adiposity in mice by down-regulation of hepatic de novo lipogenesis and adipogenesis in white adipose tissue. J. Microbiol. Biotechnol. 2017, 27, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Compounds | Retention Time(min) | Retention Index | Content (ug/mL) | Odor Intensity | Odor Description |
|---|---|---|---|---|---|
| Alcohols (5) | |||||
| 1,8-Cineole | 16.29 | 1061 | 38.91 | ||
| Linalool oxide | 17.58 | 1100 | 7.33 | 3 | Herb |
| Linalool | 18.37 | 1128 | 107.65 | 4 | Sweet, Fruit |
| Fenchyl alcohol | 18.85 | 1145 | 32.11 | ||
| trans-Anethole | 23.02 | 1291 | 14.39 | ||
| Hydrocarbons (10) | |||||
| Camphene | 13.62 | 978 | 5.02 | ||
| β-Myrcene | 14.98 | 1019 | 15.72 | ||
| γ-Terpinene | 15.42 | 1034 | 11.24 | ||
| α-Terpinene | 15.80 | 1046 | 6.22 | ||
| β-Cymene | 16.07 | 1054 | 7.77 | ||
| D-Limonene | 16.20 | 1058 | 34.64 | ||
| Ocimene | 16.44 | 1066 | 13.43 | ||
| Menthene | 20.82 | 1212 | 17.33 | 4 | Herb, Basil, Xylitol |
| 2-Hydroxy phenyl butane | 24.15 | 1335 | 22.29 | ||
| 1-Methoxy ethyl benzene | 24.34 | 1342 | 14.65 | ||
| Ketones (2) | |||||
| Menth-4-en-3-one | 22.82 | 1284 | 45.40 | ||
| D-Carvone | 22.89 | 1287 | 6.44 | 1 | Lemon |
| Total Food Intake (g) | Total Weight Gain (g) | |
|---|---|---|
| CON | 499.51 ± 10.60 a1 | 219.67 ± 2.08 a |
| POS | 483.37 ± 9.73 a | 204.67 ± 12.34 ab |
| 5 MIN | 467.21 ± 24.37 a | 201.67 ± 11.15 ab |
| 20 MIN | 479.90 ± 11.72 a | 190.67 ± 2.52 b |
| Initial (s) | Final (s) | |
|---|---|---|
| CON | 71.67 ± 22.19 a1 | 8.33 ± 5.13 b |
| POS | 44.67 ± 3.51 a | 16.67 ± 16.97 b |
| 5-MIN | 63.00 ± 6.93 a | 18.67 ± 9.87 ab |
| 20-MIN | 72.66 ± 10.26 a | 47.33 ± 14.84 a |
| Pulse (beats/min) | Initial | Final |
|---|---|---|
| CON | 406 ± 13 a1 | 420 ± 19 a |
| POS | 407 ± 17 a | 416 ± 14 a,b |
| 5 MIN | 402 ± 14 a | 351 ± 19 b |
| 20 MIN | 414 ± 3 a | 392 ± 21 a,b |
| Liver (g) | Kidney (g) | Heart (g) | WAT (g) | BAT (g) | |
|---|---|---|---|---|---|
| CON | 11.74 ± 2.87 a1 | 2.09 ± 0.03 a | 1.38 ± 0.03 a | 6.86 ± 0.40 a | 0.62 ± 0.06 a |
| POS | 8.83 ± 0.19 a | 1.97 ± 0.02 a,b | 1.33 ± 0.11 a | 5.75 ± 0.61 b | 0.71 ± 0.10 a |
| 5 MIN | 9.00 ± 0.28 a | 2.11 ± 0.05 a | 1.30 ± 0.29 a | 6.46 ± 0.29 a,b | 0.68 ± 0.05 a |
| 20 MIN | 9.96 ± 0.29 a | 1.86 ± 0.03 b | 1.38 ± 0.08 a | 6.15 ± 0.16 a,b | 0.59 ± 0.03 a |
| Cortisol (ng/mL) | Serotonin (ng/mL) | |
|---|---|---|
| CON | 25.34 ± 1.12 a1 | 8.25 ± 0.90 a |
| POS | 20.99 ± 8.96 a | 9.72 ± 0.38 a |
| 5 MIN | 24.77 ± 3.14 a | 9.09 ± 1.01 a |
| 20 MIN | 24.16 ± 4.24 a | 9.34 ± 0.86 a |
| TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | TG (mg/dL) | AI | CRF | LHR (mg/dL) | |
|---|---|---|---|---|---|---|---|
| CON | 131.23 ± 6.29 a1 | 46.79 ± 2.83 b | 84.43 ± 3.47 a | 46.78 ± 5.33 a | 1.81 ± 0.26 a | 2.81 ± 0.26 a | 180.71 ± 9.05 a |
| POS | 121.62 ± 4.43 ab | 47.17 ± 2.88 b | 73.78 ± 1.51 b | 40.81 ± 3.10 ab | 1.58 ± 0.07 ab | 2.58 ± 0.07 ab | 156.84 ± 11.28 b |
| 5 MIN | 131.10 ± 3.28 a | 55.34 ± 1.60 a | 75.76 ± 1.97 b | 43.32 ± 1.54 a | 1.37 ± 0.05 b | 2.37 ± 0.05 b | 136.91 ± 1.76 b |
| 20 MIN | 110.30 ± 0.59 b | 56.46 ± 2.70 a | 53.94 ± 2.72 c | 34.36 ± 0.17 b | 0.97 ± 0.10 c | 1.97 ± 0.10 c | 95.83 ± 9.36 c |
| AST (Karmen/mL) | ALT (Karmen/mL) | AST/ALT | |
|---|---|---|---|
| CON | 153.16 ± 1.82 a1 | 25.24 ± 0.07 a | 6.07 ± 0.09 a |
| POS | 155.65 ± 2.67 a | 24.84 ± 0.58 a | 6.27 ± 0.23 a |
| 5 MIN | 151.94 ± 1.43 a | 24.05 ± 0.55 a | 6.18 ± 0.03 a |
| 20 MIN | 151.34 ± 1.89 a | 24.58 ± 0.12 a | 6.29 ± 0.14 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Hong, S.-J.; Yoon, S.; Jo, S.-M.; Jeong, H.; Youn, M.-Y.; Kim, Y.-J.; Kim, J.-K.; Shin, E.-C. Olfactory Stimulation with Volatile Aroma Compounds of Basil (Ocimum basilicum L.) Essential Oil and Linalool Ameliorates White Fat Accumulation and Dyslipidemia in Chronically Stressed Rats. Nutrients 2022, 14, 1822. https://doi.org/10.3390/nu14091822
Kim D-S, Hong S-J, Yoon S, Jo S-M, Jeong H, Youn M-Y, Kim Y-J, Kim J-K, Shin E-C. Olfactory Stimulation with Volatile Aroma Compounds of Basil (Ocimum basilicum L.) Essential Oil and Linalool Ameliorates White Fat Accumulation and Dyslipidemia in Chronically Stressed Rats. Nutrients. 2022; 14(9):1822. https://doi.org/10.3390/nu14091822
Chicago/Turabian StyleKim, Da-Som, Seong-Jun Hong, Sojeong Yoon, Seong-Min Jo, Hyangyeon Jeong, Moon-Yeon Youn, Young-Jun Kim, Jae-Kyeom Kim, and Eui-Cheol Shin. 2022. "Olfactory Stimulation with Volatile Aroma Compounds of Basil (Ocimum basilicum L.) Essential Oil and Linalool Ameliorates White Fat Accumulation and Dyslipidemia in Chronically Stressed Rats" Nutrients 14, no. 9: 1822. https://doi.org/10.3390/nu14091822
APA StyleKim, D.-S., Hong, S.-J., Yoon, S., Jo, S.-M., Jeong, H., Youn, M.-Y., Kim, Y.-J., Kim, J.-K., & Shin, E.-C. (2022). Olfactory Stimulation with Volatile Aroma Compounds of Basil (Ocimum basilicum L.) Essential Oil and Linalool Ameliorates White Fat Accumulation and Dyslipidemia in Chronically Stressed Rats. Nutrients, 14(9), 1822. https://doi.org/10.3390/nu14091822