Curcumin Supplementation Ameliorates Bile Cholesterol Supersaturation in Hamsters by Modulating Gut Microbiota and Cholesterol Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Histological Analysis
2.3. Biochemical Analysis
2.4. Real-Time Polymerase Chain Reaction (RT-qPCR)
2.5. Western Blot
2.6. Gut Microbiota Analysis
2.7. Cholesterol Absorption Assay
2.8. Statistics
3. Results
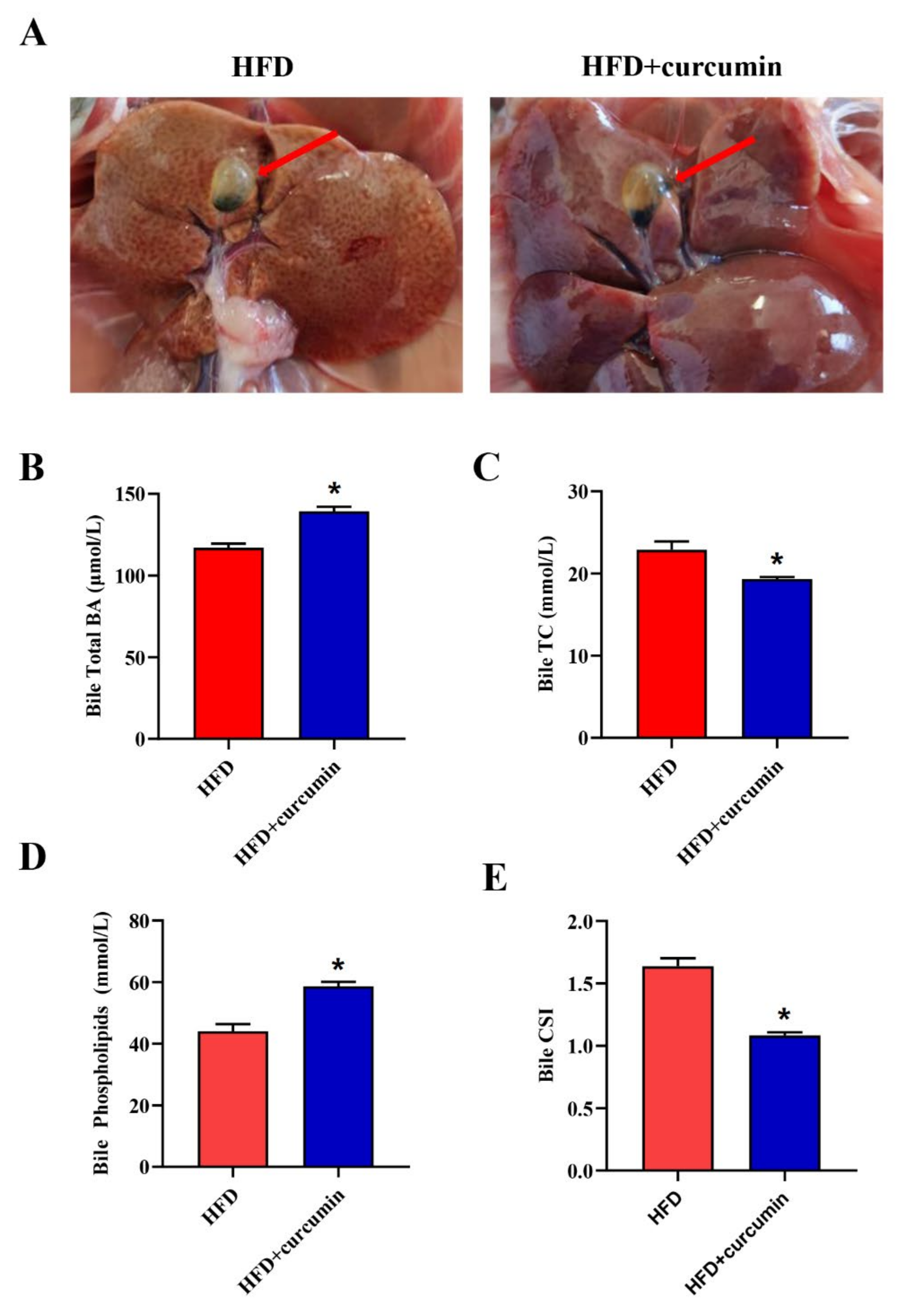
3.1. Curcumin Improved Bile Cholesterol Supersaturation in HFD-Fed Hamsters
3.2. Curcumin Ameliorated Serum and Liver Lipid Levels in HFD-Fed Hamsters
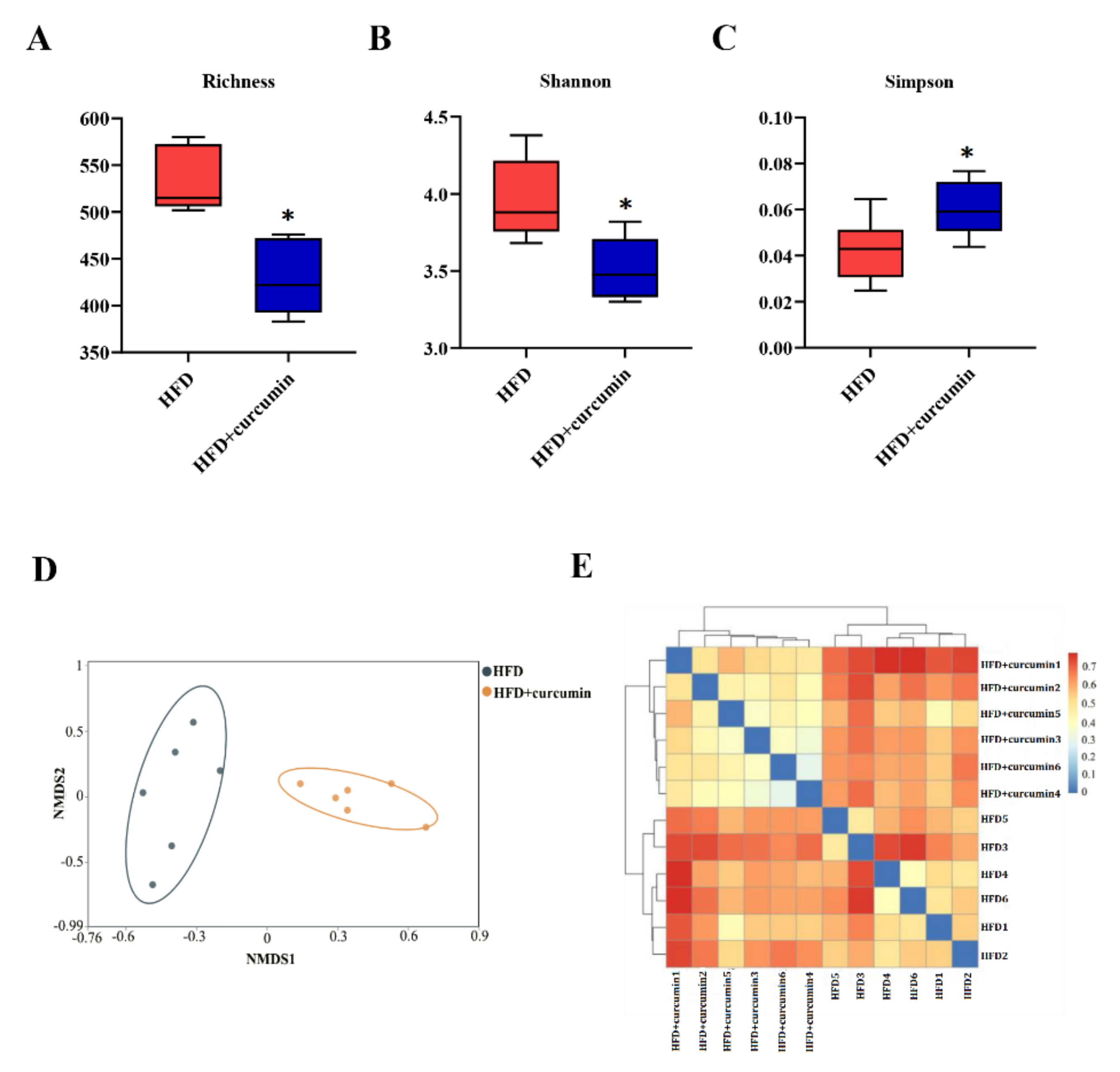
3.3. Curcumin Modulated Gut Microbiota Composition in HFD-Fed Hamsters
3.4. Curcumin Modulated Gut Microbiota Abundance in HFD-Fed Hamsters
3.5. Curcumin Up-Regulated the Expression of Liver CYP7A1 in HFD-Fed Hamsters
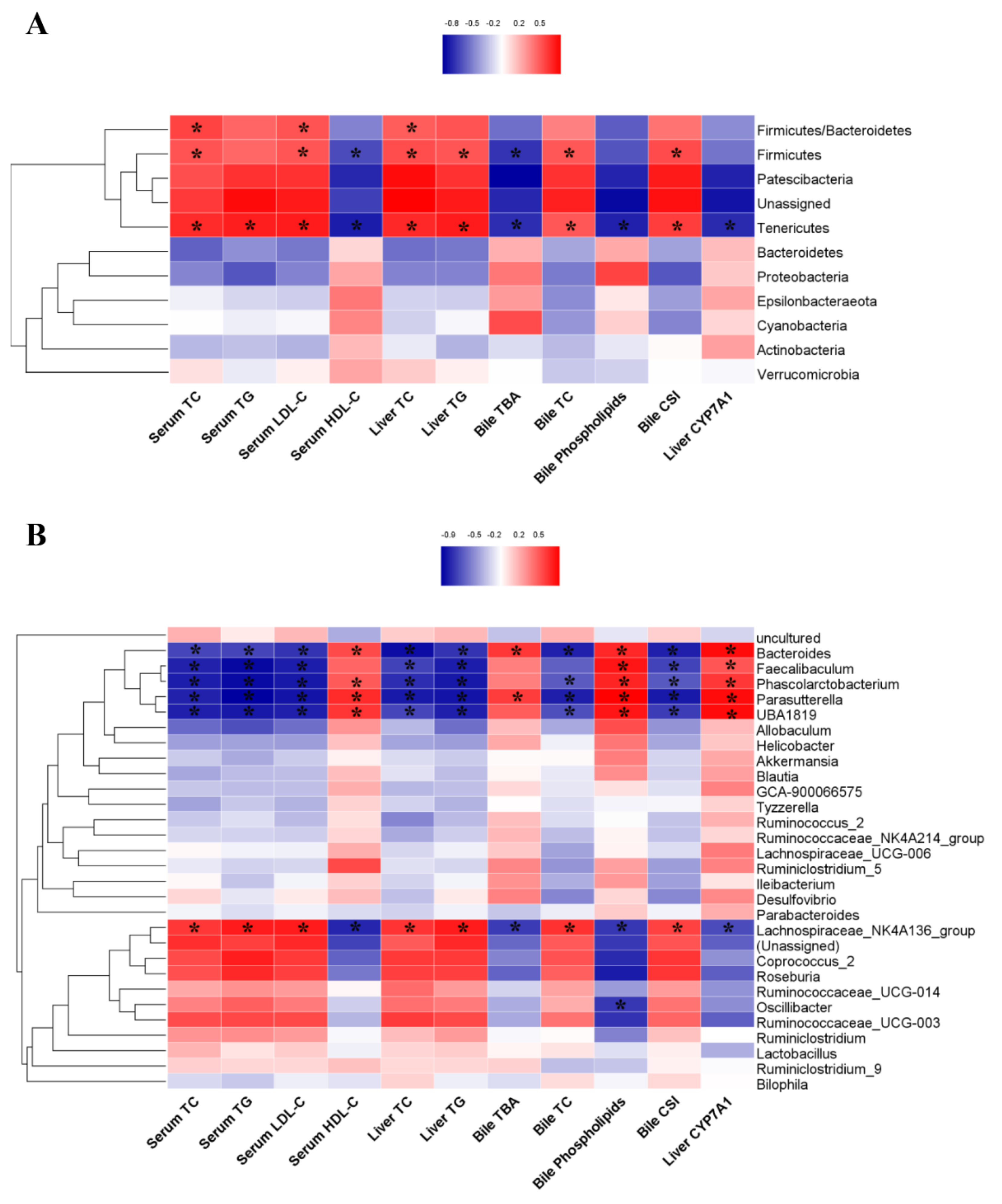
3.6. The Correlations between the Gut Microbiota and Biochemical Markers
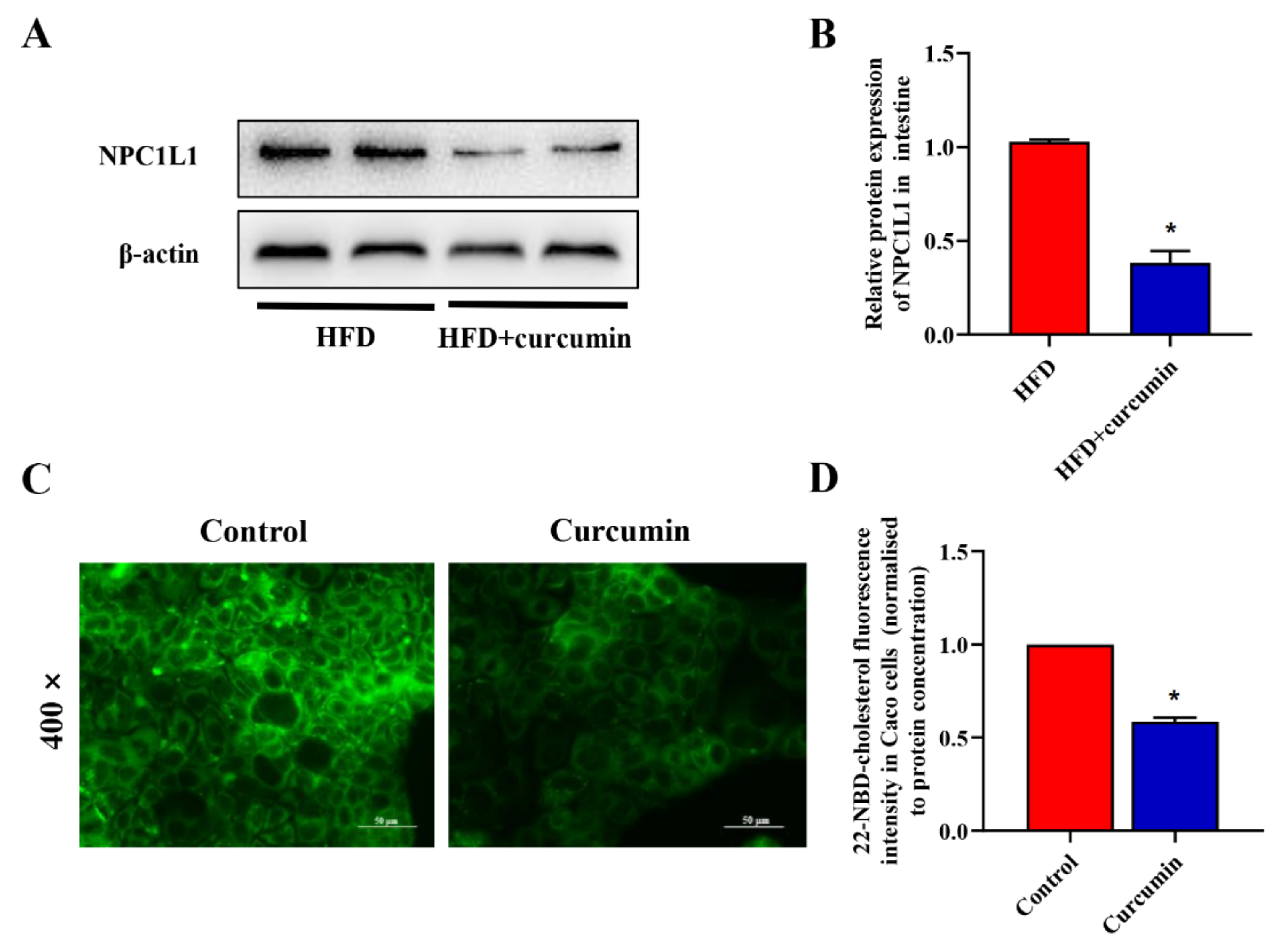
3.7. Curcumin Reduced Intestinal Cholesterol Absorption in HFD-Fed Hamsters and Caco-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.F.; Méndez-Sánchez, N.; Portincasa, P.; van Erpecum, K.J.; van Laarhoven, C.J.; Wang, D.Q. Gallstones. Nat. Rev. Dis. Prim. 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed]
- Rudling, M.; Laskar, A.; Straniero, S. Gallbladder bile supersaturated with cholesterol in gallstone patients preferentially develops from shortage of bile acids. J. Lipid. Res. 2019, 60, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zák, A.; Zeman, M.; Hrubant, K.; Vecka, M.; Tvrzická, E. Effect of hypolipidemic treatment on the composition of bile and the risk or cholesterol gallstone disease. Cas. Lek. Cesk. 2007, 146, 24–34. [Google Scholar] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Sadeghi, A.; Radinnia, E.; Naseh, A.; Gholamrezaei, Z.; Azizmohammad Looha, M.; Yadegar, A. The association between gut microbiota, cholesterol gallstones, and colorectal cancer. Gastroenterol. Hepatol. Bed Bench 2019, 12, S8–S13. [Google Scholar] [PubMed]
- Wang, Q.; Hao, C.; Yao, W.; Zhu, D.; Lu, H.; Li, L.; Ma, B.; Sun, B.; Xue, D.; Zhang, W. Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; He, C.; Sun, H.; Cai, Q.; Han, T.; Hu, H. Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 2017, 17, 74. [Google Scholar] [CrossRef]
- Fremont-Rahl, J.J.; Ge, Z.; Umana, C.; Whary, M.T.; Taylor, N.S.; Muthupalani, S.; Carey, M.C.; Fox, J.G.; Maurer, K.J. An analysis of the role of the indigenous microbiota in cholesterol gallstone pathogenesis. PLoS ONE 2013, 8, e70657. [Google Scholar] [CrossRef] [Green Version]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, g554–g573. [Google Scholar] [CrossRef]
- Fushimi, T.; Suruga, K.; Oshima, Y.; Fukiharu, M.; Tsukamoto, Y.; Goda, T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br. J. Nutr. 2006, 95, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- Srinivasan, K. Anti-cholelithogenic potential of dietary spices and their bioactives. Crit. Rev. Food Sci. Nutr. 2017, 57, 1749–1758. [Google Scholar] [CrossRef]
- Marciani, L.; Cox, E.F.; Hoad, C.L.; Totman, J.J.; Costigan, C.; Singh, G.; Shepherd, V.; Chalkley, L.; Robinson, M.; Ison, R.; et al. Effects of various food ingredients on gall bladder emptying. Eur. J. Clin. Nutr. 2013, 67, 1182–1187. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, M.; Wu, S.; Tian, Y. Combination of curcumin and piperine prevents formation of gallstones in C57BL6 mice fed on lithogenic diet: Whether NPC1L1/SREBP2 participates in this process? Lipids Health Dis. 2015, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Zou, J.; Zhang, S.; Li, X.; Lu, M. Hypocholesterolemic Activity of Curcumin Is Mediated by Down-regulating the Expression of Niemann-Pick C1-like 1 in Hamsters. J. Agric. Food Chem. 2017, 65, 276–280. [Google Scholar] [CrossRef]
- Ghiamati Yazdi, F.; Soleimanian-Zad, S.; van den Worm, E.; Folkerts, G. Turmeric Extract: Potential Use as a Prebiotic and Anti-Inflammatory Compound? Plant Foods Hum. Nutr. 2019, 74, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Shubha, M.C.; Reddy, R.R.; Srinivasan, K. Antilithogenic influence of dietary capsaicin and curcumin during experimental induction of cholesterol gallstone in mice. Appl. Physiol. Nutr. Metab. 2011, 36, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Portincasa, P. An update on the pathogenesis of cholesterol gallstone disease. Curr. Opin. Gastroenterol. 2018, 34, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, Y.; Shao, W.; Wang, Q.; Jiang, Z.; Hu, H. Dietary plant sterols prevented cholesterol gallstone formation in mice. Food Funct. 2021, 12, 11829–11837. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [Green Version]
- Gasmi Benahmed, A.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the gut and oral microbiome with obesity. Anaerobe 2021, 70, 102248. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Martin, R.C.; Aiyer, H.S.; Malik, D.; Li, Y. Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects. Food Chem. Toxicol. 2012, 50, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Marion, S.; Desharnais, L.; Studer, N.; Dong, Y.; Notter, M.D.; Poudel, S.; Menin, L.; Janowczyk, A.; Hettich, R.L.; Hapfelmeier, S.; et al. Biogeography of microbial bile acid transformations along the murine gut. J. Lipid Res. 2020, 61, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Tian, W.J.; Kwok, L.Y.; Wang, Y.L.; Shang, Y.N.; Menghe, B.; Wang, J.G. Effects of microencapsulated Lactobacillus plantarum LIP-1 on the gut microbiota of hyperlipidaemic rats. Br. J. Nutr. 2017, 118, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, T.; Liang, S.; Li, W.; Wu, X.; Jin, F. Antibiotic-induced imbalances in gut microbiota aggravates cholesterol accumulation and liver injuries in rats fed a high-cholesterol diet. Appl. Microbiol. Biotechnol. 2015, 99, 9111–9122. [Google Scholar] [CrossRef]
- Kc, D.; Sumner, R.; Lippmann, S. Gut microbiota and health. Postgrad. Med. 2020, 132, 274. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhang, Z.; Liu, B.; Hou, D.; Liang, Y.; Zhang, J.; Shi, P. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genom. 2013, 14, 669. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, T.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barceló, J.; Huby, T.; Guerin, M.; et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Wang, X.; Huang, F.; Zhao, A.; Chen, W.; Yan, J.; Zhang, Y.; Lei, S.; Ge, K.; Zheng, X.; et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int. J. Cancer 2016, 139, 1764–1775. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, T.; Zou, J.; Jiang, X.; Yang, J.; Cao, Z.; He, Y.; Feng, D. Curcumin Supplementation Ameliorates Bile Cholesterol Supersaturation in Hamsters by Modulating Gut Microbiota and Cholesterol Absorption. Nutrients 2022, 14, 1828. https://doi.org/10.3390/nu14091828
Hong T, Zou J, Jiang X, Yang J, Cao Z, He Y, Feng D. Curcumin Supplementation Ameliorates Bile Cholesterol Supersaturation in Hamsters by Modulating Gut Microbiota and Cholesterol Absorption. Nutrients. 2022; 14(9):1828. https://doi.org/10.3390/nu14091828
Chicago/Turabian StyleHong, Ting, Jun Zou, Xin Jiang, Jie Yang, Zhuo Cao, Youming He, and Dan Feng. 2022. "Curcumin Supplementation Ameliorates Bile Cholesterol Supersaturation in Hamsters by Modulating Gut Microbiota and Cholesterol Absorption" Nutrients 14, no. 9: 1828. https://doi.org/10.3390/nu14091828






