Carbohydrate Maldigestion and Intolerance
Abstract
:1. Introduction
2. Lactose Malabsorption and Intolerance
3. Sucrase-Isomaltase Deficiency
4. Fructose Malabsorption
5. Sorbitol Intolerance
6. Sugar Malabsorption and Functional Bowel Disease
7. Carbohydrate-Reduced Diets in Functional Bowel Disease
8. Mechanisms of Carbohydrate-Reduced Diet Improvement in Functional Bowel Disease
9. Final Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef] [PubMed]
- Christopher, N.L.; Bayless, T.M. Role of the Small Bowel and Colon in Lactose-Induced Diarrhea. Gastroenterology 1971, 60, 845–852. [Google Scholar] [CrossRef]
- He, T.; Venema, K.; Priebe, M.G.; Welling, G.W.; Brummer, R.J.; Vonk, R.J. The role of colonic metabolism in lactose intolerance. Eur. J. Clin. Investig. 2008, 38, 541–547. [Google Scholar] [CrossRef]
- Caspary, W.F. Diarrhoea associated with carbohydrate malabsorption. Clin. Gastroenterol. 1986, 15, 631–655. [Google Scholar] [CrossRef]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [Green Version]
- Jansson-Knodell, C.L.; Krajicek, E.J.; Savaiano, D.A.; Shin, A.S. Lactose Intolerance: A Concise Review to Skim the Surface. Mayo Clin. Proc. 2020, 95, 1499–1505. [Google Scholar] [CrossRef]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Bañares, F. Reliability of symptom analysis during carbohydrate hydrogen-breath tests. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 494–498. [Google Scholar] [CrossRef]
- Yang, J.; Deng, Y.; Chu, H.; Cong, Y.; Zhao, J.; Pohl, D.; Misselwitz, B.; Fried, M.; Dai, N.; Fox, M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 262–268. [Google Scholar] [CrossRef]
- He, T.; Priebe, M.G.; Harmsen, H.J.; Stellaard, F.; Sun, X.; Welling, G.W.; Vonk, R.J. Colonic fermentation may play a role in lactose intolerance in humans. J. Nutr. 2006, 136, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, H.J. Role of colonic short-chain fatty acid transport in diarrhea. Annu. Rev. Physiol. 2010, 72, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T.; Allison, C.; Segal, I.; Vorster, H.H.; Walker, A.R. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut 1990, 31, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Colecchia, A.; Orsola-Malpighi, P.S.; Festi, D.; Perri, F. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar]
- Hammer, H.F.; Fox, M.R.; Keller, J.; Salvatore, S.; Basilisco, G.; Hammer, J.; Lopetuso, L.; Benninga, M.; Borrelli, O.; Dumitrascu, D.; et al. European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Neurogastroenterology and Motility, and European Society for Paediatric Gastroenterology Hepatology and Nutrition consensus. United Eur. Gastroenterol. J. 2022, 10, 15–40. [Google Scholar]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Jellema, P.; Schellevis, F.G.; Van Der Windt, D.A.W.M.; Kneepkens, C.M.F.; Van Der Horst, H.E. Lactose malabsorption and intolerance: A systematic review on the diagnostic value of gastrointestinal symptoms and self-reported milk intolerance. QJM Med. 2010, 103, 555–572. [Google Scholar] [CrossRef] [Green Version]
- Vernia, P.; Ricciardi, M.R.; Frandina, C.; Bilotta, T.; Frieri, G. Lactose malabsorption and irritable bowel syndrome. Effect of a long-term lactose-free diet. Ital. J. Gastroenterol. 1995, 27, 117–121. [Google Scholar]
- Fernández-Bañares, F.; Rosinach, M.; Esteve, M.; Forné, M.; Espinós, J.C.; Viver, J.M. Sugar malabsorption in functional abdominal bloating: A pilot study on the long-term effect of dietary treatment. Clin. Nutr. 2006, 25, 824–831. [Google Scholar] [CrossRef]
- Alkalay, M.J. Nutrition in Patients with Lactose Malabsorption, Celiac Disease, and Related Disorders. Nutrients 2021, 14, 2. [Google Scholar] [CrossRef]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: Effective management strategies for lactose intolerance. Ann. Intern. Med. 2010, 152, 797–803. [Google Scholar] [CrossRef]
- Ohlsson, B. Theories behind the effect of starch- and sucrose-reduced diets on gastrointestinal symptoms in irritable bowel syndrome (Review). Mol. Med. Rep. 2021, 24, 732. [Google Scholar] [CrossRef] [PubMed]
- Treem, W.R. Clinical aspects and treatment of congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Calmet, F.H.; Garrido, J.; Garcia-Buitrago, M.T.; Moshiree, B. Sucrase-isomaltase deficiency as a potential masquerade in irritable bowel syndrome. Dig. Dis. Sci. 2000, 65, 534–540. [Google Scholar] [CrossRef]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef]
- Garcia-Etxebarria, K.; Zheng, T.; Bonfiglio, F.; Bujanda, L.; Dlugosz, A.; Lindberg, G.; Schmidt, P.T.; Karling, P.; Ohlsson, B.; Simren, M.; et al. Increased Prevalence of Rare Sucrase-isomaltase Pathogenic Variants in Irritable Bowel Syndrome Patients. Clin. Gastroenterol. Hepatol. 2018, 16, 1673–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husein, D.M.; Rizk, S.; Naim, H.Y. Differential Effects of Sucrase-Isomaltase Mutants on Its Trafficking and Function in Irritable Bowel Syndrome: Similarities to Congenital Sucrase-Isomaltase Deficiency. Nutrients 2020, 13, 9. [Google Scholar] [CrossRef]
- Burke, M. Carbohydrate Intolerance and Disaccharidase Measurement—A Mini-Review. Clin. Biochem. Rev. 2019, 40, 167–174. [Google Scholar] [CrossRef]
- Robayo-Torres, C.C.; Opekun, A.R.; Quezada-Calvillo, R.; Xavier, V.; Smith, E.B.; Navarrete, M.; Baker, S.S.; Nichols, B.L. 13C-breath tests for sucrose digestion in congenital sucrase isomaltase-deficient and sacrosidase-supplemented patients. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Davidson, G.P.; Robb, T.A. Value of breath hydrogen analysis in management of diarrheal illness in childhood: Comparison with duodenal biopsy. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 381–387. [Google Scholar] [CrossRef]
- Uhrich, S.; Wu, Z.; Huang, J.-Y.; Scott, C.R. Four mutations in the SI gene are responsible for the majority of clinical symptoms of CSID. J. Pediatr. Gastroenterol. Nutr. 2012, 55, S34–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Treem, W.R.; McAdams, L.; Stanford, L.; Kastoff, G.; Justinich, C.; Hyams, J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 1999, 28, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, H.R. Ratio scales of sugar sweetness. Percept. Psychophys. 1970, 7, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Hanover, L.M.; White, J.S. Manufacturing, composition, and applications of fructose. Am. J. Clin. Nutr. 1993, 58, 724S–732S. [Google Scholar] [CrossRef]
- Duffey, K.J.; Popkin, B.M. High-fructose corn syrup: Is this what’s for dinner? Am. J. Clin. Nutr. 2008, 88, 1722S–1732S. [Google Scholar] [CrossRef] [Green Version]
- Glucose-Fructose Syrup: An Ingredient Worth Knowing. Starch EU: Brussels, Belgium, June 2017. Available online: https://www.starch.eu/wp-content/uploads/2017/06/5247.055_starch_eu-fiche-glucose-fructose-webC-1.pdf (accessed on 8 March 2022).
- Montonen, J.; Järvinen, R.; Knekt, P.; Heliovaara, M.; Reunanen, A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J. Nutr. 2007, 137, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Sluik, D.; Engelen, A.I.; Feskens, E.J. Fructose consumption in the Netherlands: The Dutch National Food Consumption Survey 2007–2010. Eur. J. Clin. Nutr. 2015, 69, 475–481. [Google Scholar] [CrossRef]
- Vos, M.B.; Kimmons, J.E. Dietary fructose consumption among US children and adults: The third National Health and Nutrition Examination Survey. Medscape J. Med. 2008, 10, 160. [Google Scholar]
- Rumessen, J.J.; Gudmand-Hoyer, E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut 1986, 27, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Ravich, W.J.; Bayless, T.M.; Thomas, M. Fructose: Incomplete intestinal absorption in humans. Gastroenterology 1983, 84, 26–29. [Google Scholar] [CrossRef]
- Rao, S.S.; Attaluri, A.; Anderson, L.; Stumbo, P. Ability of the normal human small intestine to absorb fructose: Evaluation by breath testing. Clin. Gastroenterol. Hepatol. 2007, 5, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.F.; Butler, R.N.; Brooks, D.A. Intestinal fructose transport and malabsorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G202–G206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Banares, F.; Esteve-Pardo, M.; De Leon, R.; Humbert, P.; Cabre, E.; Llovet, J.M.; Gassull, M.A. Sugar malabsorption in functional bowel disease: Clinical implications. Am. J. Gastroenterol. 1993, 88, 2044–2050. [Google Scholar] [PubMed]
- Wang, X.J.; Camilleri, M.; Vanner, S.; Tuck, C. Review article: Biological mechanisms for symptom causation by individual FODMAP subgroups-the case for a more personalised approach to dietary restriction. Aliment. Pharmacol. Ther. 2019, 50, 517–529. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Brown, S.; Forbes, A.; Fox, M.R.; Hungin, P.; Kelman, L.; Major, G.; O’Connor, M.; Sanders, D.S.; Sinha, R.; et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut 2018, 67, 1380–1399. [Google Scholar] [CrossRef] [PubMed]
- Berg, L.K.; Fagerli, E.; Martinussen, M.; Myhre, A.O.; Florholmen, J.; Goll, R. Effect of fructose-reduced diet in patients with irritable bowel syndrome, and its correlation to a standard fructose breath test. Scand. J. Gastroenterol. 2013, 48, 936–943. [Google Scholar] [CrossRef]
- Berg, L.K.; Fagerli, E.; Myhre, A.O.; Florholmen, J.; Goll, R. Self-reported dietary fructose intolerance in irritable bowel syndrome: Proposed diagnostic criteria. World J. Gastroenterol. 2015, 21, 5677–5684. [Google Scholar] [CrossRef]
- Hammer, J.; Sonyi, M.; Engeßer, K.M.; Riedl, G.; Luong, S.; Hammer, H.F. Carbohydrate-induced gastrointestinal symptoms: Development and validation of a test-specific symptom questionnaire for an adult population, the adult Carbohydrate Perception Questionnaire. Eur. J. Gastroenterol. Hepatol. 2021, 32, 171–177. [Google Scholar] [CrossRef]
- Komericki, P.; Akkilic-Materna, M.; Strimitzer, T.; Weyermair, K.; Hammer, H.F.; Aberer, W. Oral xylose isomerase decreases breath hydrogen excretion and improves gastrointestinal symptoms in fructose malabsorption: A double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2012, 36, 980–987. [Google Scholar] [CrossRef]
- Tennant, D.R. Potential intakes of total polyols based on UK usage survey data. Food Addit. Contam. Part A 2014, 31, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Dills, W.L., Jr. Sugar alcohols as bulk sweeteners. Ann. Rev. Nutr. 1989, 9, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Strocchi, A.; Rossi, R.; Sirola, D.; Gasbarrini, G. Sorbitol malabsorption in normal volunteers and in patients with celiac disease. Gut 1998, 29, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Rumessen, J.J.; Gudmand-Hoyer, E. Malabsorption of fructose-sorbitol mixtures. Interactions causing abdominal distress. Scand. J. Gastroenterol. 1987, 22, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Rumessen, J.J.; Gudmand-Hoyer, E. Functional bowel disease: Malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology 1988, 95, 694–700. [Google Scholar] [CrossRef]
- Gibson, P.R.; Halmos, E.P.; Muir, J.G. Review article: FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment. Pharmacol. Ther. 2020, 52, 233–246. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Materna, A.; Wermelinger, C.; Schuler, J. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2013, 37, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, S.; Gibson, P. Fructose malabsorption and symptoms of Irritable Bowel Syndrome: Guidelines for effective dietary management. J. Am. Diet. Assoc. 2006, 106, 1631–1639. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kraft, N.; Zimmerman, B.; Jackson, M.; Rao, S.S. Fructose intolerance in IBS and utility of fructose-restricted diet. J. Clin. Gastroenterol. 2008, 42, 233–238. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rej, A.; Aziz, I.; Sanders, D.S. A gluten-free diet: The express route to fructan reduction. Am. J. Gastroenterol. 2019, 114, 1553. [Google Scholar] [CrossRef]
- O’keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and health care utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef] [Green Version]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a gluten-free diet in subjects with irritable bowel syndrome-diarrhea unaware of their HLA-DQ2/8 genotype. Clin. Gastroenterol. Hepatol. 2016, 14, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Barmeyer, C.; Schumann, M.; Meyer, T.; Zielinski, C.; Zuberbier, T.; Siegmund, B.; Schulzke, J.D.; Daum, S.; Ullrich, R. Long-term response to gluten-free diet as evidence for non-celiac wheat sen-sitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int. J. Colorectal Dis. 2017, 32, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Bañares, F.; Arau, B.; Raga, A.; Aceituno, M.; Tristán, E.; Carrasco, A.; Ruiz, L.; Martín-Cardona, A.; Ruiz-Ramírez, P.; Esteve, M. Long-Term Effect of a Gluten-Free Diet on Diarrhoea- or Bloating-Predominant Functional Bowel Disease: Role of the ’Low-Grade Coeliac Score’ and the ’Coeliac Lymphogram’ in the Response Rate to the Diet. Nutrients 2021, 13, 1812. [Google Scholar] [CrossRef]
- Paduano, D.; Cingolani, A.; Tanda, E.; Usai, P. Effect of Three Diets (Low-FODMAP, Gluten-free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life. Nutrients 2019, 11, 1566. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.J.; Reed, D.E.; Muir, J.G.; Vanner, S.J. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterol. Motil. 2020, 32, e13730. [Google Scholar] [CrossRef]
- Nilholm, C.; Larsson, E.; Sonestedt, E.; Roth, B.; Ohlsson, B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals with Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, X.; Cong, Y.; Chu, H.; Fried, M.; Dai, N.; Fox, M. Bloating and distention in irritable bowel syndrome: The role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am. J. Gastroenterol. 2013, 108, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Impact of diet on symptoms of the irritable bowel syndrome. Nutrients 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Törnblom, H.; Palsson, O.S.; van Tilburg, M.A.; Van Oudenhove, L.; Tack, J.; Whitehead, W.E. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: Consistent findings from five different patient cohorts. Gut 2018, 67, 255–262. [Google Scholar] [CrossRef]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a Western-style diet or drinking water supplemented with fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J. Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2016, 66, 1241–1251. [Google Scholar] [CrossRef]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
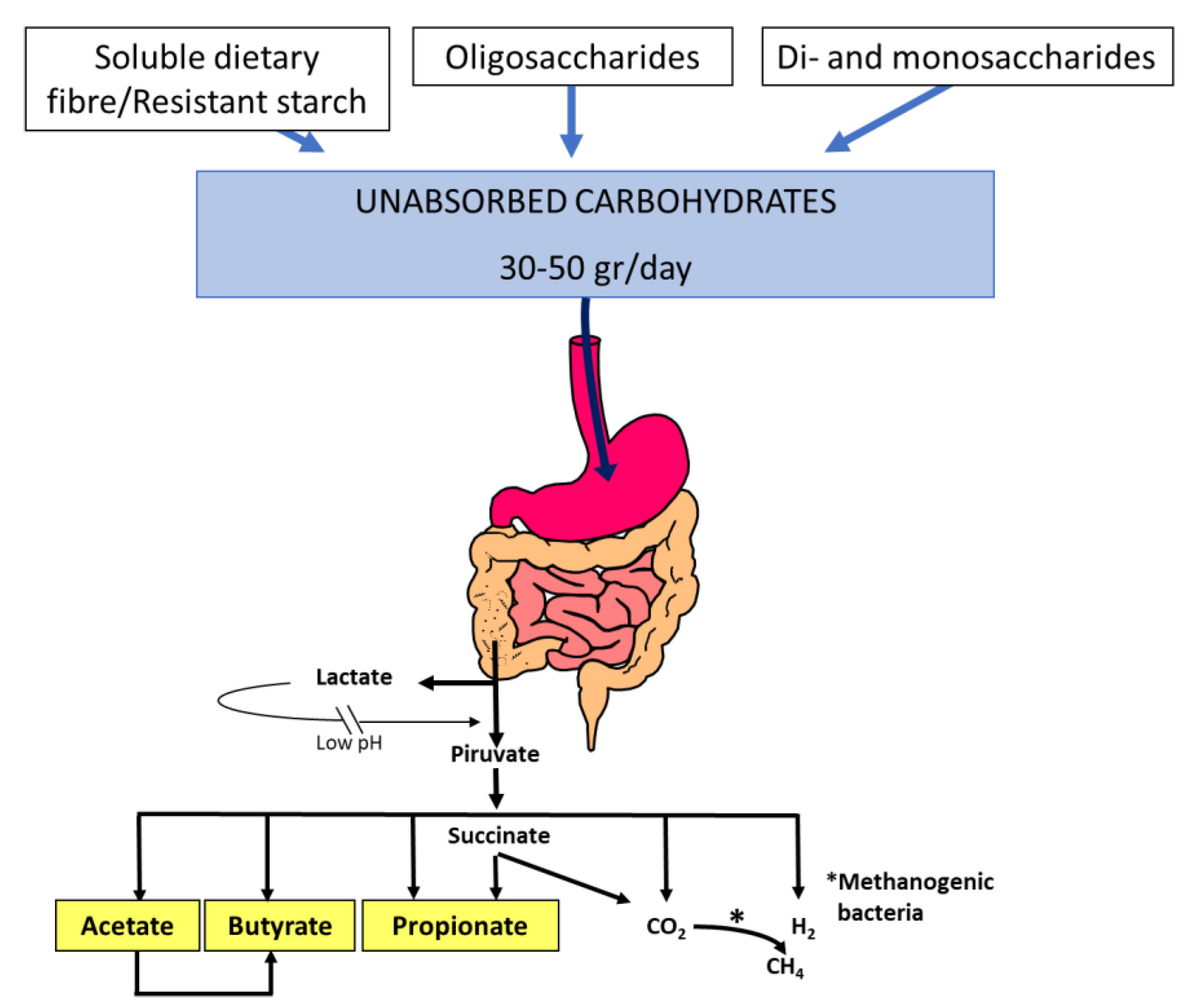
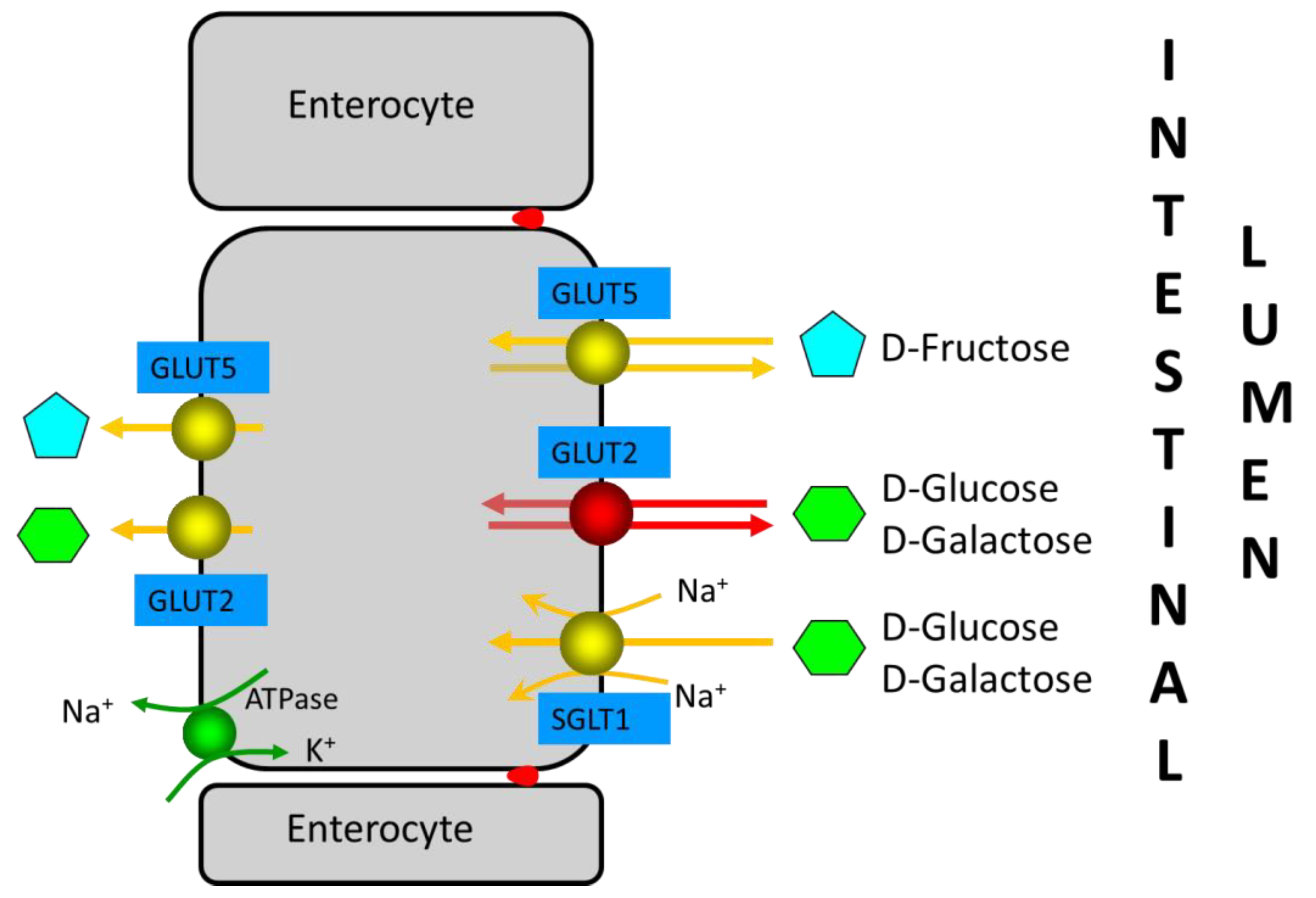

| Carbohydrate | Type | Absorption Mechanisms | Available Specific Drugs |
|---|---|---|---|
| Monosaccharides | Fructose | Absorption of excess fructose occurs in the small intestine: rapidly via GLUT-2, the sodium-dependent active transport mechanism in conjunction with glucose; slowly via GLUT-5, a specific transporter for fructose using carrier-mediated facilitated diffusion. Thus, fructose is well-absorbed in the presence of equimolar glucose in the proximal small intestine, whereas free fructose is absorbed slowly along the length of the small intestine. | Xylose-isomerase |
| Disaccharides | Lactose Sucrose | Unabsorbed in small intestine if lactase is absent. Unabsorbed in small intestine in case of sucrase-isomaltase deficiency | Lactase Sacrosidase |
| Oligosaccharides | FOS * GOS | Humans do not possess small intestinal hydrolases to hydrolyse oligosaccharides, and they are unabsorbed. | None |
| Polyols | Sorbitol Mannitol Maltitol Isomalt Lactitol Xylitol | Sugar alcohols are poorly absorbed along the length of the small intestine by slow passive diffusion. | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Bañares, F. Carbohydrate Maldigestion and Intolerance. Nutrients 2022, 14, 1923. https://doi.org/10.3390/nu14091923
Fernández-Bañares F. Carbohydrate Maldigestion and Intolerance. Nutrients. 2022; 14(9):1923. https://doi.org/10.3390/nu14091923
Chicago/Turabian StyleFernández-Bañares, Fernando. 2022. "Carbohydrate Maldigestion and Intolerance" Nutrients 14, no. 9: 1923. https://doi.org/10.3390/nu14091923
APA StyleFernández-Bañares, F. (2022). Carbohydrate Maldigestion and Intolerance. Nutrients, 14(9), 1923. https://doi.org/10.3390/nu14091923






