Effects of Energy Drink Acute Assumption in Gastrointestinal Tract of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Treatment
2.2. Examination and Isolation of Organs and Tissue
2.3. Histological Stains and Immunohistochemistry
2.4. Plasmatic Soluble Factors Analysis
2.5. Analysis of Circulating mtDNA in Plasma
2.6. Statistical Analysis
3. Results
3.1. Effects of the Dietary Treatment on Organ Macroscopic Phenotype and Metabolic Parameters
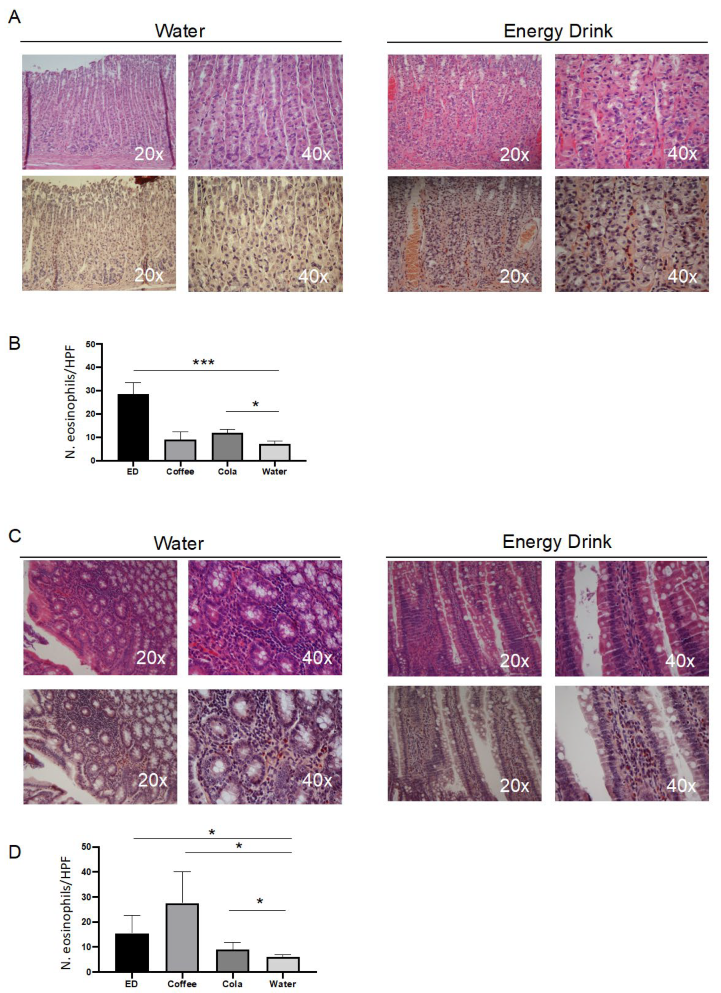
3.2. Caffeine Triggered the Eosinophilic Infiltrate in Intestinal Mucosa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mattioli, A.V.; Pennella, S.; Manenti, A.; Ballerini Puviani, M.; Farinetti, A. Influence of energy drinks on obesity: A preliminary experimental study. Prog. Nutr. 2018, 19, 369–372. [Google Scholar] [CrossRef]
- Valle, M.T.C.; Couto-Pereira, N.S.; Lampert, C.; Arcego, D.M.; Toniazzo, A.P.; Limberger, R.P.; Dallegrave, E.; Dalmaz, C.; Arbo, M.D.; Leal, M.B. Energy drinks and their component modulate attention, memory, and antioxidant defences in rats. Eur. J. Nutr. 2018, 57, 2501–2511. [Google Scholar] [CrossRef]
- Patrick, M.E.; Griffin, J.; Huntley, E.D.; Maggs, J.L. Energy Drinks and Binge Drinking Predict College Students’ Sleep Quantity, Quality, and Tiredness. Behav. Sleep Med. 2018, 16, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Goslinski, M.; Nowatkowska, K. The Effect of Acute Consumption of Energy Drinks on Blood Pressure, Heart Rate and Blood Glucose in the Group of Young Adults. Int. J. Environ. Res. Public Health 2018, 15, 544. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.; Santini, L.; Capucci, A.; Oto, A.; Santomauro, M.; Riganti, C.; Raviele, A.; Cappato, R. European Cardiac Arrhythmia Society Statement on the cardiovascular events associated with the use or abuse of energy drinks. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2019, 56, 99–115. [Google Scholar] [CrossRef]
- Subaiea, G.M.; Altebainawi, A.F.; Alshammari, T.M. Energy drinks and population health: Consumption pattern and adverse effects among Saudi population. BMC Public Health 2019, 19, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boekema, P.J.; Samsom, M.; van Berge Henegouwen, G.P.; Smout, A.J. Coffee and gastrointestinal function: Facts and fiction. A review. Scand. J. Gastroenterol. Suppl. 1999, 230, 35–39. [Google Scholar] [CrossRef]
- Shimamoto, T.; Yamamichi, N.; Kodashima, S.; Takahashi, Y.; Fujishiro, M.; Oka, M.; Mitsushima, T.; Koike, K. No association of coffee consumption with gastric ulcer, duodenal ulcer, reflux esophagitis, and non-erosive reflux disease: A cross-sectional study of 8013 healthy subjects in Japan. PLoS ONE 2013, 8, e65996. [Google Scholar] [CrossRef] [Green Version]
- Nwokediuko, S. Gastroesophageal Reflux Disease: A Population Based Study. Gastroenterol. Res. 2009, 2, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.R.; Cann, P.A.; Read, N.W. Effect of coffee on distal colon function. Gut 1990, 31, 450–453. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.; Welcher, K.; Zimmerman, B.; Stumbo, P. Is coffee a colonic stimulant? Eur. J. Gastroenterol. Hepatol. 1998, 10, 113–118. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Wald, A.; Back, C.; Bayless, T.M. Effect of caffeine on the human small intestine. Gastroenterology 1976, 71, 738–742. [Google Scholar] [CrossRef]
- Wagner, S.M.; Mekhjian, H.S.; Caldwell, J.H.; Thomas, F.B. Effects of caffeine and coffee on fluid transport in the small intestine. Gastroenterology 1978, 75, 379–381. [Google Scholar] [CrossRef]
- Manzini, R.; Schwarzfischer, M.; Bircher, A.; Niechcial, A.; Vavricka, S.R.; Atrott, K.; Lang, S.; Scharl, M.; Spalinger, M.R. Energy Drink Administration Ameliorates Intestinal Epithelial Barrier Defects and Reduces Acute DSS Colitis. Inflamm. Bowel Dis. 2021, 27, 1139–1152. [Google Scholar] [CrossRef]
- Trani, N.; Bonetti, L.R.; Gualandri, G.; Barbolini, G. Immediate anaphylactic death following antibiotics injection: Splenic eosinophilia easily revealed by pagoda red stain. Forensic Sci. Int. 2008, 181, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zordani, A.; Pisciotta, A.; Bertoni, L.; Bertani, G.; Vallarola, A.; Giuliani, D.; Puliatti, S.; Mecugni, D.; Bianchi, G.; de Pol, A.; et al. Regenerative potential of human dental pulp stem cells in the treatment of stress urinary incontinence: In vitro and in vivo study. Cell Prolif. 2019, 52, e12675. [Google Scholar] [CrossRef] [Green Version]
- Nasi, M.; Bianchini, E.; Lo Tartaro, D.; De Biasi, S.; Mattioli, M.; Paolini, A.; Gibellini, L.; Pinti, M.; De Gaetano, A.; D’Alisera, R.; et al. Effects of whole-body cryotherapy on the innate and adaptive immune response in cyclists and runners. Immunol. Res. 2020, 68, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Nasi, M.; De Gaetano, A.; Bianchini, E.; De Biasi, S.; Gibellini, L.; Neroni, A.; Mattioli, M.; Pinti, M.; Lo Tartaro, D.; Borella, R.; et al. Mitochondrial damage-associated molecular patterns stimulate reactive oxygen species production in human microglia. Mol. Cell. Neurosci. 2020, 108, 103538. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Manenti, A.; Bonetti, L.R.; Farinetti, A. Energy drinks and obesity: Preliminary results from a preclinical study. Anatol. J. Cardiol. 2018, 19, 422. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, B.; Schoeps, B.; Kruger, A. Recognizing the Molecular Multifunctionality and Interactome of TIMP-1. Trends Cell Biol. 2019, 29, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M. Soluble ICAM-1: A marker of vascular inflammation and lifestyle. Cytokine 2005, 31, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. 2020, 15, 179–209. [Google Scholar] [CrossRef] [Green Version]
- Masterson, J.C.; Menard-Katcher, C.; Larsen, L.D.; Furuta, G.T.; Spencer, L.A. Heterogeneity of Intestinal Tissue Eosinophils: Potential Considerations for Next-Generation Eosinophil-Targeting Strategies. Cells 2021, 10, 426. [Google Scholar] [CrossRef]
- Yan, B.M.; Shaffer, E.A. Primary eosinophilic disorders of the gastrointestinal tract. Gut 2009, 58, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain-Gut Axis. Nutrients 2020, 13, 88. [Google Scholar] [CrossRef]
- Furuta, G.T.; Katzka, D.A. Eosinophilic Esophagitis. N. Engl. J. Med. 2015, 373, 1640–1648. [Google Scholar] [CrossRef] [Green Version]
- Katre, R.S.; Sunnapwar, A.; Restrepo, C.S.; Katabathina, V.S.; Mumbower, A.; Baxi, A.; Sonavane, S. Cardiopulmonary and Gastrointestinal Manifestations of Eosinophil- associated Diseases and Idiopathic Hypereosinophilic Syndromes: Multimodality Imaging Approach. Radiographics 2016, 36, 433–451. [Google Scholar] [CrossRef]
- Caldwell, J.H.; Tennenbaum, J.I.; Bronstein, H.A. Serum IgE in eosinophilic gastroenteritis. Response to intestinal challenge in two cases. N. Engl. J. Med. 1975, 292, 1388–1390. [Google Scholar] [CrossRef]
- Gonsalves, N. Eosinophilic Gastrointestinal Disorders. Clin. Rev. Allergy Immunol. 2019, 57, 272–285. [Google Scholar] [CrossRef]
- Chen, P.H.; Anderson, L.; Zhang, K.; Weiss, G.A. Eosinophilic Gastritis/Gastroenteritis. Curr. Gastroenterol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef]
- Peterson, K.; Safroneeva, E.; Schoepfer, A. Emerging Therapies for Eosinophilic Gastrointestinal Diseases. J. Allergy Clin. Immunol. Pract. 2021, 9, 3276–3281. [Google Scholar] [CrossRef] [PubMed]
- Ertuglu, L.A.; Afsar, B.; Yildiz, A.B.; Demiray, A.; Ortiz, A.; Covic, A.; Kanbay, M. Substitution of Sugar-Sweetened Beverages for Other Beverages: Can It Be the Next Step Towards Healthy Aging? Curr. Nutr. Rep. 2021, 10, 399–412. [Google Scholar] [CrossRef]
- Popa, A.R.; Vesa, C.M.; Uivarosan, D.; Jurca, C.M.; Isvoranu, G.; Socea, B.; Stanescu, A.M.A.; Iancu, M.A.; Scarneciu, I.; Zaha, D.C. Cross Sectional Study Regarding the Association between Sweetened Beverages Intake, Fast-food Products, Body Mass Index, Fasting Blood Glucose and Blood Pressure in the Young Adults from North-western Romania. Rev. Chim. 2019, 70, 156–160. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fulop, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, P.; Stamerra, C.A.; Giorgini, P.; Jamialahamdi, T.; Butler, A.E.; Sahebkar, A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022, 77, 101596. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasi, M.; De Gaetano, A.; Carnevale, G.; Bertoni, L.; Selleri, V.; Zanini, G.; Pisciotta, A.; Caramaschi, S.; Reggiani Bonetti, L.; Farinetti, A.; et al. Effects of Energy Drink Acute Assumption in Gastrointestinal Tract of Rats. Nutrients 2022, 14, 1928. https://doi.org/10.3390/nu14091928
Nasi M, De Gaetano A, Carnevale G, Bertoni L, Selleri V, Zanini G, Pisciotta A, Caramaschi S, Reggiani Bonetti L, Farinetti A, et al. Effects of Energy Drink Acute Assumption in Gastrointestinal Tract of Rats. Nutrients. 2022; 14(9):1928. https://doi.org/10.3390/nu14091928
Chicago/Turabian StyleNasi, Milena, Anna De Gaetano, Gianluca Carnevale, Laura Bertoni, Valentina Selleri, Giada Zanini, Alessandra Pisciotta, Stefania Caramaschi, Luca Reggiani Bonetti, Alberto Farinetti, and et al. 2022. "Effects of Energy Drink Acute Assumption in Gastrointestinal Tract of Rats" Nutrients 14, no. 9: 1928. https://doi.org/10.3390/nu14091928
APA StyleNasi, M., De Gaetano, A., Carnevale, G., Bertoni, L., Selleri, V., Zanini, G., Pisciotta, A., Caramaschi, S., Reggiani Bonetti, L., Farinetti, A., Cossarizza, A., Pinti, M., Manenti, A., & Mattioli, A. V. (2022). Effects of Energy Drink Acute Assumption in Gastrointestinal Tract of Rats. Nutrients, 14(9), 1928. https://doi.org/10.3390/nu14091928








