Urinary Phosphorus Excretion and Cardiovascular Outcomes in Patients with Pre-Dialysis Chronic Kidney Disease: The KNOW-CKD Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection from Participants
2.3. Exposure and Study Outcome
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
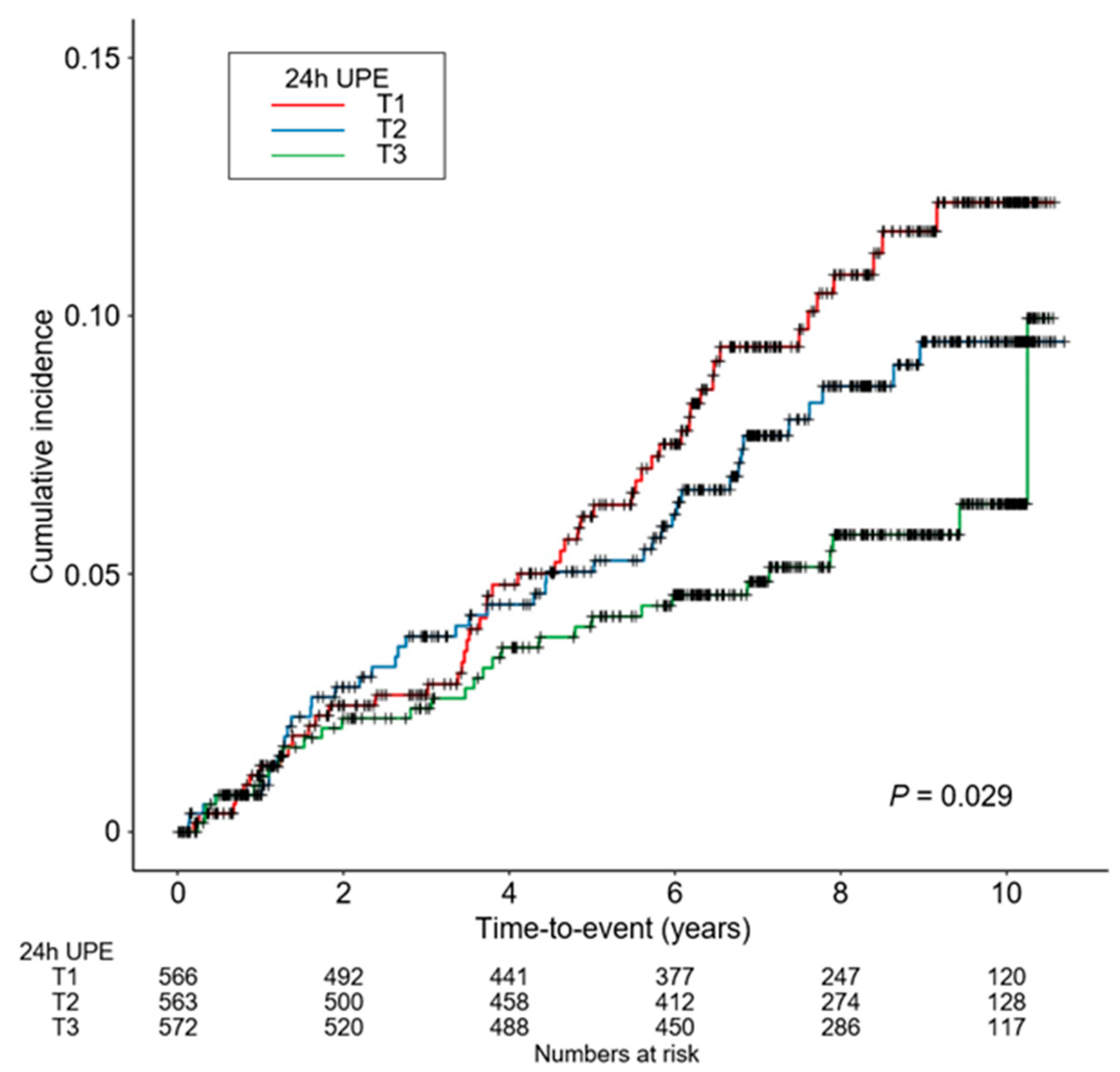
3.2. Association between 24 h UPE Level and the Risk of CVE in Patients with CKD
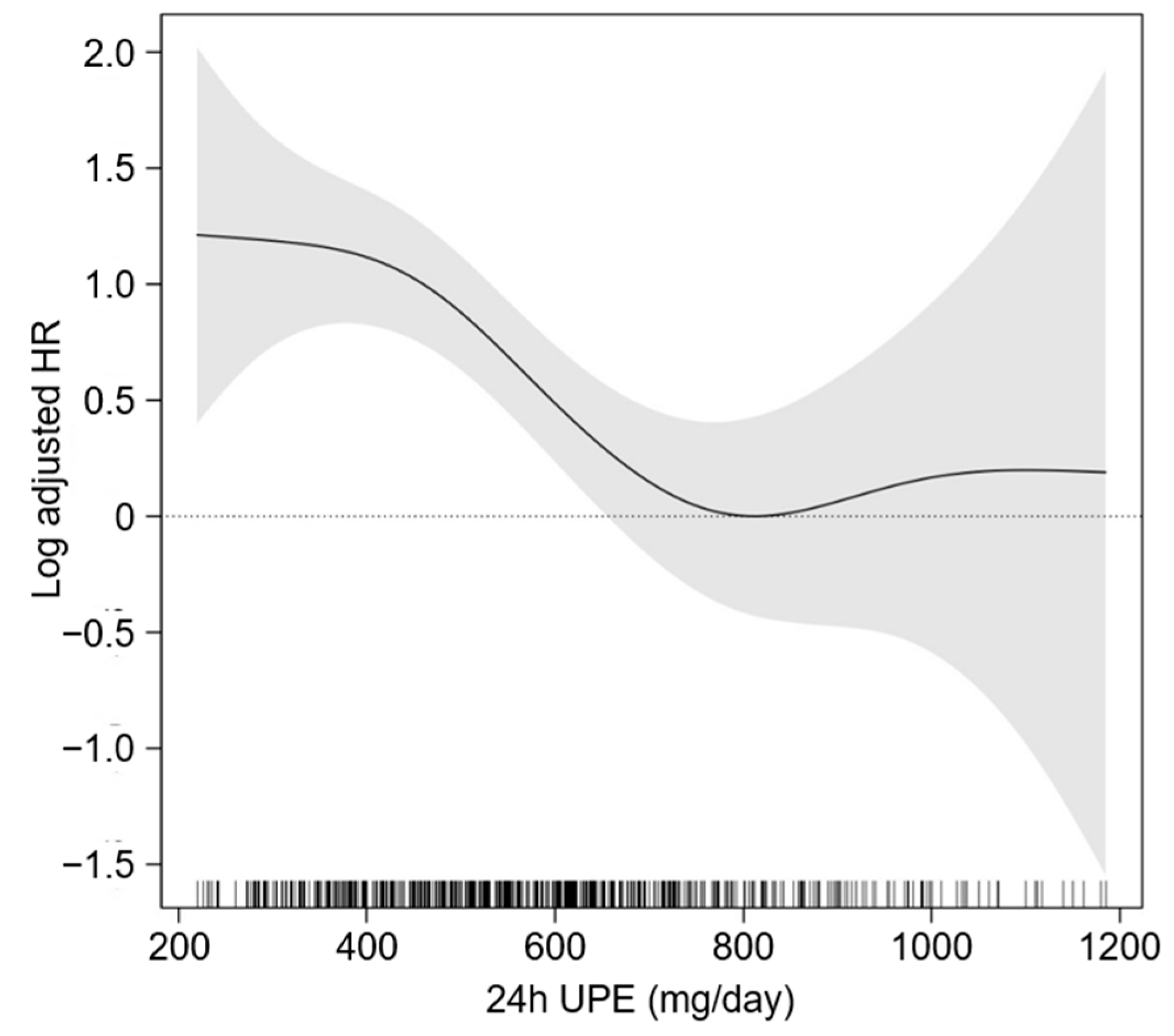
3.3. Sensitivity Analysis
3.4. Prespecified Subgroup Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stremke, E.R.; Hill Gallant, K.M. Intestinal Phosphorus Absorption in Chronic Kidney Disease. Nutrients 2018, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, S.; Miyagawa, A.; Kaneko, I.; Shiozaki, Y.; Segawa, H.; Miyamoto, K. Regulation of renal phosphate handling: Inter-organ communication in health and disease. J. Bone Miner. Metab. 2016, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M. Renal and extrarenal effects of fibroblast growth factor 23. Nat. Rev. Nephrol. 2019, 15, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Jüppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Naveh-Many, T. Phosphate and the parathyroid. Kidney Int. 2009, 75, 898–905. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J.; Herzog, C.A.; Ishani, A.; Kalra, P.A. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J. Am. Soc. Nephrol. 2009, 20, 397–404. [Google Scholar] [CrossRef]
- Park, K.S.; Chang, J.W.; Kim, T.Y.; Kim, H.W.; Lee, E.K.; Kim, H.S.; Yang, W.S.; Kim, S.B.; Park, S.K.; Lee, S.K.; et al. Lower concentrations of serum phosphorus within the normal range could be associated with less calcification of the coronary artery in Koreans with normal renal function. Am. J. Clin. Nutr. 2011, 94, 1465–1470. [Google Scholar] [CrossRef]
- Ix, J.H.; De Boer, I.H.; Peralta, C.A.; Adeney, K.L.; Duprez, D.A.; Jenny, N.S.; Siscovick, D.S.; Kestenbaum, B.R. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin. J. Am. Soc. Nephrol. 2009, 4, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Ix, J.H.; Targher, G.; Smits, G.; Chonchol, M. Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am. J. Cardiol. 2010, 106, 564–568. [Google Scholar] [CrossRef]
- Meng, J.; Wassel, C.L.; Kestenbaum, B.R.; Collins, T.C.; Criqui, M.H.; Lewis, C.E.; Cummings, S.R.; Ix, J.H. Serum phosphorus levels and the spectrum of ankle-brachial index in older men: The Osteoporotic Fractures in Men (MrOS) study. Am. J. Epidemiol. 2010, 171, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B., Sr.; Gaziano, J.M.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med. 2007, 167, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Collins, A.J.; Ishani, A.; Kalra, P.A. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 556–563. [Google Scholar] [CrossRef]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Gao, Z.; Curhan, G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005, 112, 2627–2633. [Google Scholar] [CrossRef]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef]
- Saxena, A.; Sachan, T.; Gupta, A.; Kapoor, V. Effect of Dietary Phosphorous Restriction on Fibroblast Growth 2 Factor-23 and sKlotho Levels in Patients with Stages 1-2 Chronic Kidney Disease. Nutrients 2022, 14, 3302. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Rokicki, R.; Gluba-Brzózka, A. The Influence of Dietary Interventions on Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Nutrients 2021, 13, 2065. [Google Scholar] [CrossRef]
- Shinozaki, N.; Murakami, K.; Asakura, K.; Uechi, K.; Kobayashi, S.; Masayasu, S.; Sasaki, S. Dietary phosphorus intake estimated by 4-day dietary records and two 24-hour urine collections and their associated factors in Japanese adults. Eur. J. Clin. Nutr. 2018, 72, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Salomo, L.; Kamper, A.L.; Poulsen, G.M.; Poulsen, S.K.; Astrup, A.; Rix, M. Habitual dietary phosphorus intake and urinary excretion in chronic kidney disease patients: A 3-day observational study. Eur. J. Clin. Nutr. 2017, 71, 798–800. [Google Scholar] [CrossRef]
- Palomino, H.L.; Rifkin, D.E.; Anderson, C.; Criqui, M.H.; Whooley, M.A.; Ix, J.H. 24-hour urine phosphorus excretion and mortality and cardiovascular events. Clin. J. Am. Soc. Nephrol. 2013, 8, 1202–1210. [Google Scholar] [CrossRef]
- Stremke, E.R.; McCabe, L.D.; McCabe, G.P.; Martin, B.R.; Moe, S.M.; Weaver, C.M.; Peacock, M.; Hill Gallant, K.M. Twenty-Four-Hour Urine Phosphorus as a Biomarker of Dietary Phosphorus Intake and Absorption in CKD: A Secondary Analysis from a Controlled Diet Balance Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Park, S.K.; Park, H.C.; Chin, H.J.; Chae, D.W.; Choi, K.H.; Han, S.H.; Yoo, T.H.; Lee, K.; Kim, Y.S.; et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): Design and methods. BMC Nephrol. 2014, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.T.; Lee, J.; Jung, J.Y.; Lee, K.B.; Kim, Y.H.; Yoo, T.H.; Kang, S.W.; Choi, K.H.; Oh, K.H.; et al. The difference between cystatin C- and creatinine-based eGFR is associated with adverse cardiovascular outcome in patients with chronic kidney disease. Atherosclerosis 2021, 335, 53–61. [Google Scholar] [CrossRef]
- Jung, C.Y.; Yun, H.R.; Park, J.T.; Joo, Y.S.; Kim, H.W.; Yoo, T.H.; Kang, S.W.; Lee, J.; Chae, D.W.; Chung, W.; et al. Association of coronary artery calcium with adverse cardiovascular outcomes and death in patients with chronic kidney disease: Results from the KNOW-CKD. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2022, 38, 712–721. [Google Scholar] [CrossRef]
- Craver, L.; Dusso, A.; Martinez-Alonso, M.; Sarro, F.; Valdivielso, J.M.; Fernández, E. A low fractional excretion of Phosphate/Fgf23 ratio is associated with severe abdominal Aortic calcification in stage 3 and 4 kidney disease patients. BMC Nephrol. 2013, 14, 221. [Google Scholar] [CrossRef]
- Ucha, I.; Mamven, M.; Adejumo, O.; Nwankwo, E.A. Malnutrition Inflammation Complex Syndrome in Pre-dialysis Chronic Kidney Disease Patients in a Nigerian Tertiary Hospital. West Afr. J. Med. 2022, 39, 1253–1259. [Google Scholar]
- Zhang, K.; Gao, J.; Chen, J.; Liu, X.; Cai, Q.; Liu, P.; Huang, H. MICS, an easily ignored contributor to arterial calcification in CKD patients. Am. J. Physiol. Ren. Physiol. 2016, 311, F663–F670. [Google Scholar] [CrossRef]
- Isakova, T.; Barchi-Chung, A.; Enfield, G.; Smith, K.; Vargas, G.; Houston, J.; Xie, H.; Wahl, P.; Schiavenato, E.; Dosch, A.; et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1009–1018. [Google Scholar] [CrossRef]
- Lou, L.M.; Caverni, A.; Gimeno, J.A.; Moreno, R.; Pérez, J.; Alvarez, R.; Campos, B.; García, M.; Gutiérrez, A.; Bielsa, S.; et al. Dietary intervention focused on phosphate intake in hemodialysis patients with hyperphosphoremia. Clin. Nephrol. 2012, 77, 476–483. [Google Scholar] [CrossRef]
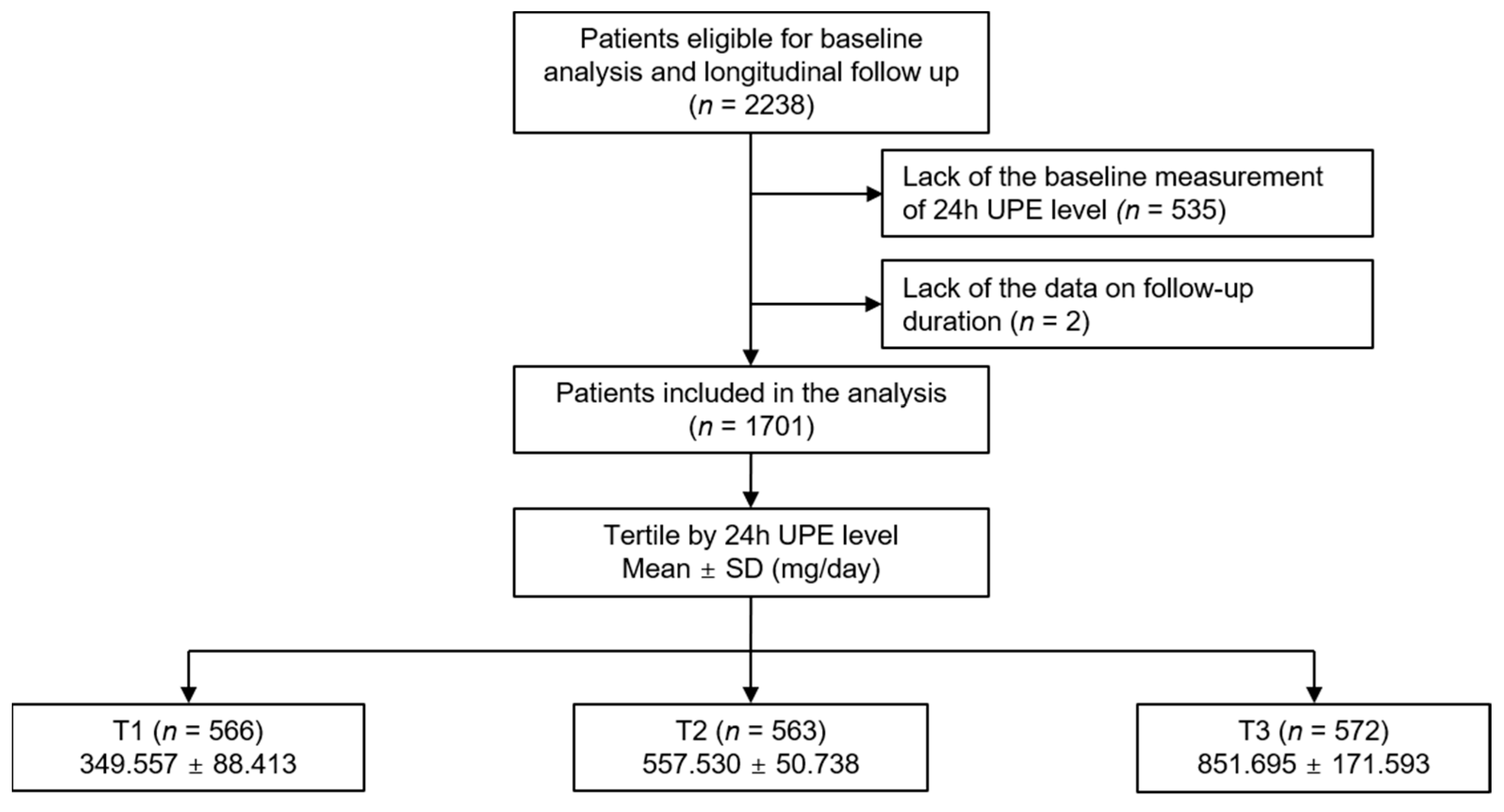
| 24 h UPE | p Value | |||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Follow-up duration (year) | 6.908 ± 3.050 | 7.259 ± 2.814 | 7.382 ± 2.747 | 0.019 |
| Age (year) | 55.981 ± 12.021 | 54.128 ± 12.030 | 52.395 ± 12.159 | <0.001 |
| Male | 249 (44.0) | 350 (62.2) | 434 (75.9) | <0.001 |
| Charlson comorbidity index | <0.001 | |||
| 0–3 | 368 (65.0) | 387 (68.7) | 449 (78.5) | |
| 4–5 | 187 (33.0) | 165 (29.3) | 115 (20.1) | |
| 6–7 | 11 (1.9) | 10 (1.8) | 8 (1.4) | |
| ≥8 | 0 (0.0) | 1 (0.2) | 0 (0.0) | |
| Primary cause of CKD | <0.001 | |||
| DM | 172 (30.4) | 153 (27.2) | 109 (19.1) | |
| HTN | 103 (18.2) | 112 (19.9) | 129 (22.6) | |
| GN | 149 (26.3) | 151 (26.8) | 195 (34.2) | |
| TID | 2 (0.4) | 3 (0.5) | 5 (0.9) | |
| PKD | 95 (16.8) | 120 (21.3) | 93 (16.3) | |
| Others | 45 (8.0) | 24 (4.3) | 40 (7.0) | |
| Smoking status | <0.01 | |||
| Non-smoker | 361 (63.8) | 292 (51.9) | 234 (41.0) | |
| Ex-smoker | 60 (10.6) | 81 (14.4) | 115 (20.1) | |
| Current smoker | 145 (25.6) | 190 (33.7) | 222 (38.9) | |
| Medication | ||||
| ACEi/ARBs | 480 (84.8) | 479 (85.1) | 511 (89.3) | 0.044 |
| Diuretics | 211 (37.3) | 165 (29.3) | 161 (28.1) | 0.002 |
| Anti-HTN drugs ≥ 3 | 202 (35.7) | 161 (28.6) | 170 (29.7) | 0.022 |
| Statins | 296 (52.3) | 289 (51.3) | 306 (53.5) | 0.765 |
| BMI (kg/m2) | 23.846 ± 3.487 | 24.430 ± 3.275 | 25.743 ± 3.263 | <0.001 |
| Waist-to-hip ratio | 0.893 ± 0.068 | 0.900 ± 0.065 | 0.908 ± 0.057 | <0.001 |
| SBP (mmHg) | 126.931 ± 17.369 | 127.652 ± 15.011 | 128.534 ± 15.096 | 0.245 |
| DBP (mmHg) | 75.342 ± 11.553 | 77.066 ± 10.517 | 78.545 ± 10.886 | <0.001 |
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 11.933 ± 1.761 | 12.840 ± 1.988 | 13.721 ± 1.840 | <0.001 |
| Albumin (g/dL) | 4.144 ± 0.427 | 4.213 ± 0.424 | 4.274 ± 0.403 | <0.001 |
| Total calcium (mg/dL) | 9.048 ± 0.612 | 9.135 ± 0.497 | 9.174 ± 0.478 | 0.001 |
| Phosphorus (mg/dL) | 3.855 ± 0.732 | 3.702 ± 0.665 | 3.540 ± 0.555 | <0.001 |
| Total cholesterol (mg/dL) | 169.637 ± 38.741 | 173.141 ± 39.863 | 176.114 ± 37.416 | 0.017 |
| HDL-C (mg/dL) | 47.919 ± 15.018 | 49.451 ± 15.015 | 49.792 ± 15.398 | 0.088 |
| LDL-C (mg/dL) | 92.842 ± 31.764 | 95.479 ± 31.351 | 97.806 ± 30.134 | 0.027 |
| TG (mg/dL) | 151.839 ± 94.815 | 156.248 ± 101.444 | 163.737 ± 100.745 | 0.124 |
| Fasting glucose (mg/dL) | 109.851 ± 38.327 | 108.729 ± 32.560 | 111.614 ± 42.695 | 0.442 |
| 25(OH)D | 16.947 ± 7.989 | 17.226 ± 7.236 | 18.156 ± 7.335 | 0.019 |
| hs-CRP (mg/dL) | 0.600 [0.200, 1.840] | 0.600 [0.200, 1.500] | 0.765 [0.300, 1.800] | 0.492 |
| Spot urine ACR (mg/g) | 391.176 [100.859, 1143.000] | 329.409 [50.776, 991.994] | 298.723 [57.699, 862.463] | 0.002 |
| Creatinine (mg/dL) | 2.131 ± 1.376 | 1.751 ± 1.013 | 1.498 ± 0.762 | <0.001 |
| eGFR (mL/min./1.73 m2) | 41.066 ± 26.853 | 51.454 ± 30.055 | 59.563 ± 29.716 | <0.001 |
| CKD stages | <0.001 | |||
| Stage 1 | 54 (9.5) | 94 (16.7) | 135 (23.6) | |
| Stage 2 | 75 (13.3) | 112 (19.9) | 129 (22.6) | |
| Stage 3a | 82 (14.5) | 86 (15.3) | 113 (19.8) | |
| Stage 3b | 123 (21.7) | 127 (22.6) | 116 (20.3) | |
| Stage 4 | 164 (29.0) | 117 (20.8) | 72 (12.6) | |
| Stage 5 | 68 (12.0) | 27 (4.8) | 7 (1.2) |
| 24 h UPE Level | Events, n (%) | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | |||
| 6-point MACE | T1 | 69 (12.2) | Reference | Reference | Reference | Reference | ||||
| T2 | 59 (10.5) | 0.741 (0.470, 1.169) | 0.198 | 0.804 (0.524, 1.233) | 0.317 | 0.843 (0.532, 1.335) | 0.467 | 0.755 (0.464, 1.229) | 0.259 | |
| T3 | 51 (8.9) | 0.417 (0.242, 0.716) | 0.002 | 0.559 (0.343, 0.910) | 0.019 | 0.468 (0.271, 0.808) | 0.006 | 0.376 (0.207, 0.683) | 0.001 | |
| 24 h UPE Level | Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | HR (95% CIs) | p Value | ||
| 6-point MACE | T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 0.803 (0.533, 1.210) | 0.297 | 0.817 (0.536, 1.247) | 0.352 | 0.823 (0.533, 1.270) | 0.380 | 0.818 (0.529, 1.265) | 0.369 | |
| T3 | 0.545 (0.346, 0.857) | 0.010 | 0.563 (0.346, 0.914) | 0.022 | 0.531 (0.321, 0.878) | 0.016 | 0.534 (0.323, 0.882) | 0.016 | |
| 24 h UPE Level | Events, n (%) | Unadjusted HR (95% CIs) | p for Interaction | Adjusted HR (95% CIs) | p for Interaction | |
|---|---|---|---|---|---|---|
| Age < 60 years | T1 | 23 (7.0) | Reference | 0.04 | Reference | 0.025 |
| T2 | 17 (4.9) | 0.705 (0.376, 1.319) | 0.348 (0.147, 0.822) | |||
| T3 | 8 (2.0) | 0.279 (0.125, 0.623) | 0.141 (0.046, 0.435) | |||
| Age ≥ 60 years | T1 | 27 (11.3) | Reference | Reference | ||
| T2 | 25 (11.5) | 0.915 (0.531, 1.578) | 1.047 (0.547, 2.005) | |||
| T3 | 22 (12.4) | 0.956 (0.544, 1.679) | 0.620 (0.295, 1.301) | |||
| Male | T1 | 32 (12.9) | Reference | 0.476 | Reference | 0.930 |
| T2 | 30 (8.6) | 0.602 (0.366, 0.991) | 0.733 (0.404, 1.331) | |||
| T3 | 27 (6.2) | 0.424 (0.254, 0.708) | 0.376 (0.192, 0.738) | |||
| Female | T1 | 18 (5.7) | Reference | Reference | ||
| T2 | 12 (5.6) | 0.972 (0.468, 2.019) | 0.971 (0.384, 2.457) | |||
| T3 | 3 (2.2) | 0.348 (0.103, 1.183) | 0.216 (0.040, 1.177) | |||
| BMI < 23 kg/m2 | T1 | 26 (10.3) | Reference | 0.341 | Reference | 0.856 |
| T2 | 14 (7.3) | 0.663 (0.346, 1.270) | 0.826 (0.302, 2.261) | |||
| T3 | 4 (3.7) | 0.305 (0.107, 0.875) | 0.337 (0.081, 1.396) | |||
| BMI ≥ 23 kg/m2 | T1 | 24 (7.7) | Reference | Reference | ||
| T2 | 28 (7.6) | 0.955 (0.554, 1.647) | 0.791 (0.412, 1.519) | |||
| T3 | 26 (5.6) | 0.696 (0.399, 1.212) | 0.406 (0.192, 0.860) | |||
| eGFR ≥ 45 mL/min./1.73 m2 | T1 | 18 (9.1) | Reference | 0.028 | Reference | 0.261 |
| T2 | 23 (8.3) | 0.865 (0.467, 1.604) | 0.779 (0.349, 1.738) | |||
| T3 | 11 (3.1) | 0.320 (0.151, 0.677) | 0.229 (0.081, 0.653) | |||
| eGFR < 45 mL/min./1.73 m2 | T1 | 32 (8.7) | Reference | Reference | ||
| T2 | 19 (6.7) | 0.741 (0.420, 1.307) | 0.638 (0.325, 1.255) | |||
| T3 | 19 (8.7) | 0.932 (0.528, 1.645) | 0.480 (0.219, 1.052) | |||
| Spot urine ACR < 300 mg/g | T1 | 27 (10.8) | Reference | 0.066 | Reference | 0.146 |
| T2 | 15 (5.6) | 0.470 (0.250, 0.884) | 0.577 (0.281, 1.186) | |||
| T3 | 11 (4.0) | 0.333 (0.165, 0.671) | 0.293 (0.118, 0.723) | |||
| Spot urine ACR ≥ 300 mg/g | T1 | 22 (7.2) | Reference | Reference | ||
| T2 | 25 (8.7) | 1.187 (0.669, 2.106) | 0.938 (0.457, 1.924) | |||
| T3 | 17 (6.2) | 0.797 (0.423, 1.501) | 0.447 (0.182, 1.098) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Oh, K.-H.; Hyun, Y.Y.; Sung, S.; Kim, S.W., on behalf of the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD) Investigators. Urinary Phosphorus Excretion and Cardiovascular Outcomes in Patients with Pre-Dialysis Chronic Kidney Disease: The KNOW-CKD Study. Nutrients 2023, 15, 2267. https://doi.org/10.3390/nu15102267
Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Ma SK, Oh K-H, Hyun YY, Sung S, Kim SW on behalf of the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD) Investigators. Urinary Phosphorus Excretion and Cardiovascular Outcomes in Patients with Pre-Dialysis Chronic Kidney Disease: The KNOW-CKD Study. Nutrients. 2023; 15(10):2267. https://doi.org/10.3390/nu15102267
Chicago/Turabian StyleSuh, Sang Heon, Tae Ryom Oh, Hong Sang Choi, Chang Seong Kim, Eun Hui Bae, Seong Kwon Ma, Kook-Hwan Oh, Young Youl Hyun, Suah Sung, and Soo Wan Kim on behalf of the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD) Investigators. 2023. "Urinary Phosphorus Excretion and Cardiovascular Outcomes in Patients with Pre-Dialysis Chronic Kidney Disease: The KNOW-CKD Study" Nutrients 15, no. 10: 2267. https://doi.org/10.3390/nu15102267
APA StyleSuh, S. H., Oh, T. R., Choi, H. S., Kim, C. S., Bae, E. H., Ma, S. K., Oh, K.-H., Hyun, Y. Y., Sung, S., & Kim, S. W., on behalf of the Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD) Investigators. (2023). Urinary Phosphorus Excretion and Cardiovascular Outcomes in Patients with Pre-Dialysis Chronic Kidney Disease: The KNOW-CKD Study. Nutrients, 15(10), 2267. https://doi.org/10.3390/nu15102267






