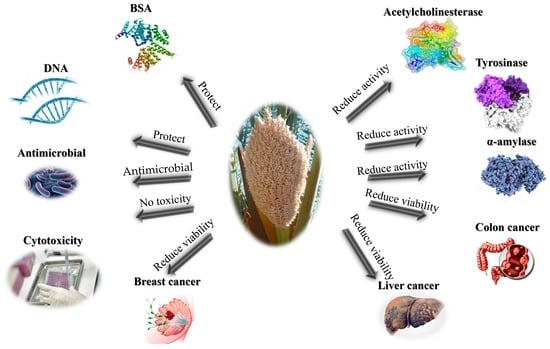
Palm Fruit (Phoenix dactylifera L.) Pollen Extract Inhibits Cancer Cell and Enzyme Activities and DNA and Protein Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Materials
2.2. Methods
2.2.1. Plant Material Extraction and Preparation
2.2.2. Total Phenolic Content (TPC) Assay
2.2.3. Total Flavonoids Content (TFC) Assay
2.3. Antioxidants Activity
2.3.1. DPPH•- Free Radical-Scavenging Evaluate
2.3.2. ABTS•- Free Radical-Scavenging Quantitative Analysis
2.3.3. Ferric-Reducing/Antioxidant Power (FRAP) Analysis
2.3.4. Nitric Oxide Radical (NO) Test
2.3.5. Total Antioxidant Capacity (TAC)
2.4. Enzyme Inhibitory Activity
2.4.1. The assay for Inhibiting Tyrosinase Enzyme Activity
2.4.2. The Assay for Inhibiting Porcine α-amylase Enzyme Activity
2.4.3. The Assay for Inhibiting Acetylcholinesterase Enzyme Activity
2.5. Assay for DNA Damage Caused by Free Radicals
2.6. Assay for Protein Oxidation Produced by AAPH
2.7. Antimicrobial Activity
2.7.1. Test for Susceptibility by Disk Diffusion
2.7.2. Assay of Minimum Inhibitory Concentrations (MICs)
2.8. Anticancer Activity
2.8.1. Cytotoxicity of PFPE on Normal Cells
2.8.2. Anticancer Activity of PFPE against Cancer Cells (MTT) Assay
2.8.3. Nuclear Staining Analysis
2.8.4. Cell Cycle Analysis
2.8.5. Analysis of Quantitative Changes in Oncogene Expression
3. Statistical Analysis
4. Results and Discussion
4.1. Bioactive Compounds
4.1.1. Total Phenolics Content
4.1.2. Total Flavonoids Content
4.1.3. HPLC Quantification of Phenolic Acids and Flavonoids
4.2. Antioxidants Activity
4.2.1. DPPH•- Free Radical-Scavenging Activity
4.2.2. ABTS•- Free Radical-Scavenging Activity
4.2.3. Ferric-Reducing/Antioxidant Power (FRAP) Activity
4.2.4. Total Antioxidant Capacity (TAC)
4.2.5. Nitric Oxide Radical (NO)-Scavenging Activity
4.3. Enzyme Inhibitory Activity
4.3.1. Tyrosinase Inhibition Activity
4.3.2. Porcine α-Amylase Inhibition Activity
4.3.3. Acetylcholinesterase Inhibition Activity
4.4. Free Radical-Induced Damage to DNA
4.5. Protein Oxidation Produced by AAPH
4.6. Antimicrobial Activity
4.7. Anticancer Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waly, M. Phytochemical Characterization and Health Benefits of Omani Date Pollen. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Benouamane, O.; Vergara-Barberán, M.; Benaziza, A.; García-Alvarez-Coque, M.C.; Simó-Alfonso, E.; China, B.; Lerma-García, M.J. Characterization of different cultivars of Algerian date palm (Phoenix dactylifera L.) leaves and pollen by comprehensive two-dimensional liquid chromatography of phenolic compounds extracted with different solvents. Microchem. J. 2022, 182, 107874. [Google Scholar] [CrossRef]
- Bentrad, N.; Gaceb-Terrak, R.; Benmalek, Y.; Rahmania, F. Studies on chemical composition and antimicrobial activities of bioactive molecules from date palm (Phoenix dactylifera L.) Pollens and seeds. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 242–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Reidah, I.M.; Gil-Izquierdo, Á.; Medina, S.; Ferreres, F. Phenolic composition profiling of different edible parts and by-products of date palm (Phoenix dactylifera L.) by using HPLC-DAD-ESI/MSn. Food Res. Int. 2017, 100, 494–500. [Google Scholar] [CrossRef]
- Karim, K.; Awad, M.A.; Manar, A.; Monia, J.; Karim, A.; Mohammed, E. Effect of flowering stage and storage conditions on pollen quality of six male date palm genotypes. Saudi J. Biol. Sci. 2022, 29, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Nandi, P.; Omar, A.; Ugwuagbo, K.C.; Lala, P.K. EP4 as a Therapeutic Target for Aggressive Human Breast Cancer. Int. J. Mol. Sci. 2018, 19, 1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadry, M.; Megeed, R.M.A.; Ghanem, H.; Abdoon, A.; Abdel-Hamid, A.-H. Does glycogen synthase kinase-3 β signaling pathway has a significant role in date palm pollen cancer therapy? Egypt. Pharm. J. 2019, 18, 208. [Google Scholar] [CrossRef]
- El-Far, A.H.; Oyinloye, B.E.; Sepehrimanesh, M.; Allah, M.A.G.; Abu-Reidah, I.; Shaheen, H.M.; Razeghian-Jahromi, I.; Alsenosy, A.E.-W.A.; Noreldin, A.E.; Al Jaouni, S.K.; et al. Date Palm (Phoenix dactylifera): Novel Findings and Future Directions for Food and Drug Discovery. Curr. Drug Discov. Technol. 2019, 16, 2–10. [Google Scholar] [CrossRef]
- Abdallah, W.E.; AbdelMohsen, M.M.; Awad, H.M. Phytochemical Composition, Antioxidant and Antitumor Activities of some Date Palm Pollen Extracts. Egypt. J. Chem. 2022. [Google Scholar] [CrossRef]
- Teo, D.; Abdullah, B.; Zainatul, N.; Binti Zainol, N.; Hasraf, N.; Nayan, B.M.; Muhammad, N.B. A Systematic Literature Review: The Effect of Date Palms (Phoenix Dactylifera) toward Breast Cancer MCF-7 Cell Line. Ann. Rom. Soc. Cell Biol. 2021, 25, 5387–5393. [Google Scholar]
- El-Far, A.H.; Ragab, R.F.; Mousa, S.A. Date Palm Bioactive Compounds: Nutraceuticals, Functional Nutrients, and Pharmaceuticals. In The Date Palm Genome; Springer International Publishing: Cham, Switzerland, 2021; pp. 27–50. [Google Scholar] [CrossRef]
- Karra, S.; Sebii, H.; Jardak, M.; Bouaziz, M.A.; Attia, H.; Blecker, C.; Besbes, S. Male date palm flowers: Valuable nutritional food ingredients and alternative antioxidant source and antimicrobial agent. S. Afr. J. Bot. 2020, 131, 181–187. [Google Scholar] [CrossRef]
- El Azim, M.H.M.A. Identification Phenolic and Biological Activities of Methanolic Extract of Date Palm Pollen (Phoenix dactylifera). J. Microb. Biochem. Technol. 2015, 7, 1. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Bicas, J.L.; Pastore, G.M.; Marostica, M.R., Jr. Introduction. In Bioactive Food Components Activity in Mechanistic Approach; Academic Press: Cambridge, MA, USA, 2021; pp. 1–3. [Google Scholar] [CrossRef]
- Abdel-Shaheed, M.M.; Abdalla, E.S.; Khalil, A.F.; El-Hadidy, E.M. Effect of Egyptian Date Palm Pollen (Phoenix Dactylifera L.) and Its Hydroethanolic Extracts on Serum Glucose and Lipid Profiles in Induced Diabetic Rats. Food Nutr. Sci. 2021, 12, 147–161. [Google Scholar] [CrossRef]
- Bentrad, N.; Hamida-Ferhat, A. Date palm fruit (Phoenix dactylifera): Nutritional values and potential benefits on health. In The Mediterranean Diet; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–255. [Google Scholar] [CrossRef]
- Mia, M.A.-T.; Mosaib, M.G.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Potentials and Safety of Date Palm Fruit against Diabetes: A Critical Review. Foods 2020, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Waly, M. Health Benefits and Nutritional Aspects of Date Palm Pollen. Can. J. Clin. Nutr. 2020, 8, 1–3. [Google Scholar] [CrossRef]
- Farag, M.A.; Otify, A.; Baky, M.H. Phoenix Dactylifera L. Date Fruit By-products Outgoing and Potential Novel Trends of Phytochemical, Nutritive and Medicinal Merits. Food Rev. Int. 2023, 39, 488–510. [Google Scholar] [CrossRef]
- Zain, M.R.A.M.; Kari, Z.A.; Dawood, M.A.O.; Ariff, N.S.N.A.; Salmuna, Z.N.; Ismail, N.; Ibrahim, A.H.; Krishnan, K.T.; Mat, N.F.C.; Edinur, H.A.; et al. Bioactivity and Pharmacological Potential of Date Palm (Phoenix dactylifera L.) Against Pandemic COVID-19: A Comprehensive Review. Appl. Biochem. Biotechnol. 2022, 194, 4587–4624. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Inam ur Raheem, M.; Khalid, W.; Zubair Khalid, M.; Sajid Saleem, F.; Sabir, A.; Sharif, I.; Zafar, K.-W. Health Benefits, Male Fertility, Nutritional Aspects of Dates and Date Palm Pollens: An Overview. JPAA 2022, 7, 4. [Google Scholar]
- Shahriarinour, M.; Divsar, F. Release Kinetics and Antibacterial Property of Curcumin-Loaded Date Palm (Phoenix dactylifera L.) Pollen. Arab. J. Sci. Eng. 2023, 48, 7263–7272. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El-Sayed, Y.S.; Soliman, M.M.; Shukry, M. Date Palm Pollen Extract Avert Doxorubicin-Induced Cardiomyopathy Fibrosis and Associated Oxidative/Nitrosative Stress, Inflammatory Cascade, and Apoptosis-Targeting Bax/Bcl-2 and Caspase-3 Signaling Pathways. Animals 2021, 11, 886. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef] [Green Version]
- Hachef, A.; Bourguiba, H.; Cherif, E.; Ivorra, S.; Terral, J.-F.; Zehdi-Azouzi, S. Agro-morphological traits assessment of Tunisian male date palms (Phœnix dactylifera L.) for preservation and sustainable utilization of local germplasm. Saudi J. Biol. Sci. 2023, 30, 103574. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Shariati, M.; Tešić, Ž.L.j.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Al-Mssallem, M.Q.; Alqurashi, R.M.; Al-Khayri, J.M. Bioactive Compounds of Date Palm (Phoenix dactylifera L.). In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Sporchia, F.; Patrizi, N.; Pulselli, F.M. Date Fruit Production and Consumption: A Perspective on Global Trends and Drivers from a Multidimensional Footprint Assessment. Sustainability 2023, 15, 4358. [Google Scholar] [CrossRef]
- Paszke, M.Z. Date Palm and Date Palm Inflorescences in the Late Uruk Period (c. 3300 b.c.): Botany and Archaic Script. Iraq 2019, 81, 221–239. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.M.; Hashim, I.B.; Kamal, H.; AlMaqbali, F.; Souka, U.; Ibrahim, W.H. Production of functional pita bread using date seed powder. J. Food Sci. Technol. 2015, 52, 6375–6384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, H.M.; Ibrahim, W.H. Effect of date seeds on oxidative damage and antioxidant status in vivo. J. Sci. Food Agric. 2011, 91, 1674–1679. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Souka, U.D.; Elsebaee, F.M.; El-Ziney, M.G.; Ibrahim, W.H. Polyphenol-Rich Date Palm Fruit Seed (Phoenix Dactylifera L.) Extract Inhibits Labile Iron, Enzyme, and Cancer Cell Activities, and DNA and Protein Damage. Nutrients 2022, 2022, 3536. [Google Scholar] [CrossRef] [PubMed]
- Al Meqbaali, F.T.; Habib, H.; Othman, A.; Al-Marzooqi, S.; Al-Bawardi, A.; Pathan, J.Y.; Hilary, S.; Souka, U.; Al-Hammadi, S.; Ibrahim, W.; et al. The antioxidant activity of date seed: Preliminary results of a preclinical in vivo study. Emir. J. Food Agric. 2017, 822, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.; Mohamed, F.E. DNA and BSA damage inhibitory activities, and anti-acetylcholinesterase, anti-porcine α-amylase and antioxidant properties of Dolichos lablab beans. Food Funct. 2016, 8, 881–887. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, W.H.; Schneider-Stock, R.; Hassan, H.M. Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities. Food Chem. 2013, 141, 148–152. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.; Al Maqbali, F.D.; Jaber, N.N.; Ibrahim, W.H. Identification of Date Seeds Varieties Patterns to Optimize Nutritional Benefits of Date Seeds. J. Nutr. Food Sci. 2014, S8, 8. [Google Scholar] [CrossRef]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W. Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chem. 2014, 153, 28–34. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape seed proanthocyanidin extract inhibits DNA and protein damage and labile iron, enzyme, and cancer cell activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.E.; Mohamed, F.E. Functional, bioactive, biochemical, and physicochemical properties of the Dolichos lablab bean. Food Funct. 2016, 8, 872–880. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arch. Ind. Hyg. Toxicol. 2020, 71, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, N.T.A.; El-Fakharany, E.M.; Abdallah, A.E.; El-Gendi, H.; Lotfy, D.R. Synthesis, and docking studies of novel heterocycles incorporating the indazolylthiazole moiety as antimicrobial and anticancer agents. Sci. Rep. 2022, 12, 3424. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.K.; El-Gendi, H.; El-Fakharany, E.M.; Owda, M.E.; Awad, M.A.; Kamoun, E.A. Exploitation of cantaloupe peels for bacterial cellulose production and functionalization with green synthesized Copper oxide nanoparticles for diverse biological applications. Sci. Rep. 2022, 12, 19241. [Google Scholar] [CrossRef]
- Omer, A.M.; Eltaweil, A.S.; El-Fakharany, E.M.; El-Monaem, E.M.A.; Ismail, M.M.F.; Mohy-Eldin, M.S.; Ayoup, M.S. Novel Cytocompatible Chitosan Schiff Base Derivative as a Potent Antibacterial, Antidiabetic, and Anticancer Agent. Arab. J. Sci. Eng. 2023, 1–15. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Abu-Serie, M.M.; Litus, E.A.; Permyakov, S.E.; Permyakov, E.A.; Uversky, V.N.; Redwan, E.M. The Use of Human, Bovine, and Camel Milk Albumins in Anticancer Complexes with Oleic Acid. Protein J. 2018, 37, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; El-Fakharany, E.M. Efficiency of novel nanocombinations of bovine milk proteins (lactoperoxidase and lactoferrin) for combating different human cancer cell lines. Sci. Rep. 2017, 7, 16769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Fakharany, E.M.; Abu-Serie, M.M.; Habashy, N.H.; Eltarahony, M. Augmenting apoptosis-mediated anticancer activity of lactoperoxidase and lactoferrin by nanocombination with copper and iron hybrid nanometals. Sci. Rep. 2022, 12, 13153. [Google Scholar] [CrossRef]
- Hilary, S.; Habib, H.; Souka, U.; Ibrahim, W.; Platat, C. Bioactivity of arid region honey: An in vitro study. BMC Complement. Altern. Med. 2017, 17, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouhlali, E.D.T.; Alem, C.; Ennassir, J.; Benlyas, M.; Mbark, A.N.; Zegzouti, Y.F. Phytochemical compositions and antioxidant capacity of three date (Phoenix dactylifera L.) seeds varieties grown in the South East Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 350–357. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Derouich, M.; Meziani, R.; Bourkhis, B.; Filali-Zegzouti, Y.; Alem, C. Nutritional, mineral and organic acid composition of syrups produced from six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. Food Compos. Anal. 2020, 93, 103591. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Hmidani, A.; Bourkhis, B.; Khouya, T.; Ramchoun, M.; Filali-Zegzouti, Y.; Alem, C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 2020, 6, e03436. [Google Scholar] [CrossRef]
- Sebii, H.; Karra, S.; Bchir, B.; Ghribi, A.M.; Danthine, S.M.; Blecker, C.; Attia, H.; Besbes, S. Physico-Chemical, Surface and Thermal Properties of Date Palm Pollen as a Novel Nutritive Ingredient. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 84–91. [Google Scholar] [CrossRef]
- Zeid, H.M.A.; Shiha, M.A.; Shehata, A.A. Comparative Study of Pollen Grains Morphology and Phytochemical Constituents of Some Saudi Arabian Date Palm (Phoenix dactylifera L.) Cultivars. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2800–2809. [Google Scholar] [CrossRef]
- Aybastıer, Ö.; Dawbaa, S.; Demir, C. Investigation of antioxidant ability of grape seeds extract to prevent oxidatively induced DNA damage by gas chromatography-tandem mass spectrometry. J. Chromatogr. B 2018, 1072, 328–335. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Soliman, T.N.; Darwish, A.M.G. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS ONE 2019, 14, e0222789. [Google Scholar] [CrossRef] [Green Version]
- Echegaray, N.; Pateiro, M.; Gullón, B.; Amarowicz, R.; Misihairabgwi, J.M.; Lorenzo, J.M. Phoenix dactylifera products in human health—A review. Trends Food Sci. Technol. 2020, 105, 238–250. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and Combined Antioxidant Activity of Spices and Spice Phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Banan-MwineDaliri, E.; Oh, D.-H. Screening for Antioxidant Activity: Total Antioxidant Assay. In Methods in Actinobacteriology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 459–460. [Google Scholar] [CrossRef]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gülçin, I.; Çekiç, S.D.; Güçlü, K.; Özyürek, M.; Çelik, S.E.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species (IUPAC Technical Report). Pure Appl. Chem. 2021, 94, 87–144. [Google Scholar] [CrossRef]
- McDonald, A.G.; Tipton, K.F. Enzymes: Irreversible Inhibition. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2020; pp. 1–17. [Google Scholar]
- Habib, H.M.; Kheadr, E.; Ibrahim, W.H. Inhibitory effects of honey from arid land on some enzymes and protein damage. Food Chem. 2021, 364, 130415. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L. Understanding the combined effect and inhibition mechanism of 4-hydroxycinnamic acid and ferulic acid as tyrosinase inhibitors. Food Chem. 2021, 352, 129369. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2019, 64, 103587. [Google Scholar] [CrossRef]
- Niu, F.; Xie, W.; Zhang, W.; Kawuki, J.; Yu, X. Vitamin C, vitamin E, β-carotene and risk of Parkinson’s disease: A systematic review and dose–response meta-analysis of observational studies. Nutr. Neurosci. 2023, 1–13. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]








| Bioactive Compounds (mg/100 g Palm Fruit Pollen) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TPC | TFC | Gallic Acid | 4-Hydroxy-3- Methoxybenzoic Acid | Catechin | Syringic Acid | Epicatechin | P-Coumaric Acid | Ferulic Acid | Rutin | Cinnamic Acid |
| 1474.33 ± 11.85 | 771.00 ± 48.28 | 0.052 ± 0.003 | 0.007 ± 0.000 | 0.928 ± 0.032 | 0.147 ± 0.003 | 0.347 ± 0.021 | 0.153 ± 0.003 | 0.020 ± 0.001 | 6.23 ± 0.111 | 1.51 ± 0.016 |
| Radicals | Antioxidant Inhibition Activity IC50 (µg/mL) | ||
|---|---|---|---|
| PFPE | VC | Rutin | |
| DPPH | 974.00 ± 28.02 | 881.70 ± 13.38 | 417.80 ± 5.78 |
| ABTS | 106.60 ± 1.05 | 0.69 ± 0.06 | 6.50 ± 0.08 |
| FRAP | 1671.00 ± 161.8 | 743.60 ± 30.11 | 391.00 ± 7.46 |
| Total Antioxidant Capacity | 1210.00 ± 57.32 | 3005.00 ± 72.45 | 17,600 ± 41.64 |
| Nitric Oxide | 249.20 ± 18.67 | 2710.00 ±13.35 | 270.10 ± 3.51 |
| Enzyme’s Inhibition Activity IC50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Enzymes | PFPE | VC | Rutin | Kojic Acid | Acarbose | Galantamine |
| Tyrosinase | 618.60 ± 34.54 | 1881.00 ± 48.56 | 494.40 ± 27.86 | 379.30 ± 29.28 | -- | -- |
| Porcine α-amylase | 1084.00 ± 36.22 | 97.62 ± 8.14 | 86.76 ± 4.43 | -- | 197.90 ±17.45 | -- |
| Acetylcholinesterase | 653.60 ± 18.54 | 721.40 ± 7.96 | 911.70 ± 17.21 | -- | 319.60 ± 10.38 | |
| DNA, BSA Damage Inhibition Activity IC50 (µg/mL) | |||
|---|---|---|---|
| PFPE | VC | Rutin | |
| DNA | 1428.00 ± 16.57 | 505.00 ± 21.63 | 15.00 ± 0.22 |
| BSA | 64.81 ± 1.64 | 25.32 ± 1.29 | 3.68 ± 0.35 |
| Organism | Control 1 | Control 2 | PFPE (mg/Well) | MIC (mg/mL) | ||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | ||||
| S. aureus | −ve | −ve | 12.50 ± 1.10 | 19.00 ± 2.10 | 22.00 ± 1.30 | 25.00 |
| S. mutans | 37.00 ± 2.80 | 18.00 ± 1.48 | 14.00 ± 1.50 | 18.00 ± 2.10 | 23.00 ± 1.90 | 12.50 |
| E. coli | 25.00 ± 2.40 | −ve | −ve | 10.00 ± 0.49 | 11.00 ± 0.89 | 100.00 |
| S. typhimurium | 30.00 ± 1.30 | 10.00 ± 0.80 | 13.00 ± 0.47 | 17.00 ± 0.47 | 21.60 ± 1.80 | 50.00 |
| P. aeruginosa | 28.00 ± 2.76 | 13.20 ± 0.68 | 14.20 ± 1.23 | 17.30 ± 2.10 | 20.60 ± 1.90 | 50.00 |
| C. albicans | - | 25.50 ± 2.30 | −ve | −ve | −ve | - |
| 24 h | 48 h | |||||
|---|---|---|---|---|---|---|
| Cells | EC100 | IC50 | SI | EC100 | IC50 | SI |
| HSF | 133.24 ± 4.49 | 1232 ± 44.93 | - | 123.43 ± 3.52 | 1114 ± 39.02 | - |
| HepG-2 | 41.04 ± 1.29 | 430.3 ± 12.95 | 2.86 ± 0.11 | 27.05 ± 1.16 | 250.4 ± 11.58 | 4.45 ± 0.16 |
| Caco-2 | 32.51 ± 1.68 | 394.9 ± 16.81 | 3.12 ± 0.12 | 29.36 ± 2.17 | 246.9 ± 25.42 | 4.51 ± 0.15 |
| MDA | 31.46 ± 1.74 | 334.5 ± 17.37 | 3.68 ± 0.14 | 25.69 ± 2.54 | 223.5 ± 21.72 | 4.98 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, H.M.; El-Fakharany, E.M.; El-Gendi, H.; El-Ziney, M.G.; El-Yazbi, A.F.; Ibrahim, W.H. Palm Fruit (Phoenix dactylifera L.) Pollen Extract Inhibits Cancer Cell and Enzyme Activities and DNA and Protein Damage. Nutrients 2023, 15, 2614. https://doi.org/10.3390/nu15112614
Habib HM, El-Fakharany EM, El-Gendi H, El-Ziney MG, El-Yazbi AF, Ibrahim WH. Palm Fruit (Phoenix dactylifera L.) Pollen Extract Inhibits Cancer Cell and Enzyme Activities and DNA and Protein Damage. Nutrients. 2023; 15(11):2614. https://doi.org/10.3390/nu15112614
Chicago/Turabian StyleHabib, Hosam M., Esmail M. El-Fakharany, Hamada El-Gendi, Mohamed G. El-Ziney, Ahmed F. El-Yazbi, and Wissam H. Ibrahim. 2023. "Palm Fruit (Phoenix dactylifera L.) Pollen Extract Inhibits Cancer Cell and Enzyme Activities and DNA and Protein Damage" Nutrients 15, no. 11: 2614. https://doi.org/10.3390/nu15112614
APA StyleHabib, H. M., El-Fakharany, E. M., El-Gendi, H., El-Ziney, M. G., El-Yazbi, A. F., & Ibrahim, W. H. (2023). Palm Fruit (Phoenix dactylifera L.) Pollen Extract Inhibits Cancer Cell and Enzyme Activities and DNA and Protein Damage. Nutrients, 15(11), 2614. https://doi.org/10.3390/nu15112614