Fermented Rice Bran Supplementation Inhibits LPS-Induced Osteoclast Formation and Bone Resorption in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Animals
2.3. Preparation for Histological Evaluation
2.4. Analysis of Bone Resorption Area by Micro-CT
2.5. Osteoclast Culture
2.6. Analysis of Expression Level Using Real-Time RT-PCR
2.7. Cell Viability Assay
2.8. Primary Osteoblasts Isolation from Mice
2.9. Primary Peritoneal Macrophages Isolation from Mice
2.10. Western Blotting Analysis
2.11. Statistical Analysis
3. Results
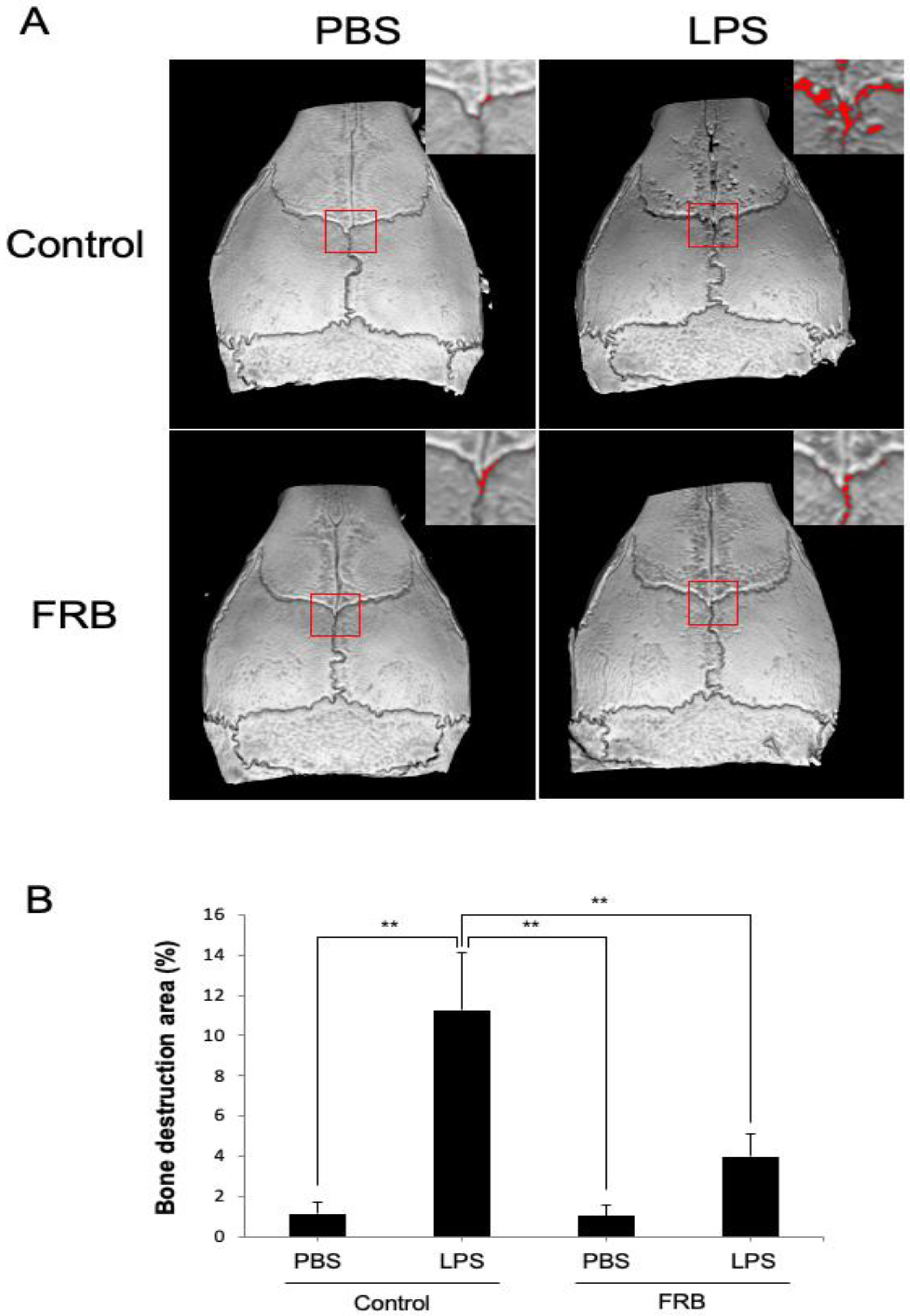
3.1. FRB Supplementation Protected against LPS-Induced Inflammatory Osteoclastogenesis In Vivo
3.2. FRB Supplementation Protected against LPS-Induced Bone Resorption In Vivo
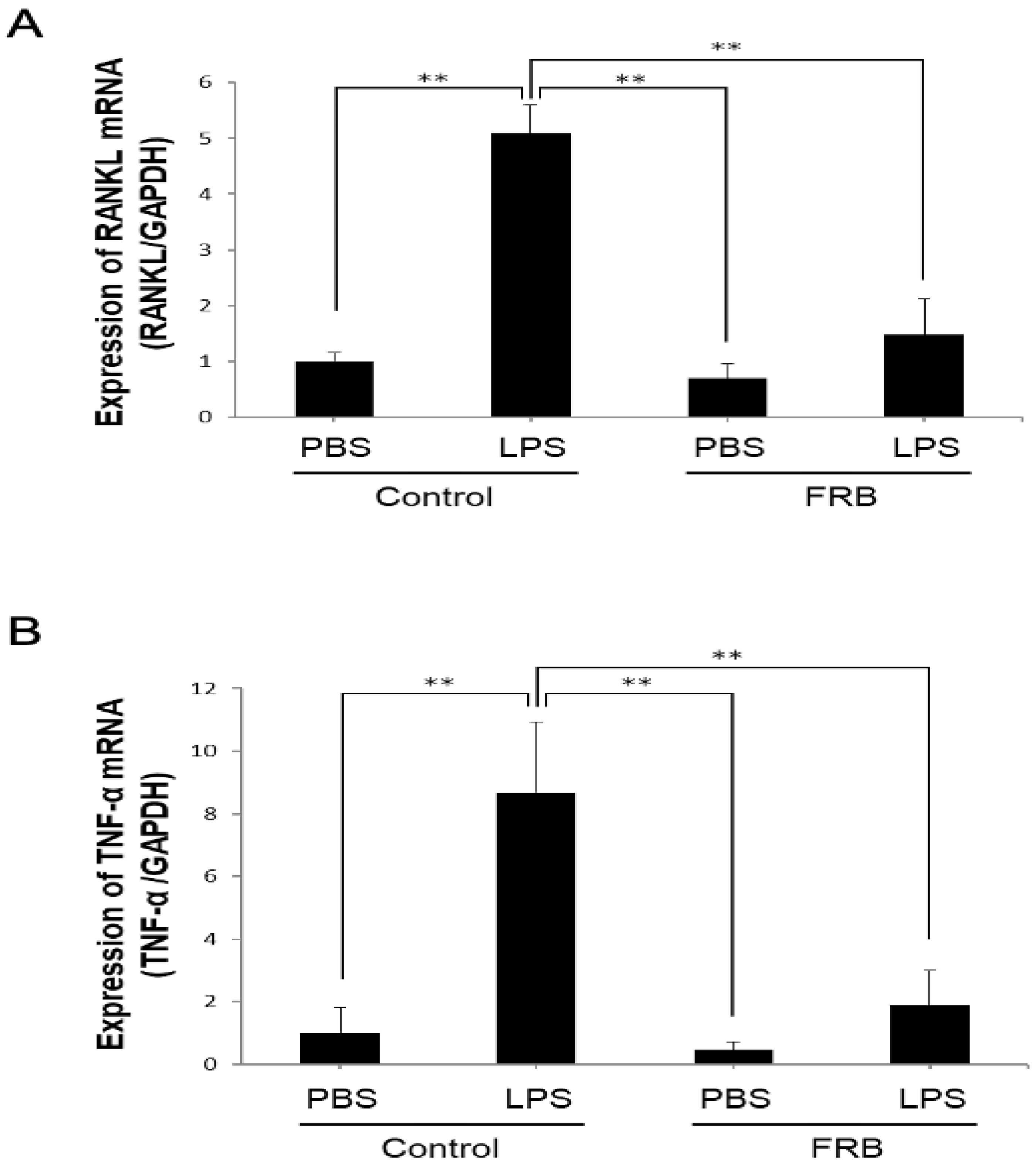
3.3. FRB Supplementation Downregulated the Expression of LPS-Induced Osteoclast-Associated Cytokines In Vivo
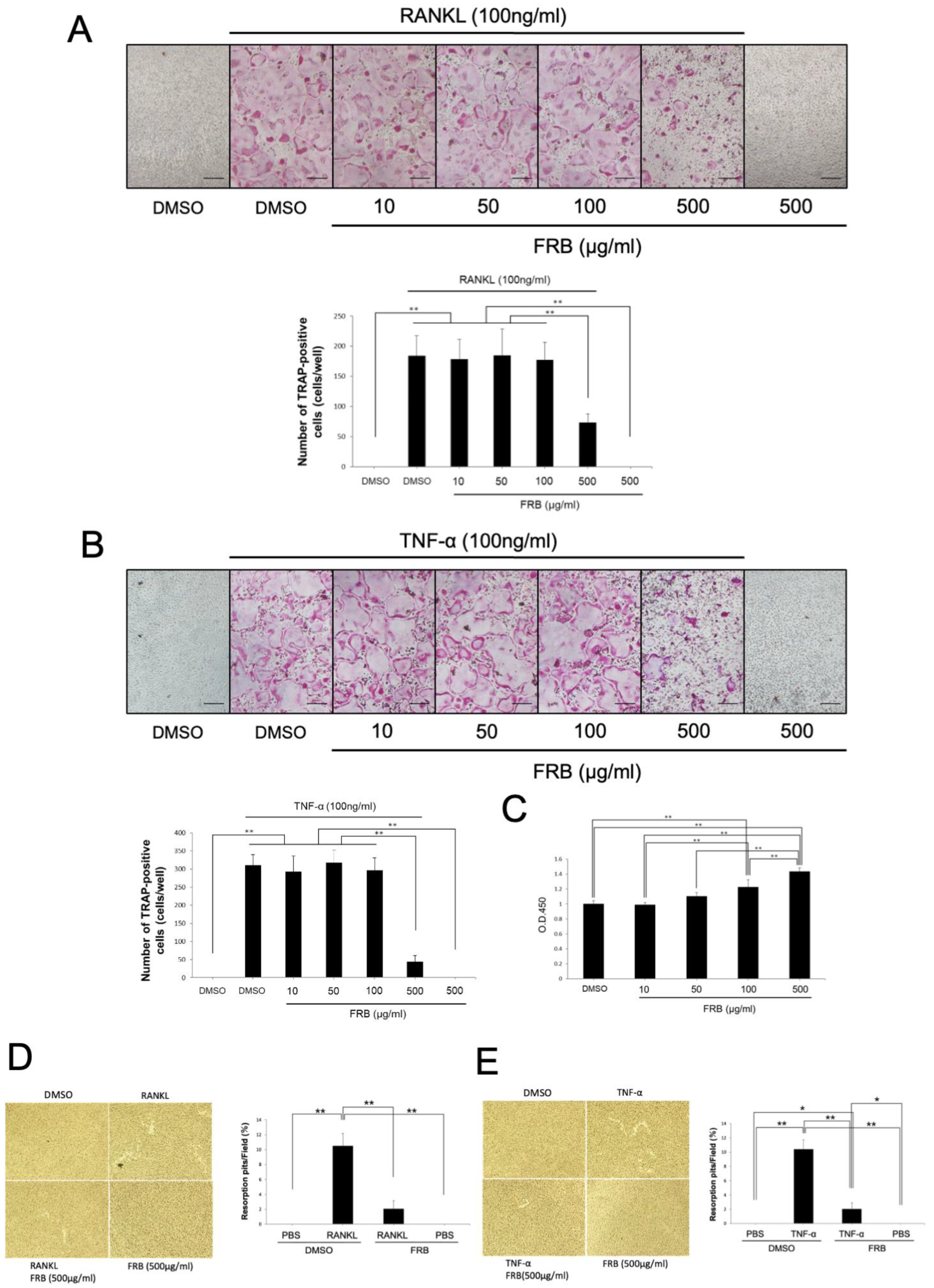
3.4. FRB Inhibited Osteoclast Formation Caused by RANKL and TNF-α but Not Cell Viability of Osteoclast Precursor Cells
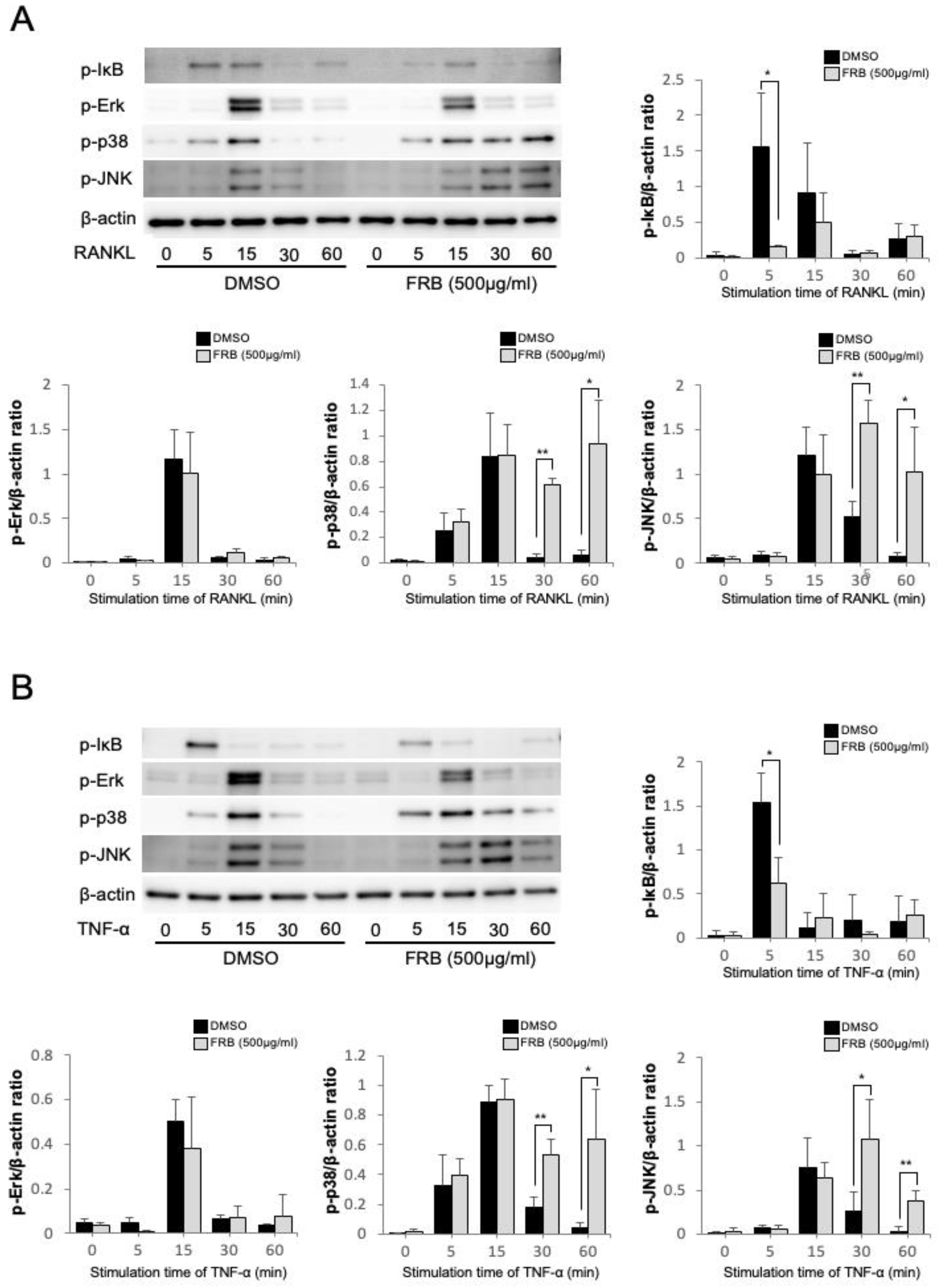
3.5. FRB Inhibited the Phosphorylation of IκB Caused by RANKL and TNF-α in Osteoclast Precursor Cells
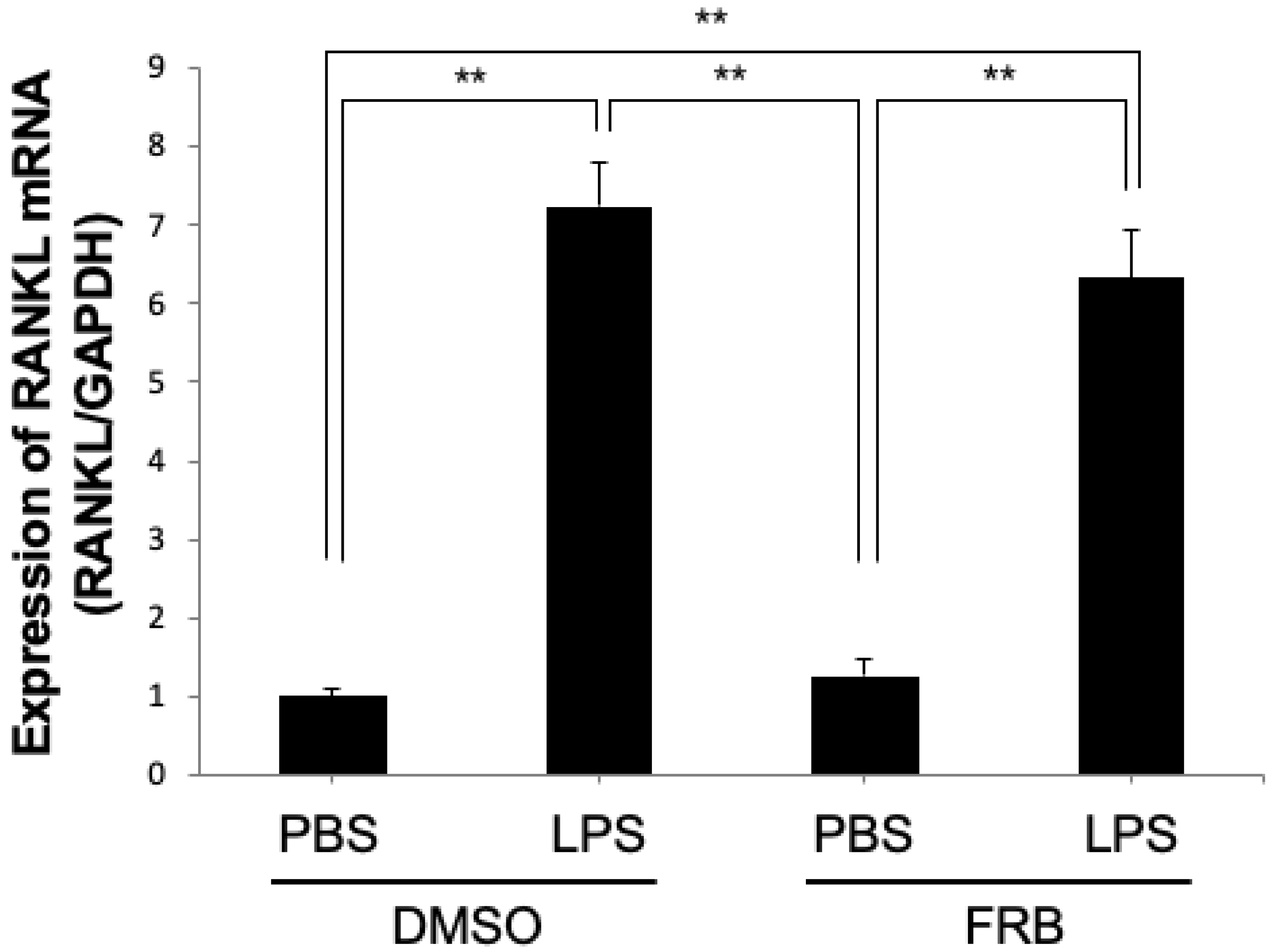
3.6. FRB Did Not Affect LPS-Induced RANKL Expression in Osteoblasts
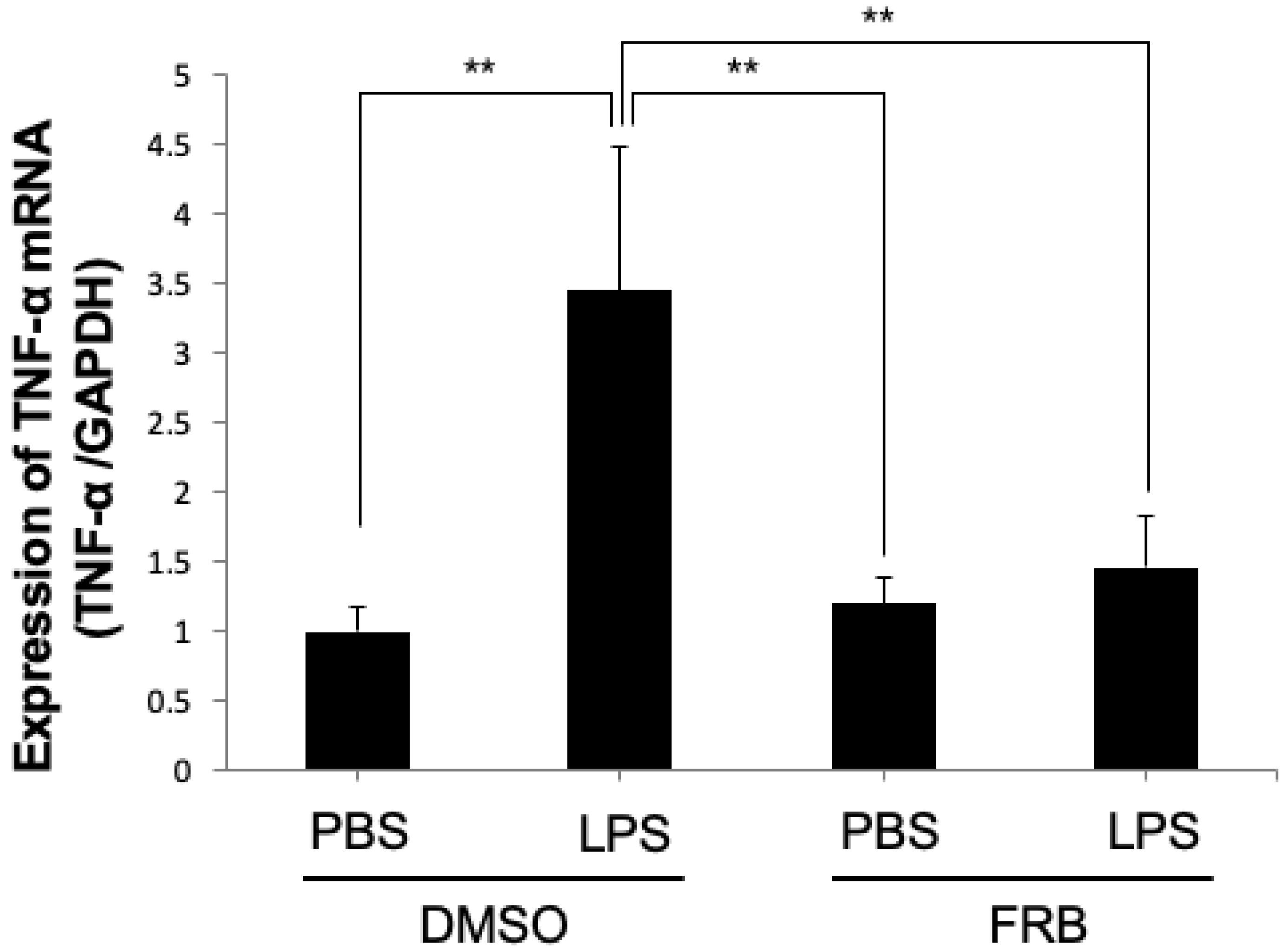
3.7. FRB Suppressed LPS-Induced TNF-α Expression in Macrophages
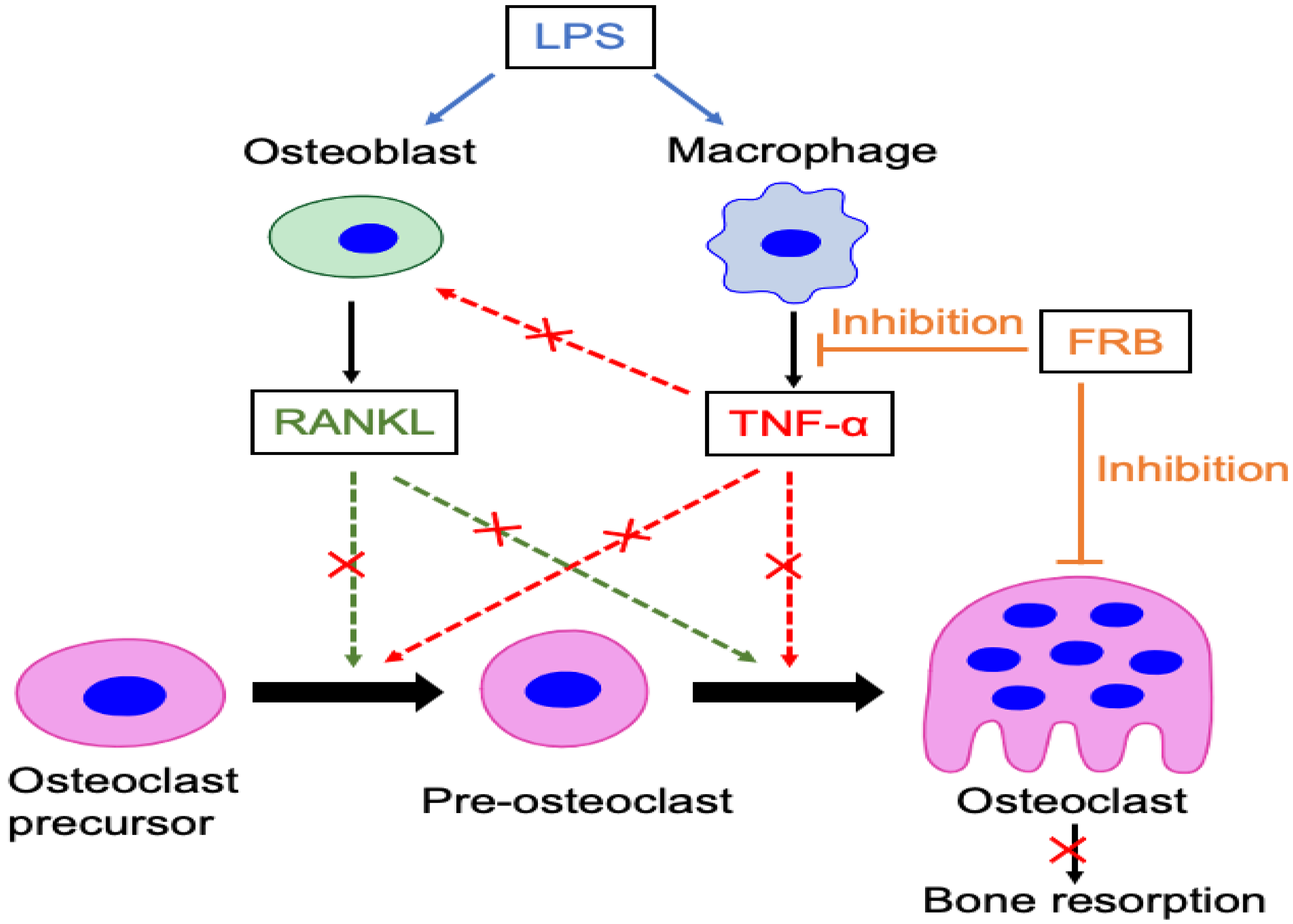
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyce, B.F.; Li, J.; Xing, L.; Yao, Z. Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption. Front. Immunol. 2018, 9, 2263. [Google Scholar] [CrossRef] [Green Version]
- Sirisereephap, K.; Maekawa, T.; Tamura, H.; Hiyoshi, T.; Domon, H.; Isono, T.; Terao, Y.; Maeda, T.; Tabeta, K. Osteoimmunology in Periodontitis: Local Proteins and Compounds to Alleviate Periodontitis. Int. J. Mol. Sci. 2022, 23, 5540. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, J.; Wang, L.; Liu, J.; Zhang, L. Regulation of differentiation and generation of osteoclasts in rheumatoid arthritis. Front. Immunol. 2022, 13, 1034050. [Google Scholar] [CrossRef]
- Crotti, T.N.; Dharmapatni, A.A.; Alias, E.; Haynes, D.R. Osteoimmunology: Major and Costimulatory Pathway Expression Associated with Chronic Inflammatory Induced Bone Loss. J. Immunol. Res. 2015, 2015, 281287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Kitaura, H.; Zhou, P.; Kim, H.J.; Novack, D.V.; Ross, F.P.; Teitelbaum, S.L. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Investig. 2005, 115, 3418–3427. [Google Scholar] [CrossRef] [Green Version]
- Kitaura, H.; Sands, M.S.; Aya, K.; Zhou, P.; Hirayama, T.; Uthgenannt, B.; Wei, S.; Takeshita, S.; Novack, D.V.; Silva, M.J.; et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J. Immunol. 2004, 173, 4838–4846. [Google Scholar] [CrossRef] [Green Version]
- Xing, Q.; de Vos, P.; Faas, M.M.; Ye, Q.; Ren, Y. LPS promotes pre-osteoclast activity by up-regulating CXCR4 via TLR-4. J. Dent. Res. 2011, 90, 157–162. [Google Scholar] [CrossRef]
- Islam, S.; Hassan, F.; Tumurkhuu, G.; Dagvadorj, J.; Koide, N.; Naiki, Y.; Mori, I.; Yoshida, T.; Yokochi, T. Bacterial lipopolysaccharide induces osteoclast formation in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 360, 346–351. [Google Scholar] [CrossRef]
- Mormann, M.; Thederan, M.; Nackchbandi, I.; Giese, T.; Wagner, C.; Hansch, G.M. Lipopolysaccharides (LPS) induce the differentiation of human monocytes to osteoclasts in a tumour necrosis factor (TNF) alpha-dependent manner: A link between infection and pathological bone resorption. Mol. Immunol. 2008, 45, 3330–3337. [Google Scholar] [CrossRef]
- Zou, W.; Bar-Shavit, Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J. Bone Miner. Res. 2002, 17, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kitaura, H.; Fujii, T.; Hakami, Z.W.; Takano-Yamamoto, T. Anti-c-Fms antibody inhibits lipopolysaccharide-induced osteoclastogenesis in vivo. FEMS Immunol. Med. Microbiol. 2012, 64, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Matsuguchi, T.; Tsuboi, N.; Mitani, A.; Tanaka, S.; Matsuoka, M.; Yamamoto, G.; Hishikawa, T.; Noguchi, T.; Yoshikai, Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J. Immunol. 2001, 166, 3574–3579. [Google Scholar] [CrossRef]
- Nason, R.; Jung, J.Y.; Chole, R.A. Lipopolysaccharide-induced osteoclastogenesis from mononuclear precursors: A mechanism for osteolysis in chronic otitis. J. Assoc. Res. Otolaryngol. 2009, 10, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberts, L.; Delcour, J.A. Carotenoids in raw and parboiled brown and milled rice. J. Agric. Food Chem. 2008, 56, 11914–11919. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Verschoyle, R.D.; Greaves, P.; Cai, H.; Edwards, R.E.; Steward, W.P.; Gescher, A.J. Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br. J. Cancer 2007, 96, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Flight, I.; Clifton, P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Justo, M.L.; Candiracci, M.; Dantas, A.P.; de Sotomayor, M.A.; Parrado, J.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Rice bran enzymatic extract restores endothelial function and vascular contractility in obese rats by reducing vascular inflammation and oxidative stress. J. Nutr. Biochem. 2013, 24, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Kurtys, E.; Eisel, U.L.M.; Hageman, R.J.J.; Verkuyl, J.M.; Broersen, L.M.; Dierckx, R.; de Vries, E.F.J. Anti-inflammatory effects of rice bran components. Nutr. Rev. 2018, 76, 372–379. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Zhou, Z.K.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2019, 59, 2458–2466. [Google Scholar] [CrossRef]
- Ramezanzadeh, F.M.; Rao, R.M.; Windhauser, M.; Prinyawiwatkul, W.; Tulley, R.; Marshall, W.E. Prevention of hydrolytic rancidity in rice bran during storage. J. Agric. Food Chem. 1999, 47, 3050–3052. [Google Scholar] [CrossRef]
- Kum, S.J.; Yang, S.O.; Lee, S.M.; Chang, P.S.; Choi, Y.H.; Lee, J.J.; Hurh, B.S.; Kim, Y.S. Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang). J. Agric. Food Chem. 2015, 63, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Futagami, T.; Mori, K.; Wada, S.; Ida, H.; Kajiwara, Y.; Takashita, H.; Tashiro, K.; Yamada, O.; Omori, T.; Kuhara, S.; et al. Transcriptomic analysis of temperature responses of Aspergillus kawachii during barley koji production. Appl. Environ. Microbiol. 2015, 81, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Erkkila, S.; Suihko, M.L.; Eerola, S.; Petaja, E.; Mattila-Sandholm, T. Dry sausage fermented by Lactobacillus rhamnosus strains. Int. J. Food Microbiol. 2001, 64, 205–210. [Google Scholar] [CrossRef]
- Villena, J.; Chiba, E.; Tomosada, Y.; Salva, S.; Marranzino, G.; Kitazawa, H.; Alvarez, S. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol. 2012, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Zommiti, M.; Cambronel, M.; Maillot, O.; Barreau, M.; Sebei, K.; Feuilloley, M.; Ferchichi, M.; Connil, N. Evaluation of Probiotic Properties and Safety of Enterococcus faecium Isolated from Artisanal Tunisian Meat “Dried Ossban”. Front. Microbiol. 2018, 9, 1685. [Google Scholar] [CrossRef] [Green Version]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Agista, A.Z.; Watanabe, K.; Nochi, T.; Aso, H.; Ohsaki, Y.; Koseki, T.; Komai, M.; Shirakawa, H. Fermented rice bran supplementation attenuates chronic colitis-associated extraintestinal manifestations in female C57BL/6N mice. J. Nutr. Biochem. 2022, 99, 108855. [Google Scholar] [CrossRef] [PubMed]
- Agista, A.Z.; Rusbana, T.B.; Islam, J.; Ohsaki, Y.; Sultana, H.; Hirakawa, R.; Watanabe, K.; Nochi, T.; Ardiansyah; Budijanto, S.; et al. Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice. Nutrients 2021, 13, 1869. [Google Scholar] [CrossRef]
- Rusbana, T.B.; Agista, A.Z.; Saputra, W.D.; Ohsaki, Y.; Watanabe, K.; Ardiansyah, A.; Budijanto, S.; Koseki, T.; Aso, H.; Komai, M.; et al. Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model. Nutrients 2020, 12, 2409. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Ardiansyah; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 442. [Google Scholar] [CrossRef] [Green Version]
- Agista, A.Z.; Tanuseputero, S.A.; Koseki, T.; Ardiansyah; Budijanto, S.; Sultana, H.; Ohsaki, Y.; Yeh, C.L.; Yang, S.C.; Komai, M.; et al. Tryptamine, a Microbial Metabolite in Fermented Rice Bran Suppressed Lipopolysaccharide-Induced Inflammation in a Murine Macrophage Model. Int. J. Mol. Sci. 2022, 23, 11209. [Google Scholar] [CrossRef]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef]
- Saeed, J.; Kitaura, H.; Kimura, K.; Ishida, M.; Sugisawa, H.; Ochi, Y.; Kishikawa, A.; Takano-Yamamoto, T. IL-37 inhibits lipopolysaccharide-induced osteoclast formation and bone resorption in vivo. Immunol. Lett. 2016, 175, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ohori, F.; Kitaura, H.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. IL-33 Inhibits TNF-alpha-Induced Osteoclastogenesis and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 1130. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Kinjo, R.; Kanou, K.; et al. Docosahexaenoic acid inhibits TNF-alpha-induced osteoclast formation and orthodontic tooth movement through GPR120. Front. Immunol. 2022, 13, 929690. [Google Scholar] [CrossRef]
- Shen, W.R.; Kimura, K.; Ishida, M.; Sugisawa, H.; Kishikawa, A.; Shima, K.; Ogawa, S.; Qi, J.; Kitaura, H. The Glucagon-Like Peptide-1 Receptor Agonist Exendin-4 Inhibits Lipopolysaccharide-Induced Osteoclast Formation and Bone Resorption via Inhibition of TNF-alpha Expression in Macrophages. J. Immunol. Res. 2018, 2018, 5783639. [Google Scholar] [CrossRef] [Green Version]
- Kishikawa, A.; Kitaura, H.; Kimura, K.; Ogawa, S.; Qi, J.; Shen, W.R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; et al. Docosahexaenoic Acid Inhibits Inflammation-Induced Osteoclast Formation and Bone Resorption in vivo Through GPR120 by Inhibiting TNF-alpha Production in Macrophages and Directly Inhibiting Osteoclast Formation. Front. Endocrinol. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, I.S.; Lim, B.O. Black Rice (Oryza sativa L.) Fermented with Lactobacillus casei Attenuates Osteoclastogenesis and Ovariectomy-Induced Osteoporosis. BioMed Res. Int. 2019, 2019, 5073085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Song, J.H.; Park, S.H.; Lee, J.H.; Park, H.W.; Kim, T.W. Effects of Brown Rice Extract Treated with Lactobacillus sakei Wikim001 on Osteoblast Differentiation and Osteoclast Formation. Prev. Nutr. Food Sci. 2014, 19, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, N.; Maeda, H.; Yoshimine, Y.; Akamine, A. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone 2004, 35, 629–635. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah; Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, T.; Kitaura, H.; Marahleh, A.; Agista, A.Z.; Ohsaki, Y.; Shirakawa, H.; Mizoguchi, I. Fermented Rice Bran Supplementation Inhibits LPS-Induced Osteoclast Formation and Bone Resorption in Mice. Nutrients 2023, 15, 3044. https://doi.org/10.3390/nu15133044
Noguchi T, Kitaura H, Marahleh A, Agista AZ, Ohsaki Y, Shirakawa H, Mizoguchi I. Fermented Rice Bran Supplementation Inhibits LPS-Induced Osteoclast Formation and Bone Resorption in Mice. Nutrients. 2023; 15(13):3044. https://doi.org/10.3390/nu15133044
Chicago/Turabian StyleNoguchi, Takahiro, Hideki Kitaura, Aseel Marahleh, Afifah Zahra Agista, Yusuke Ohsaki, Hitoshi Shirakawa, and Itaru Mizoguchi. 2023. "Fermented Rice Bran Supplementation Inhibits LPS-Induced Osteoclast Formation and Bone Resorption in Mice" Nutrients 15, no. 13: 3044. https://doi.org/10.3390/nu15133044




