Evaluating the Potential of Casein Glycomacropeptide in Adult Irritable Bowel Syndrome Management: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Study Interventions
2.3. Study Design
2.4. Fecal Samples
2.5. Microbiota: Extraction of DNA and Library Preparation
2.6. Microbiota: Bioinformatics
2.7. Calprotectin ELISA and Lactoferrin ELISA
2.8. Blood Samples
2.9. Multiplex ELISA
2.10. Gastrointestinal Symptom Rating Scale for Irritable Bowel Syndrome Questionnaire
2.11. Statistical Analysis
3. Results
3.1. Participant Demographics and Compliance
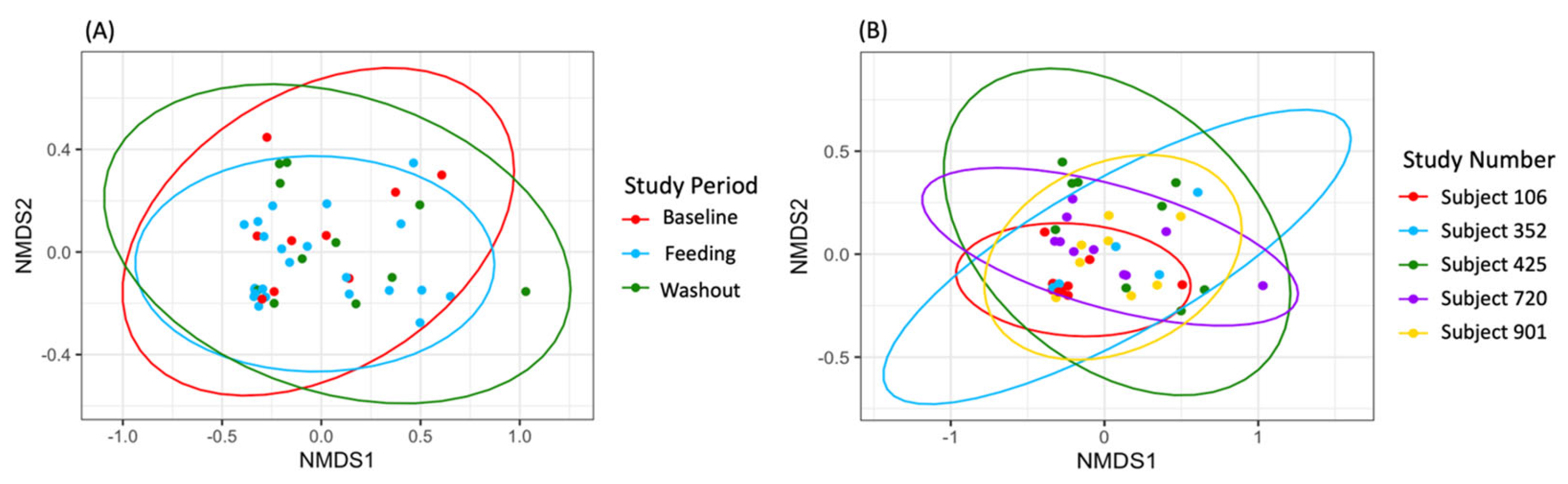
3.2. Microbial Diversity
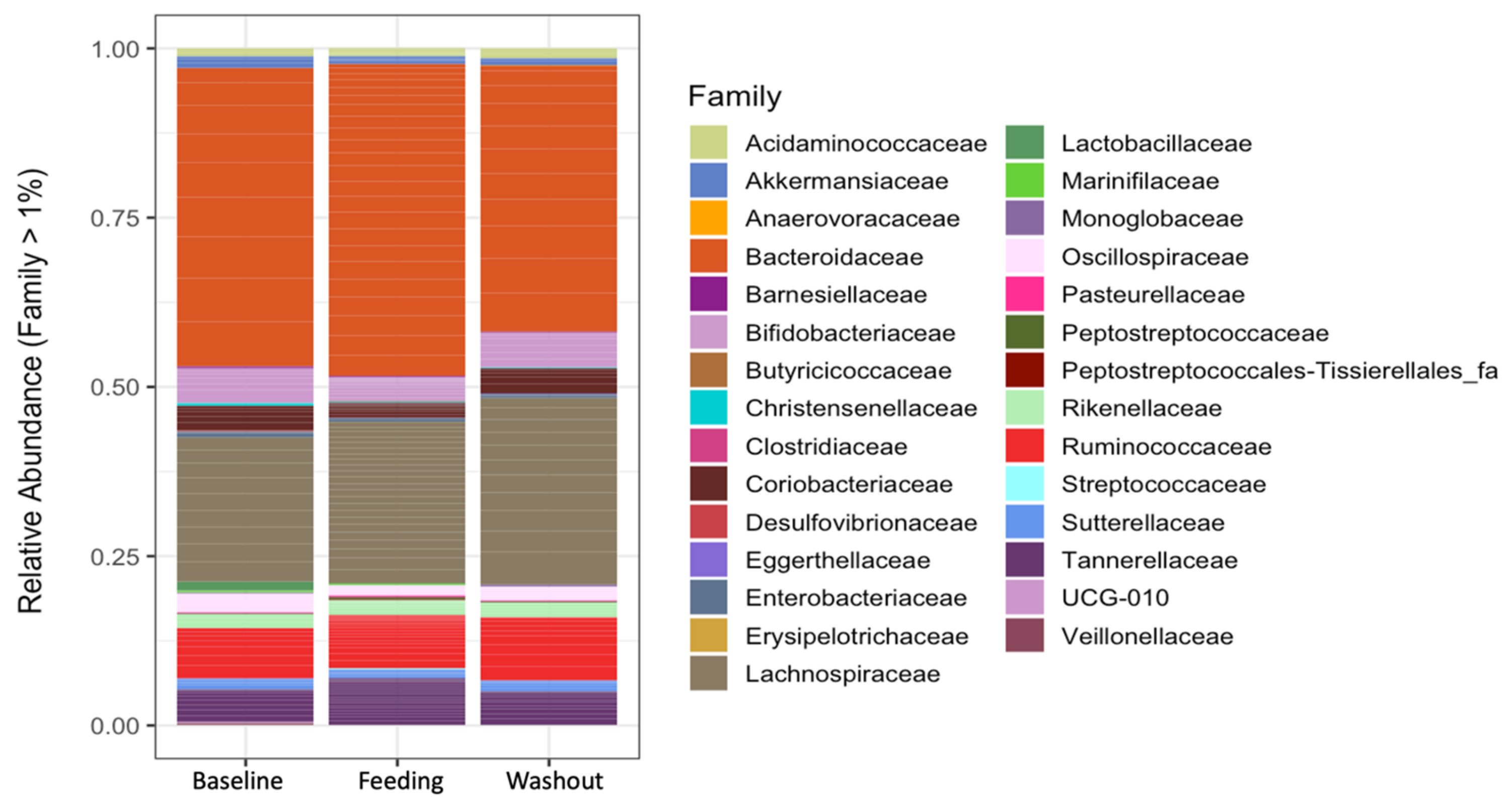
3.3. Taxonomic Analysis
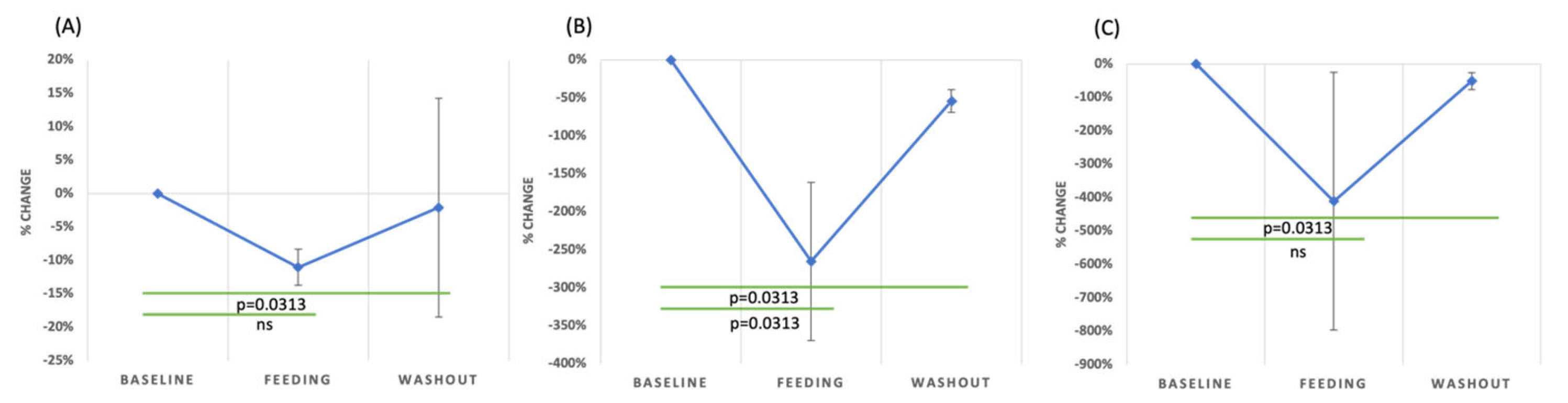
3.4. Inflammatory Markers
3.5. GSRS-IBS Questionnaire Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choung, R.S.; Locke, G.R. Epidemiology of IBS. Gastroenterol. Clin. North Am. 2011, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable Bowel Syndrome. Nat. Rev. Dis. Primers 2016, 2, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global Prevalence of Irritable Bowel Syndrome According to Rome III or IV Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Almario, C.V.; Sharabi, E.; Chey, W.D.; Lauzon, M.; Higgins, C.S.; Spiegel, B.M.R. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results from a Nationwide Cross-Sectional Study. Gastroenterology 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The Kinetics of Gut Microbial Community Composition in Patients with Irritable Bowel Syndrome Following Fecal Microbiota Transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef] [PubMed]
- Saha, L. Irritable Bowel Syndrome: Pathogenesis, Diagnosis, Treatment, and Evidence-Based Medicine. World J. Gastroenterol. WJG 2014, 20, 6759. [Google Scholar] [CrossRef]
- Gazouli, M.; Wouters, M.M.; Kapur-Pojskić, L.; Bengtson, M.B.; Friedman, E.; Nikčević, G.; Demetriou, C.A.; Mulak, A.; Santos, J.; Niesler, B. Lessons Learned—Resolving the Enigma of Genetic Factors in IBS. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 77–87. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable Bowel Syndrome: A Disease Still Searching for Pathogenesis, Diagnosis and Therapy. World J. Gastroenterol. WJG 2014, 20, 8807. [Google Scholar]
- Florea, R.; Linnstaedt, S.D.; Géranton, S.M. Editorial: Mechanisms Underlying the Interactions between Stress and Pain. Front. Pain Res. 2023, 4, 1285257. [Google Scholar] [CrossRef]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut Dysbiosis and Irritable Bowel Syndrome: The Potential Role of Probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef]
- Zhou, G.Q.; Huang, M.J.; Yu, X.; Zhang, N.N.; Tao, S.; Zhang, M. Early Life Adverse Exposures in Irritable Bowel Syndrome: New Insights and Opportunities. Front. Pediatr. 2023, 11, 1241801. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Lo, B.; Cold, F.; Christensen, A.H.; Holster, S.; König, J.; Brummer, R.J.; Aroniadis, O.C.; Lahtinen, P.; Holvoet, T.; et al. Fecal Microbiota Transplantation for the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2023, 29, 3185. [Google Scholar] [CrossRef]
- Akiho, H.; Ihara, E.; Nakamura, K. Low-Grade Inflammation Plays a Pivotal Role in Gastrointestinal Dysfunction in Irritable Bowel Syndrome. World J. Gastrointest. Pathophysiol. 2010, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, V.S.; Chen, W.; Shu, D.; Paulus, B.; Bethwaite, P.; Tie, A.; Wilson, I. Activation of the Mucosal Immune System in Irritable Bowel Syndrome. Gastroenterology 2002, 122, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Choghakhori, R.; Abbasnezhad, A.; Hasanvand, A.; Amani, R. Inflammatory Cytokines and Oxidative Stress Biomarkers in Irritable Bowel Syndrome: Association with Digestive Symptoms and Quality of Life. Cytokine 2017, 93, 34–43. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jeong, S.J. Is Fecal Calprotectin Always Normal in Children with Irritable Bowel Syndrome? Intest. Res. 2019, 17, 546–553. [Google Scholar] [CrossRef]
- Hasler, W.L.; Grabauskas, G.; Singh, P.; Owyang, C. Mast Cell Mediation of Visceral Sensation and Permeability in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2022, 34, e14339. [Google Scholar] [CrossRef]
- Vara, E.J.; Brokstad, K.A.; Hausken, T.; Lied, G.A. Altered Levels of Cytokines in Patients with Irritable Bowel Syndrome Are Not Correlated with Fatigue. Int. J. Gen. Med. 2018, 11, 285–291. [Google Scholar] [CrossRef]
- Bashashati, M.; Moradi, M.; Sarosiek, I. Interleukin-6 in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of IL-6 (-G174C) and Circulating IL-6 Levels. Cytokine 2017, 99, 132–138. [Google Scholar] [CrossRef]
- Patel, S.; Singh, A.; Misra, V.; Misra, S.P.; Dwivedi, M.; Trivedi, P. Levels of Interleukins 2, 6, 8, and 10 in Patients with Irritable Bowel Syndrome. Indian J. Pathol. Microbiol. 2017, 60, 385. [Google Scholar] [CrossRef]
- Furlanetti, A.M.; Prata, L.F. Free and Total GMP (Glycomacropeptide) Contents of Milk during Bovine Lactation. Food Sci. Technol. 2003, 23, 121–125. [Google Scholar] [CrossRef]
- Yvon, M.; Beucher, S.; Guilloteau, P.; Le Huerou-Luron, I.; Corring, T. Effects of Caseinomacropeptide (CMP) on Digestion Regulation. Reprod. Nutr. Dev. 1994, 34, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Kim, B.-J.; Koh, J.; Dallas, D.C. Comparison of Solid-Phase Extraction Sorbents for Monitoring the In Vivo Intestinal Survival and Digestion of Kappa-Casein-Derived Caseinomacropeptide. Foods 2023, 12, 299. [Google Scholar] [CrossRef]
- Eigel, W.N.; Butler, J.E.; Ernstrom, C.A.; Farrell, H.M., Jr.; Harwalkar, V.R.; Jenness, R.; Whitney, R.M. Nomenclature of Proteins of Cow’s Milk: Fifth Revision. J. Dairy Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Qu, Y.; Kim, B.J.; Koh, J.; Dallas, D.C. Analysis of Bovine Kappa-Casein Glycomacropeptide by Liquid Chromatography–Tandem Mass Spectrometry. Foods 2021, 10, 2028. [Google Scholar] [CrossRef]
- Koh, J.; Kim, B.J.; Qu, Y.; Dallas, D.C. Mass Spectral Profiling of Caseinomacropeptide Extracted from Feeding Material and Jejunal Fluid Using Three Methods-Ethanol Precipitation, Perchloric Acid Precipitation, and Ultrafiltration. Food Chem. 2023, 398, 133864. [Google Scholar] [CrossRef]
- Koh, J.; Kim, B.J.; Qu, Y.; Huang, H.; Dallas, D.C. Top-Down Glycopeptidomics Reveals Intact Glycomacropeptide Is Digested to a Wide Array of Peptides in Human Jejunum. J. Nutr. 2022, 152, 429–438. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, S.; Dong, L.; Liu, Y.; Pan, D.; Wu, Z.; Wu, Z.; Zhou, Y.; Ren, Y.; Zhang, J.; et al. Characterization and Determination of Casein Glycomacropeptide in Dairy Products by UHPLC–MS/MS Based on Its Characteristic Peptide. Food Chem. 2024, 430, 137049. [Google Scholar] [CrossRef]
- Brück, W.M.; Graverholt, G.; Gibson, G.R. A Two-Stage Continuous Culture System to Study the Effect of Supplemental Alpha-Lactalbumin and Glycomacropeptide on Mixed Cultures of Human Gut Bacteria Challenged with Enteropathogenic Escherichia coli and Salmonella Serotype Typhimurium. J. Appl. Microbiol. 2003, 95, 44–53. [Google Scholar] [CrossRef]
- Feeney, S.; Ryan, J.T.; Kilcoyne, M.; Joshi, L.; Hickey, R. Glycomacropeptide Reduces Intestinal Epithelial Cell Barrier Dysfunction and Adhesion of Entero-Hemorrhagic and Entero-Pathogenic Escherichia coli in Vitro. Foods 2017, 6, 93. [Google Scholar] [CrossRef]
- Gustavo Hermes, R.; Molist, F.; Francisco Pérez, J.; De Segura, A.G.; Ywazaki, M.; Davin, R.; Nofrarías, M.; Korhonen, T.K.; Virkola, R.; Martín-Orúe, S.M. Casein Glycomacropeptide in the Diet May Reduce Escherichia coli Attachment to the Intestinal Mucosa and Increase the Intestinal Lactobacilli of Early Weaned Piglets after an Enterotoxigenic E. coli K88 Challenge. Br. J. Nutr. 2013, 109, 1001–1012. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Isoda, H.; Tanimoto, M.; Dosako, S.; Idota, T.; Ahiko, K. Inhibition by Lactoferrin and κ-Casein Glycomacropeptide of Binding of Cholera Toxin to Its Receptor. Biosci. Biotechnol. Biochem. 1992, 56, 195–198. [Google Scholar] [CrossRef]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of Intestinal Infection by Glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.R.; Gibson, G.R.; Formentin, K.; Beer, M.; Greenberg, N.; Rastall, R.A. Caseinoglycomacropeptide Inhibits Adhesion of Pathogenic Escherichia coli Strains to Human Cells in Culture. J. Dairy Sci. 2005, 88, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Althnaibat, R.M.; Koch, M.; Bruce, H.L.; Wefers, D.; Gänzle, M.G. Glycomacropeptide from Camel Milk Inhibits the Adhesion of Enterotoxigenic Escherichia coli K88 to Porcine Cells. Int. Dairy J. 2022, 134, 105448. [Google Scholar] [CrossRef]
- Azuma, N.; Yamauchi, K.; Mitsuoka, T. Bifidus Growth-Promoting Activity of a Glycomacropeptide Derived from Human K-Casein. Agric. Biol. Chem. 1984, 48, 2159–2162. [Google Scholar] [CrossRef]
- Bomba, A.; Nemcová, R.; Kastel, R.; Herich, R.; Pataky, J.; Cízek, M. Interactions of Lactobacillus spp. and Enteropathogenic Escherichia coli under in Vitro and in Vivo Conditions. Vet. Med. 1996, 41, 155–158. [Google Scholar]
- Crociani, J.; Grill, J.P.; Huppert, M.; Ballongue, J. Adhesion of Different Bifidobacteria Strains to Human Enterocyte-like Caco-2 Cells and Comparison with in Vivo Study. Lett. Appl. Microbiol. 1995, 21, 146–148. [Google Scholar] [CrossRef]
- Janer, C.; Díaz, J.; Peláez, C.; Requena, T. The effect of caseinomacropeptide and whey protein concentrate on streptococcus mutans adhesion to polystyrene surfaces and cell aggregation. J. Food Qual. 2004, 27, 233–238. [Google Scholar] [CrossRef]
- Robitaille, G. Growth-Promoting Effects of Caseinomacropeptide from Cow and Goat Milk on Probiotics. J. Dairy Res. 2013, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial Whey Products Promote Intestinal Barrier Function with Glycomacropeptide Enhanced Activity in Downregulating Bacterial Endotoxin Lipopolysaccharides (LPS)-Induced Inflammation in Vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef] [PubMed]
- López-Posadas, R.; Requena, P.; González, R.; Suárez, M.D.; Zarzuelo, A.; Sánchez de Medina, F.; Martínez-Augustin, O. Bovine Glycomacropeptide Has Intestinal Antiinflammatory Effects in Rats with Dextran Sulfate-Induced Colitis. J. Nutr. 2010, 140, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, M.; Capitán-Cañadas, F.; Requena, P.; Ocón, B.; Romero-Calvo, I.; Aranda, C.; Suárez, M.D.; Zarzuelo, A.; Sánchez De Medina, F.; Martínez-Augustin, O. Validation of Bovine Glycomacropeptide as an Intestinal Anti-Inflammatory Nutraceutical in the Lymphocyte-Transfer Model of Colitis. Br. J. Nutr. 2014, 111, 1202–1212. [Google Scholar] [CrossRef]
- Requena, P.; González, R.; López-Posadas, R.; Abadía-Molina, A.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. The Intestinal Antiinflammatory Agent Glycomacropeptide Has Immunomodulatory Actions on Rat Splenocytes. Biochem. Pharmacol. 2010, 79, 1797–1804. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Fiori, L.; Li, J.; Fanzaga, M.; d’Adduzio, L.; Tosi, M.; Burlina, A.; Zuccotti, G.; Verduci, E. Glycomacropeptide (GMP) Rescued the Oxidative and Inflammatory Activity of Free L-AAs in Human Caco-2 Cells: New Insights That Support GMP as a Valid and Health-Promoting Product for the Dietary Management of Phenylketonuria (PKU) Patients. Food Res. Int. 2023, 173, 113258. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dige, A.; Bendix, M.; Wernlund, P.G.; Christensen, L.A.; Dahlerup, J.F.; Agnholt, J. Casein Glycomacropeptide for Active Distal Ulcerative Colitis: A Randomized Pilot Study. Eur. J. Clin. Invest. 2016, 46, 555–563. [Google Scholar] [CrossRef]
- Wernlund, P.G.; Hvas, C.L.; Dahlerup, J.F.; Bahl, M.I.; Licht, T.R.; Knudsen, K.E.B.; Agnholt, J.S. Casein Glycomacropeptide Is Well Tolerated in Healthy Adults and Changes Neither High-Sensitive C-Reactive Protein, Gut Microbiota nor Faecal Butyrate: A Restricted Randomised Trial. Br. J. Nutr. 2021, 125, 1374–1385. [Google Scholar] [CrossRef]
- Montanari, C.; Ceccarani, C.; Corsello, A.; Zuvadelli, J.; Ottaviano, E.; Dei Cas, M.; Banderali, G.; Zuccotti, G.; Borghi, E.; Verduci, E. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef]
- Hansen, K.E.; Murali, S.; Chaves, I.Z.; Suen, G.; Ney, D.M. Glycomacropeptide Impacts Amylin-Mediated Satiety, Postprandial Markers of Glucose Homeostasis, and the Fecal Microbiome in Obese Postmenopausal Women. J. Nutr. 2023, 153, 1915–1929. [Google Scholar] [CrossRef]
- Qu, Y.; Park, S.H.; Dallas, D.C. The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome. Nutrients 2023, 15, 3991. [Google Scholar] [CrossRef]
- Wiklund, I.K.; Fullerton, S.; Hawkey, C.J.; Jones, R.H.; Longstreth, G.F.; Mayer, E.A.; Peacock, R.A.; Wilson, I.K.; Naesdal, J. An Irritable Bowel Syndrome-Specific Symptom Questionnaire: Development and Validation. Scand. J. Gastroenterol. 2003, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the Miseq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, L.; Mu, Z.; Liu, L.; Yang, J.; Wu, Z.; Pan, D.; Liu, L. Research Advances of Lactoferrin in Electrostatic Spinning, Nano Self-Assembly, and Immune and Gut Microbiota Regulation. J. Agric. Food Chem. 2022, 70, 10075–10089. [Google Scholar] [CrossRef]
- Simioni, J.; Skare, T.L.; Campos, A.P.B.; Kotze, L.; Messias-Reason, I.; Ioshii, S.O.; Nisihara, R. Fecal Calprotectin, Gut Inflammation and Spondyloarthritis. Arch. Med. Res. 2019, 50, 41–46. [Google Scholar] [CrossRef]
- Klingberg, E.; Magnusson, M.K.; Strid, H.; Deminger, A.; Ståhl, A.; Sundin, J.; Simrén, M.; Carlsten, H.; Öhman, L.; Forsblad-D’Elia, H. A Distinct Gut Microbiota Composition in Patients with Ankylosing Spondylitis Is Associated with Increased Levels of Fecal Calprotectin. Arthritis Res. Ther. 2019, 21, 248. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From Biomarker to Biological Function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Bermejo, F.; Pérez-Calle, J.L.; Taxonera, C.; Vera, I.; McNicholl, A.G.; Algaba, A.; López, P.; López-Palacios, N.; Calvo, M.; et al. Fecal Calprotectin and Lactoferrin for the Prediction of Inflammatory Bowel Disease Relapse. Inflamm. Bowel Dis. 2009, 15, 1190–1198. [Google Scholar] [CrossRef]
- Gisbert, J.P.; McNicholl, A.G.; Gomollon, F. Questions and Answers on the Role of Fecal Lactoferrin as a Biological Marker in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 1746–1754. [Google Scholar] [CrossRef]
- Kane, S. Fecal Lactoferrin Is a Sensitive and Specific Marker in Identifying Intestinal Inflammation. Am. J. Gastroenterol. 2003, 98, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Mansfield, J.C. Measurement of Faecal Calprotectin and Lactoferrin in Inflammatory Bowel Disease. Frontline Gastroenterol. 2011, 2, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, D.A. Interleukin 15: Its Role in Intestinal Inflammation. Gut 2006, 55, 444–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Geboes, K.; Colpaert, S.; D’Haens, G.R.; Rutgeerts, P.; Ceuppens, J.L. IL-15 Is Highly Expressed in Inflammatory Bowel Disease and Regulates Local T Cell-Dependent Cytokine Production. J. Immunol. 2000, 164, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Kim, S.M.; Lejeune, T.; Palanski, B.A.; Ernest, J.D.; Tastet, O.; Voisine, J.; Discepolo, V.; Marietta, E.V.; Hawash, M.B.F.; et al. IL-15, Gluten and HLA-DQ8 Drive Tissue Destruction in Coeliac Disease. Nature 2020, 578, 600–604. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Polster, A.; Törnblom, H.; Isaksson, S.; Capronnier, S.; Tessier, A.; Le Nevé, B.; Simrén, M.; Öhman, L. Global Cytokine Profiles and Association With Clinical Characteristics in Patients With Irritable Bowel Syndrome. Am. J. Gastroenterol. 2016, 111, 1165–1176. [Google Scholar] [CrossRef]
- Chang, L. The Role of Stress on Physiologic Responses and Clinical Symptoms in Irritable Bowel Syndrome. Gastroenterology 2011, 140, 761–765. [Google Scholar] [CrossRef]
- Gonsalkorale, W.M.; Miller, V.; Afzal, A.; Whorwell, P.J. Long Term Benefits of Hypnotherapy for Irritable Bowel Syndrome. Gut 2003, 52, 1623–1629. [Google Scholar] [CrossRef]
- Darkoh, C.; Comer, L.; Zewdie, G.; Harold, S.; Snyder, N.; DuPont, H.L. Chemotactic Chemokines Are Important in the Pathogenesis of Irritable Bowel Syndrome. PLoS ONE 2014, 9, e93144. [Google Scholar] [CrossRef]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-García, D.; Jiménez, M.; Ventura-Juárez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef] [PubMed]
- Melchior, C.; Aziz, M.; Aubry, T.; Gourcerol, G.; Quillard, M.; Zalar, A.; Coëffier, M.; Dechelotte, P.; Leroi, A.-M.; Ducrotté, P. Does Calprotectin Level Identify a Subgroup among Patients Suffering from Irritable Bowel Syndrome? Results of a Prospective Study. United Eur. Gastroenterol. J. 2017, 5, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Chou, J.W.; Chen, S.M.; Tsai, M.C.; Sun, Y.S.; Lin, C.C.; Lin, C.P. Faecal Calprotectin as a Novel Biomarker for Differentiating between Inflammatory Bowel Disease and Irritable Bowel Syndrome. Mol. Med. Rep. 2014, 10, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Manz, M.; Burri, E.; Rothen, C.; Tchanguizi, N.; Niederberger, C.; Rossi, L.; Beglinger, C.; Lehmann, F.S. Value of Fecal Calprotectin in the Evaluation of Patients with Abdominal Discomfort: An Observational Study. BMC Gastroenterol. 2012, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Jin, C.X.; Ko, S.B.H.; Kitagawa, M.; Ishiguro, H. Lactoferrin in Gastrointestinal Disease. Intern. Med. 2009, 48, 1251–1254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sidhu, R.; Mcalindon, M.E.; Wilson, P.; Wright, A.; Yau, C.W.; A D’cruz, F.; Lobo, A.J.; Morley, S.; Foye, L. Faecal Lactoferrin-A Novel Test to Differentiate between the Irritable and Inflamed Bowel? Aliment. Pharmacol. Ther. 2010, 31, 1365–1370. [Google Scholar] [CrossRef]
- Abraham, B.P. Fecal Lactoferrin Testing. Gastroenterol. Hepatol. 2018, 14, 713. [Google Scholar]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Park, S.H.; Dallas, D.C. Evaluating the Potential of Casein Glycomacropeptide in Adult Irritable Bowel Syndrome Management: A Pilot Study. Nutrients 2023, 15, 4174. https://doi.org/10.3390/nu15194174
Qu Y, Park SH, Dallas DC. Evaluating the Potential of Casein Glycomacropeptide in Adult Irritable Bowel Syndrome Management: A Pilot Study. Nutrients. 2023; 15(19):4174. https://doi.org/10.3390/nu15194174
Chicago/Turabian StyleQu, Yunyao, Si Hong Park, and David C. Dallas. 2023. "Evaluating the Potential of Casein Glycomacropeptide in Adult Irritable Bowel Syndrome Management: A Pilot Study" Nutrients 15, no. 19: 4174. https://doi.org/10.3390/nu15194174
APA StyleQu, Y., Park, S. H., & Dallas, D. C. (2023). Evaluating the Potential of Casein Glycomacropeptide in Adult Irritable Bowel Syndrome Management: A Pilot Study. Nutrients, 15(19), 4174. https://doi.org/10.3390/nu15194174




