Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Type
2.2. Samples
2.3. In Vitro Digestion
2.4. Feeding and Intestinal Sample Collection (In Vivo Digestion)
2.5. Peptide Extraction
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Immunomodulatory Assay
2.9. Quantification of TNF-α and IL-1β by Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistics
3. Results
3.1. Toxicity Assay
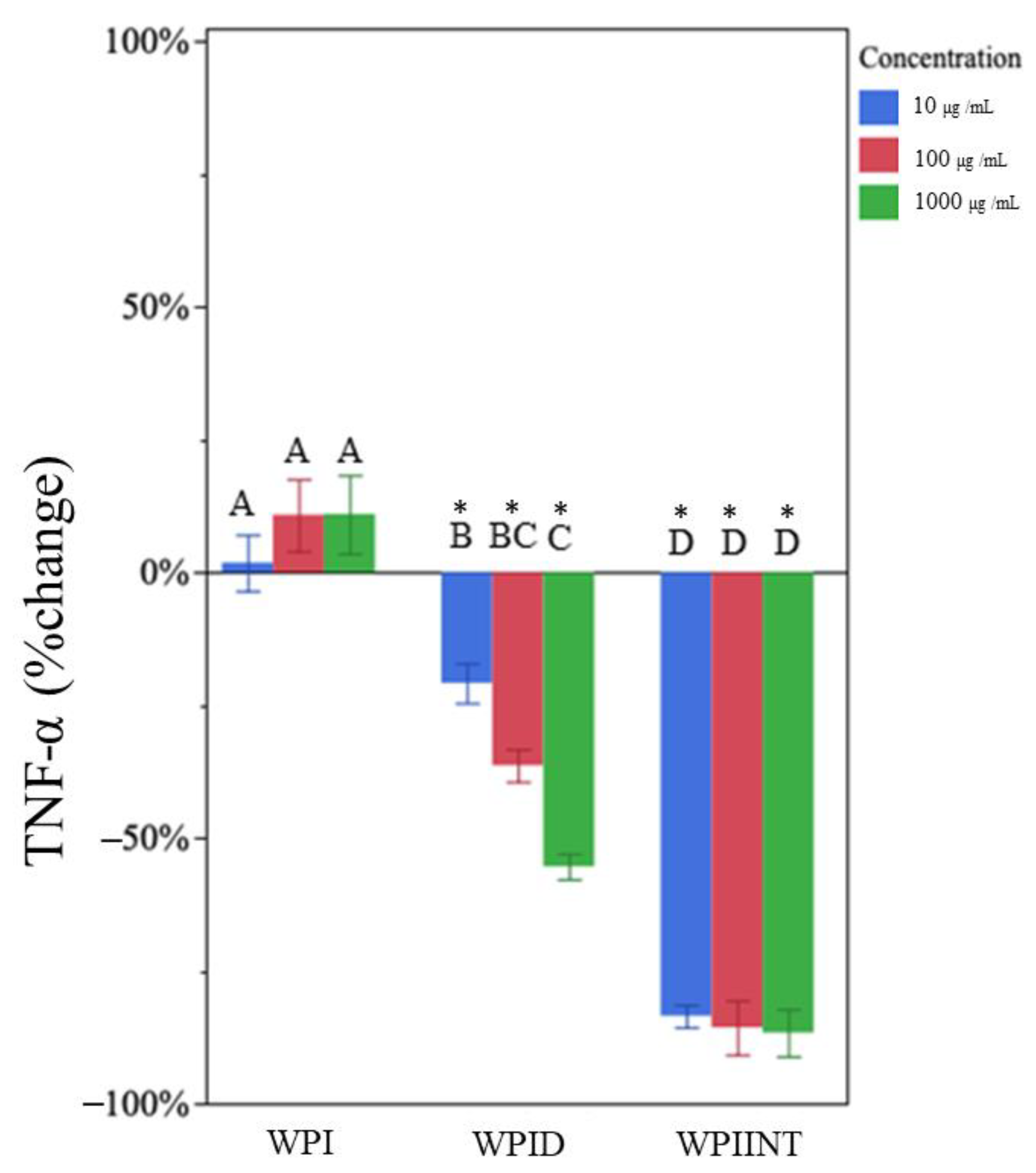
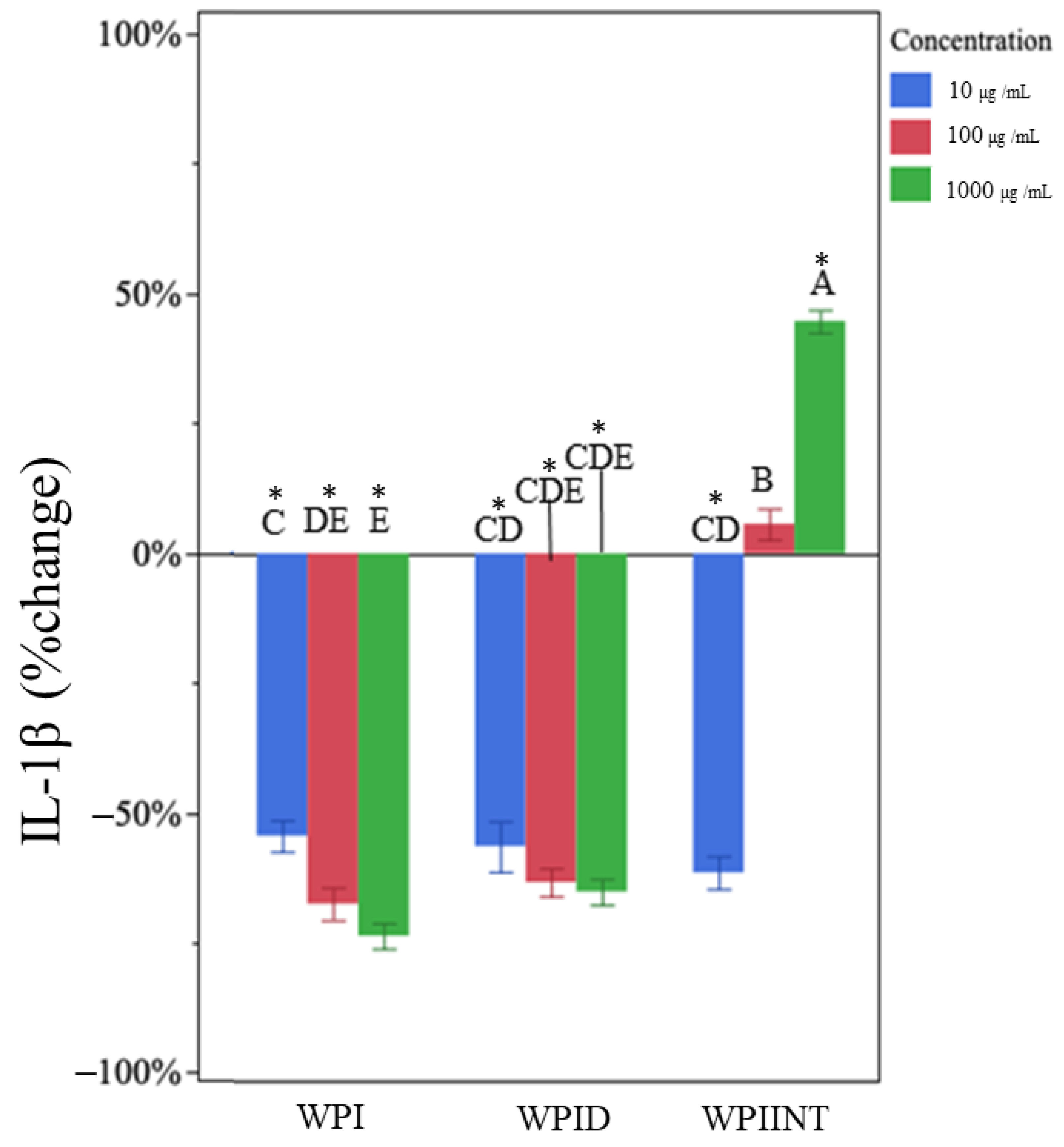
3.2. Undigested WPI Did Not Impact TNF-α but Decreased IL-1β Production
3.3. In Vitro-Digested WPI Decreased TNF-α and IL-1β Production
3.4. Intestinally Digested WPI Decreased TNF-α and Caused Dose-Dependent Changes in IL-1β Production
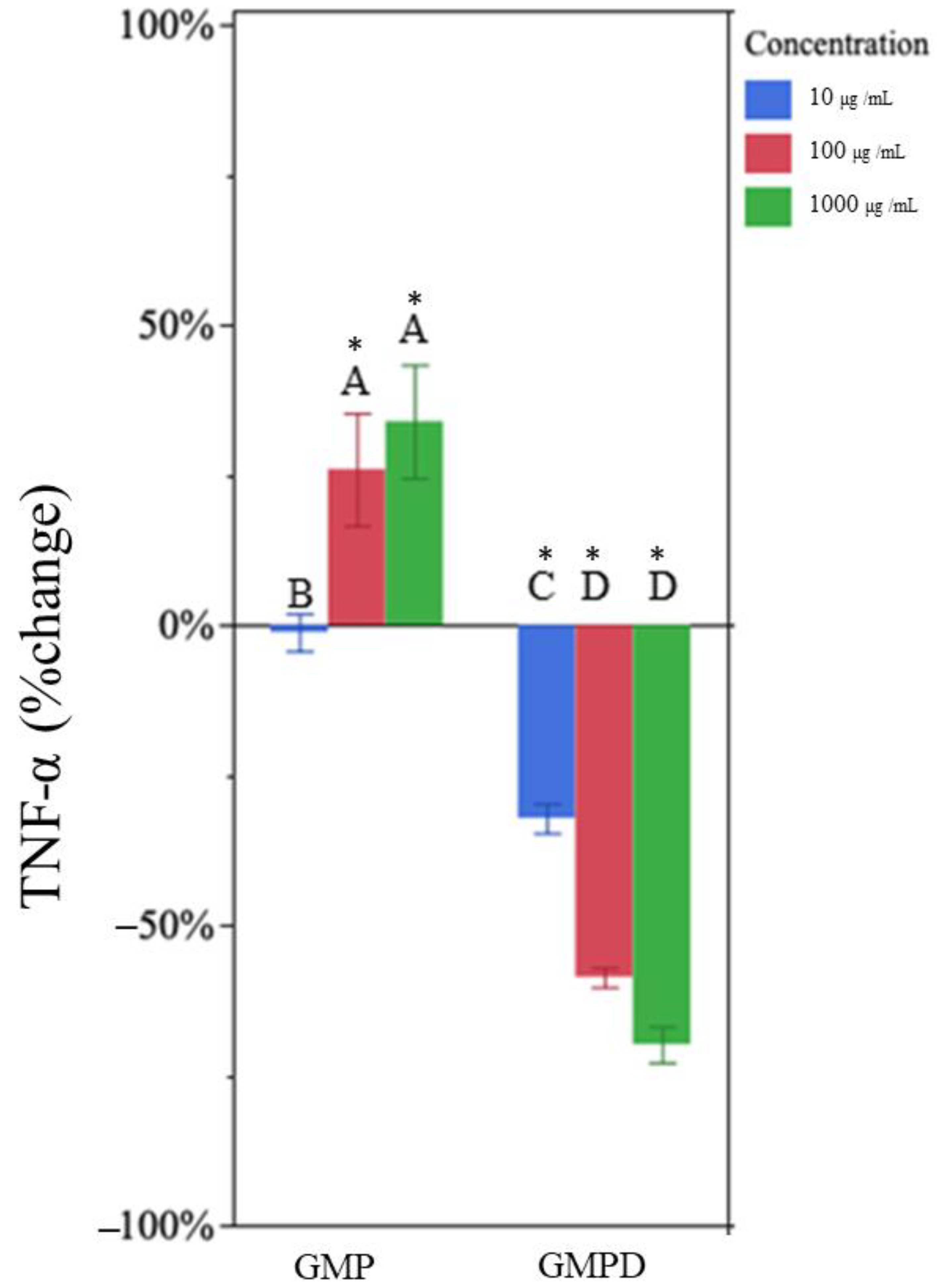
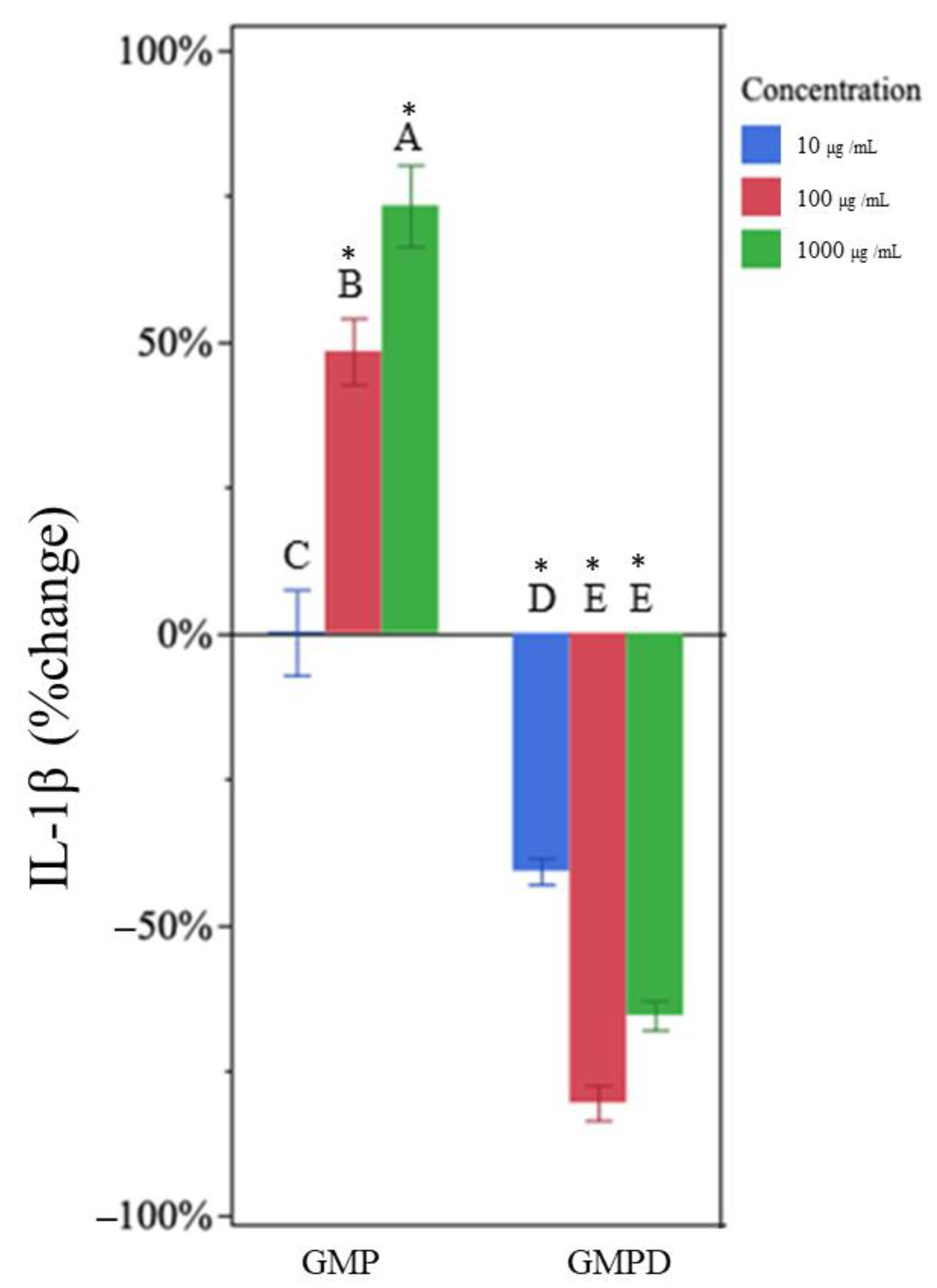
3.5. Undigested GMP Increased TNF-α and IL-1β Production
3.6. In Vitro-Digested GMP Decreased TNF-α and IL-1β Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine Whey Proteins—Overview on Their Main Biological Properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Chen, B.; Gao, P.; Song, B.; Zhang, S.; Pang, X.; Hettinga, K.; Lyu, J. Comparison of Whey Proteome and Glycoproteome in Bovine Colostrum and Mature Milk. J. Agric. Food Chem. 2023, 71, 10863–10876. [Google Scholar] [CrossRef]
- Conneely, O.M. Anti-inflammatory Activities of Lactoferrin. J. Am. Coll. Nutr. 2001, 20, 389S–395S. [Google Scholar] [CrossRef]
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tome, D.; Leonil, J. Sequential Release of Milk Protein–Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk Bioactive Peptide Database: A Comprehensive Database of Milk Protein-Derived Bioactive Peptides and Novel Visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Shi, H.; Yu, L. Isolation and Characterization of Anti-inflammatory Peptides Derived from Whey Protein. J. Dairy Sci. 2016, 99, 6902–6912. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Bigo, C.; Barbier, O.; Rudkowska, I. Whey Protein Hydrolysate and Branched-Chain Amino Acids Downregulate Inflammation-Related Genes in Vascular Endothelial Cells. Nutr. Res. 2017, 38, 43–51. [Google Scholar] [CrossRef]
- Thomä, C.; Krause, I.; Kulozik, U. Precipitation Behaviour of Caseinomacropeptides and their Simultaneous Determination with Whey Proteins by RP-HPLC. Int. Dairy J. 2006, 16, 285–293. [Google Scholar] [CrossRef]
- Chandan, R.C. Cheese|Cheese in the Marketplace. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 384–394. [Google Scholar]
- Ney, D.M.; Hull, A.K.; Van Calcar, S.C.; Liu, X.; Etzel, M.R. Dietary Glycomacropeptide Supports Growth and Reduces the Concentrations of Phenylalanine in Plasma and Brain in a Murine Model of Phenylketonuria. J. Nutr. 2008, 138, 316–322. [Google Scholar] [CrossRef]
- Azuma, N.; Kaminogawa, S.; Yamauchi, K. Properties of Glycomacropeptide and Para-K-casein Derived from Human K-Casein and Comparison of Human and Bovine K-Caseins as to Susceptibility to Chymosin and Pepsin. Agric. Biol. Chem. 1984, 48, 2025–2031. [Google Scholar] [CrossRef]
- Qu, Y.; Kim, B.-J.; Koh, J.; Dallas, D.C. Analysis of Bovine Kappa-Casein Glycomacropeptide by Liquid Chromatography–Tandem Mass Spectrometry. Foods 2021, 10, 2028. [Google Scholar] [CrossRef]
- Kreuß, M.; Strixner, T.; Kulozik, U. The Effect of Glycosylation on the Interfacial Properties of Bovine Caseinomacropeptide. Food Hyrdocoll. 2009, 23, 1818–1826. [Google Scholar] [CrossRef]
- Córdova-Dávalos, L.E.; Jiménez, M.; Salinas, E. Glycomacropeptide Bioactivity and Health: A Review Highlighting Action Mechanisms and Signaling Pathways. Nutrients 2019, 11, 598. [Google Scholar] [CrossRef]
- Otani, H.; Hata, I. Inhibition of Proliferative Responses of Mouse Spleen Lymphocytes and Rabbit Peyer’s Patch Cells by Bovine Milk Caseins and their Digests. J. Dairy Res. 1995, 62, 339–348. [Google Scholar] [CrossRef]
- Qu, Y.; Park, S.H.; Dallas, D.C. The Role of Bovine Kappa-Casein Glycomacropeptide in Modulating the Microbiome and Inflammatory Responses of Irritable Bowel Syndrome. Nutrients 2023, 15, 3991. [Google Scholar] [CrossRef]
- Requena, P.; Daddaoua, A.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; De Medina, F.S.; Martínez-Augustin, O. Bovine Glycomacropeptide Induces Cytokine Production in Human Monocytes Through the Stimulation of the MAPK and the NF-κB Signal Transduction Pathways. Br. J. Pharmacol. 2009, 157, 1232–1240. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, C.; Tu, Z.; Xu, Q.; Chen, S.; Zhang, Y.; Wang, X.; Zhang, J.; Hu, C.-A.A.; Liu, Y. Activation of the NF-κB and MAPK Signaling Pathways Contributes to the Inflammatory Responses, but Not Cell Injury, in IPEC-1 Cells Challenged with Hydrogen Peroxide. Oxid. Med. Cell. Longev. 2020, 2020, 5803639. [Google Scholar] [CrossRef]
- Zimecki, M.; Kruzel, M.L. Milk-derived Proteins and Peptides of Potential therapeutic and Nutritive Value. J. Exp. Ther. Oncol. 2007, 6, 89–106. [Google Scholar]
- Feeney, S.; Ryan, J.T.; Kilcoyne, M.; Joshi, L.; Hickey, R. Glycomacropeptide Reduces Intestinal Epithelial Cell Barrier Dysfunction and Adhesion of Entero-hemorrhagic and Entero-pathogenic Escherichia coli In Vitro. Foods 2017, 6, 93. [Google Scholar] [CrossRef]
- Wernlund, P.G.; Hvas, C.L.; Dahlerup, J.F.; Bahl, M.I.; Licht, T.R.; Knudsen, K.E.B.; Agnholt, J.S. Casein Glycomacropeptide is Well Tolerated in Healthy Adults and Changes Neither High-sensitive C-reactive Protein, Gut Microbiota Nor Faecal Butyrate: A Restricted Randomised Trial. Br. J. Nutr. 2021, 125, 1374–1385. [Google Scholar] [CrossRef]
- Li, E.W.Y. Recovery of Bovine Glycomacropeptide as Nutraceutical and its Enhancement in Human Immunity. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2003. [Google Scholar]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk Derived Bioactive Peptides and their Impact on Human Health—A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Saint-Sauveur, D.; Gauthier, S.F.; Boutin, Y.; Montoni, A. Immunomodulating Properties of a Whey Protein Isolate, its Enzymatic Digest and Peptide Fractions. Int. Dairy J. 2008, 18, 260–270. [Google Scholar] [CrossRef]
- Mercier, A.; Gauthier, S.F.; Fliss, I.l. Immunomodulating Effects of Whey Proteins and their Enzymatic Digests. Int. Dairy J. 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Koh, J.; Kim, B.J.; Qu, Y.; Huang, H.; Dallas, D.C. Top-Down Glycopeptidomics Reveals Intact Glycomacropeptide Is Digested to a Wide Array of Peptides in Human Jejunum. J. Nutr. 2022, 152, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s Disease and Ulcerative Colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory Cytokines in the Pathogenesis of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1756–1767.e1751. [Google Scholar] [CrossRef]
- Noth, R.; Stüber, E.; Häsler, R.; Nikolaus, S.; Kühbacher, T.; Hampe, J.; Bewig, B.; Schreiber, S.; Arlt, A. Anti-TNF-α Antibodies Improve Intestinal Barrier Function in Crohn’s Disease. J. Crohn’s Colitis 2012, 6, 464–469. [Google Scholar] [CrossRef]
- Xu, C.-T.; Meng, S.-Y.; Pan, B.-R. Drug Therapy for Ulcerative Colitis. World J. Gastroenterol. 2004, 10, 2311. [Google Scholar] [CrossRef]
- Pabst, R. The Anatomical Basis for the Immune Function of the Gut. Anat. Embryol. 1987, 176, 135–144. [Google Scholar] [CrossRef]
- Delfini, M.; Stakenborg, N.; Viola, M.F.; Boeckxstaens, G. Macrophages in the Gut: Masters in Multitasking. Immunity 2022, 55, 1530–1548. [Google Scholar] [CrossRef]
- Mann, E.R.; Li, X. Intestinal Antigen-presenting Cells in Mucosal Immune Homeostasis: Crosstalk Between Dendritic Cells, Macrophages and B-cells. World J. Gastroenterol. 2014, 20, 9653. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. Public Libr. Sci. 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.A.; Wassall, H.J.; Hall, L.S.; Cao, H.; Wilson, H.M.; Barker, R.N.; Vickers, M.A. Similarities and Differences in Surface Receptor Expression by THP-1 Monocytes and Differentiated Macrophages Polarized Using Seven Different Conditioning Regimens. Cell. Immunol. 2018, 332, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Qu, Y.; Kim, B.-J.; Koh, J.; Dallas, D.C. Comparison of Solid-Phase Extraction Sorbents for Monitoring the In Vivo Intestinal Survival and Digestion of Kappa-Casein-Derived Caseinomacropeptide. Foods 2023, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Beverly, R.L.; Scottoline, B.P.; Dallas, D.C. Peptides Derived from In Vitro and In Vivo Digestion of Human Milk Are Immunomodulatory in THP-1 Human Macrophages. J. Nutr. 2022, 152, 331–342. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
- Saint-Sauveur, D.; Gauthier, S.F.; Boutin, Y.; Montoni, A.; Fliss, I. Effect of Feeding Whey Peptide Fractions on the Immune Response in Healthy and Escherichia coli Infected Mice. Int. Dairy J. 2009, 19, 537–544. [Google Scholar] [CrossRef]
- Fan, W.Y.; Gong, H.Y.; Qu, Y.H.; Miao, Y.P.; Liu, Z.D. Immunomodulating Effects of the Peptides Derived from Whey Protein. Adv. Mater. Res. 2012, 345, 228–232. [Google Scholar] [CrossRef]
- Ali, A.; Ain, Q.; Saeed, A.; Khalid, W.; Ahmed, M.; Bostani, A. Bio-Molecular Characteristics of Whey Proteins with Relation to Inflammation. In New Advances in the Dairy Industry; IntechOpen: London, UK, 2021. [Google Scholar]
- Jayatilake, S.; Arai, K.; Kumada, N.; Ishida, Y.; Tanaka, I.; Iwatsuki, S.; Ohwada, T.; Ohnishi, M.; Tokuji, Y.; Kinoshita, M. The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice. Foods 2014, 3, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide is a Prebiotic that Reduces Desulfovibrio Bacteria, Increases Cecal Short-Chain Fatty Acids, and is Anti-inflammatory in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.c.; Zarzuelo, A.; Suárez, M.a.D.; Sánchez de Medina, F.n.; Martínez-Augustin, O. Bovine Glycomacropeptide is Anti-inflammatory in Rats with Hapten-Induced Colitis. J. Nutr. 2005, 135, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, M.; Capitán-Cañadas, F.; Requena, P.; Ocón, B.; Romero-Calvo, I.; Aranda, C.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. Validation of Bovine Glycomacropeptide as an Intestinal Anti-inflammatory Nutraceutical in the Lymphocyte-Transfer Model of Colitis. Br. J. Nutr. 2014, 111, 1202–1212. [Google Scholar] [CrossRef]
- Li, E.W.Y.; Mine, Y. Immunoenhancing Effects of Bovine Glycomacropeptide and Its Derivatives on the Proliferative Response and Phagocytic Activities of Human Macrophagelike Cells, U937. J. Agric. Food Chem. 2004, 52, 2704–2708. [Google Scholar] [CrossRef]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of Whey and Soy Protein Supplementation on Inflammatory Cytokines in Older Adults: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Park, S.H.; Dallas, D.C. Evaluating the Potential of Casein Glycomacropeptide in Adult Irritable Bowel Syndrome Management: A Pilot Study. Nutrients 2023, 15, 4174. [Google Scholar] [CrossRef] [PubMed]
| Treatment Outcomes | |||||
|---|---|---|---|---|---|
| Percentage Difference | |||||
| Treatment (µg/mL) | TNF-α | IL-1β | |||
| Avg. Diff. | SD | Avg. Diff. | SD | ||
| WPI a,b | 10 | 1% | 6% | −55% * | 10% |
| 100 | 10% | 10% | −68% * | 6% | |
| 1000 | 10% | 7% | −74% * | 7% | |
| WPID c | 10 | −21% * | 10% | −57% * | 10% |
| 100 | −37% * | 8% | −63% * | 8% | |
| 1000 | −55% * | 7% | −65% * | 6% | |
| GMP d | 10 | −1% | 9% | −1% | 18% |
| 100 | 26% * | 20% | 47% * | 24% | |
| 1000 | 33% * | 17% | 73% * | 32% | |
| GMPD e | 10 | −32% * | 6% | −41% * | 11% |
| 100 | −58% * | 3% | −81% * | 8% | |
| 1000 | −69% * | 7% | −66% * | 6% | |
| WPIINT f | 10 | −84% * | 3% | −62% * | 7% |
| 100 | −85% * | 12% | 6% | 18% | |
| 1000 | −86% * | 11% | 44% * | 21% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsen, W.; Liang, N.; Dallas, D.C. Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests. Nutrients 2023, 15, 4942. https://doi.org/10.3390/nu15234942
Olsen W, Liang N, Dallas DC. Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests. Nutrients. 2023; 15(23):4942. https://doi.org/10.3390/nu15234942
Chicago/Turabian StyleOlsen, Wyatt, Ningjian Liang, and David C. Dallas. 2023. "Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests" Nutrients 15, no. 23: 4942. https://doi.org/10.3390/nu15234942
APA StyleOlsen, W., Liang, N., & Dallas, D. C. (2023). Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests. Nutrients, 15(23), 4942. https://doi.org/10.3390/nu15234942





