Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Migration Assay
2.4. Western Blotting
2.5. Colony Formation
2.6. Cell Viability Assay
2.7. Monocyte Binding Assay
2.8. Human Macrophage Conditioned Medium (HMCM) Collection
2.9. Cell Transfection
2.10. ELISA Assay
2.11. Tumor Xenograft
2.12. Statistics
3. Result
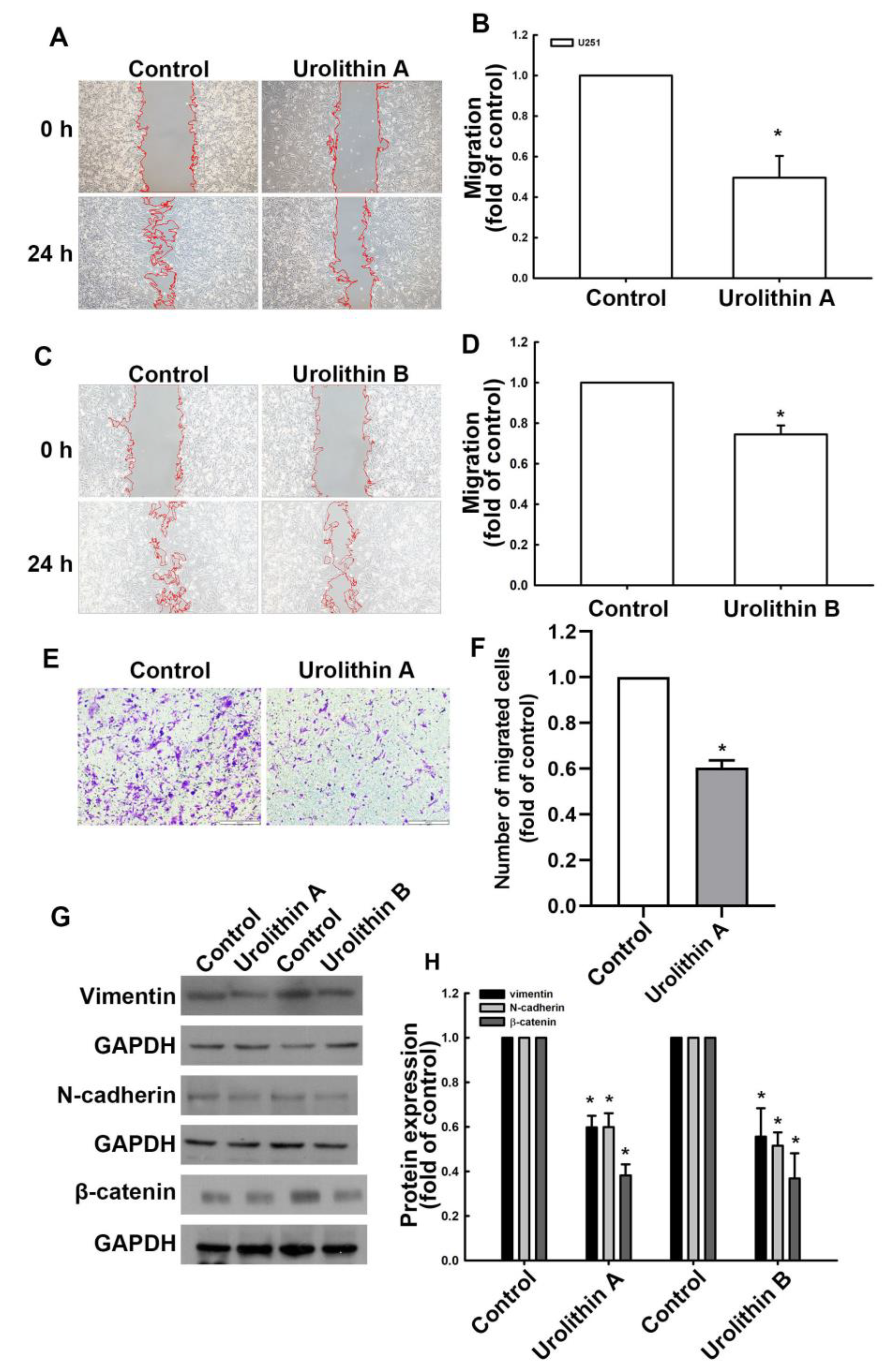
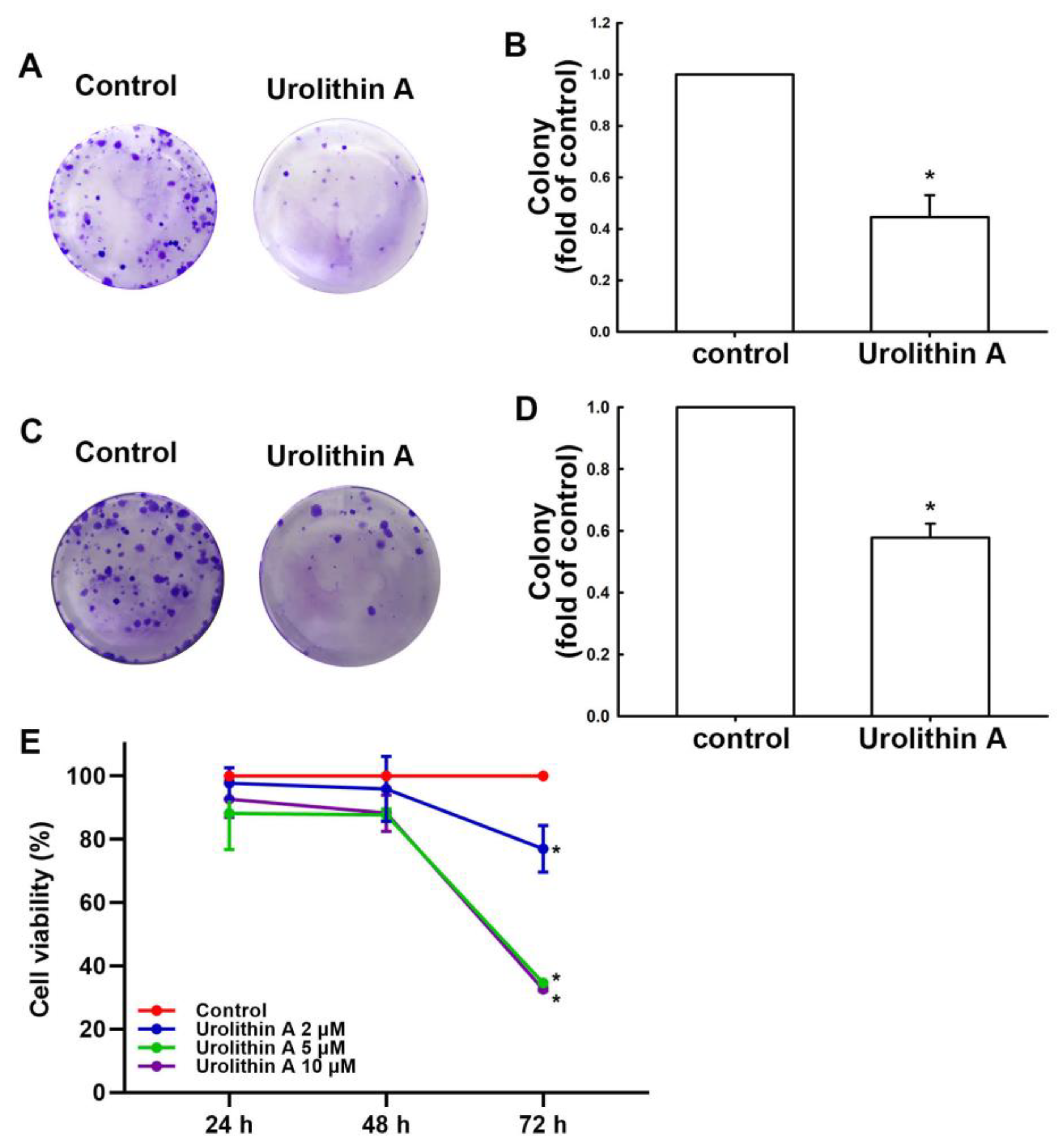
3.1. Urolithins Inhibit Migration Ability and Cell Proliferation in GBM
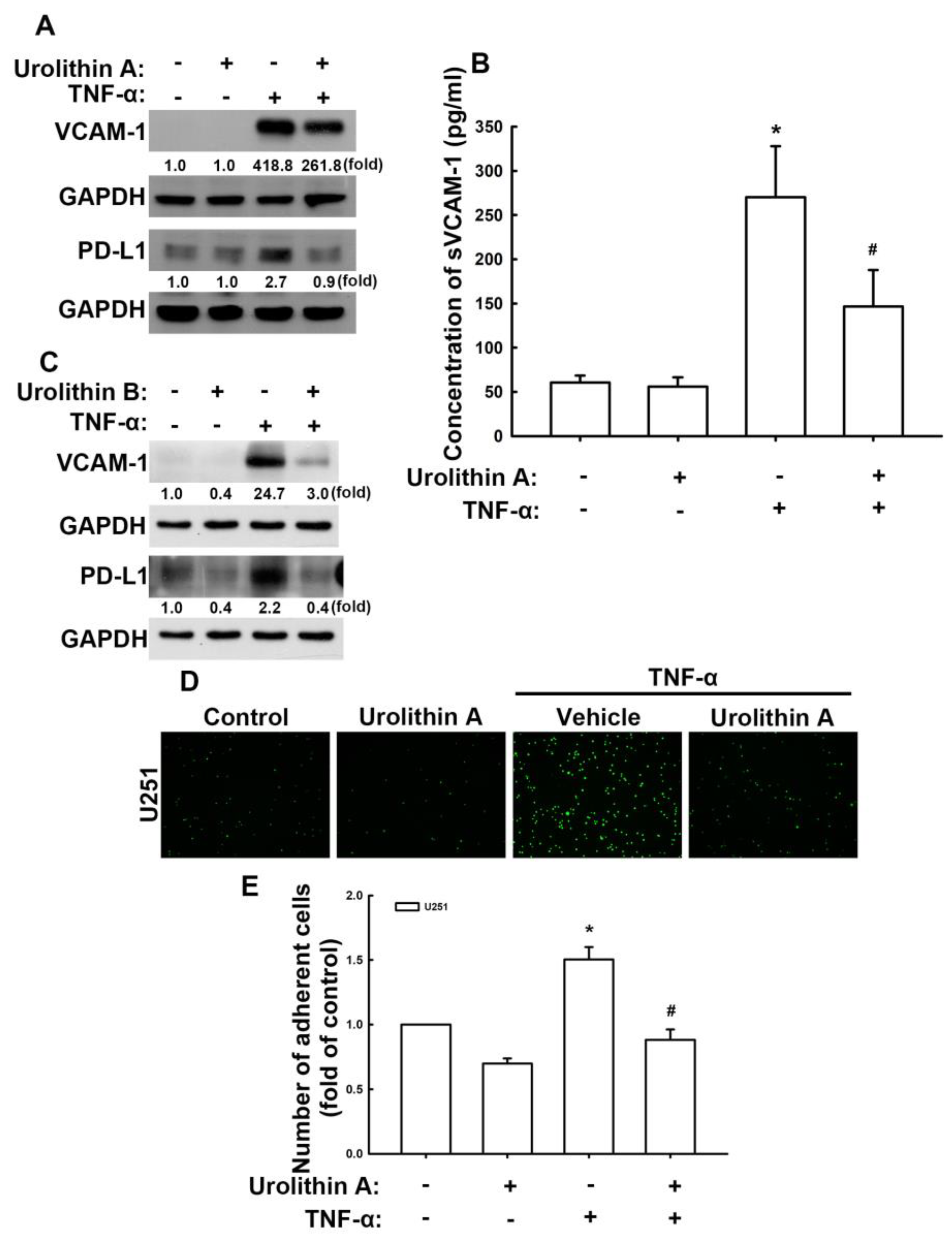
3.2. Urolithins Reduce Expression of VCAM-1 and PD-L1 in GBM Cells
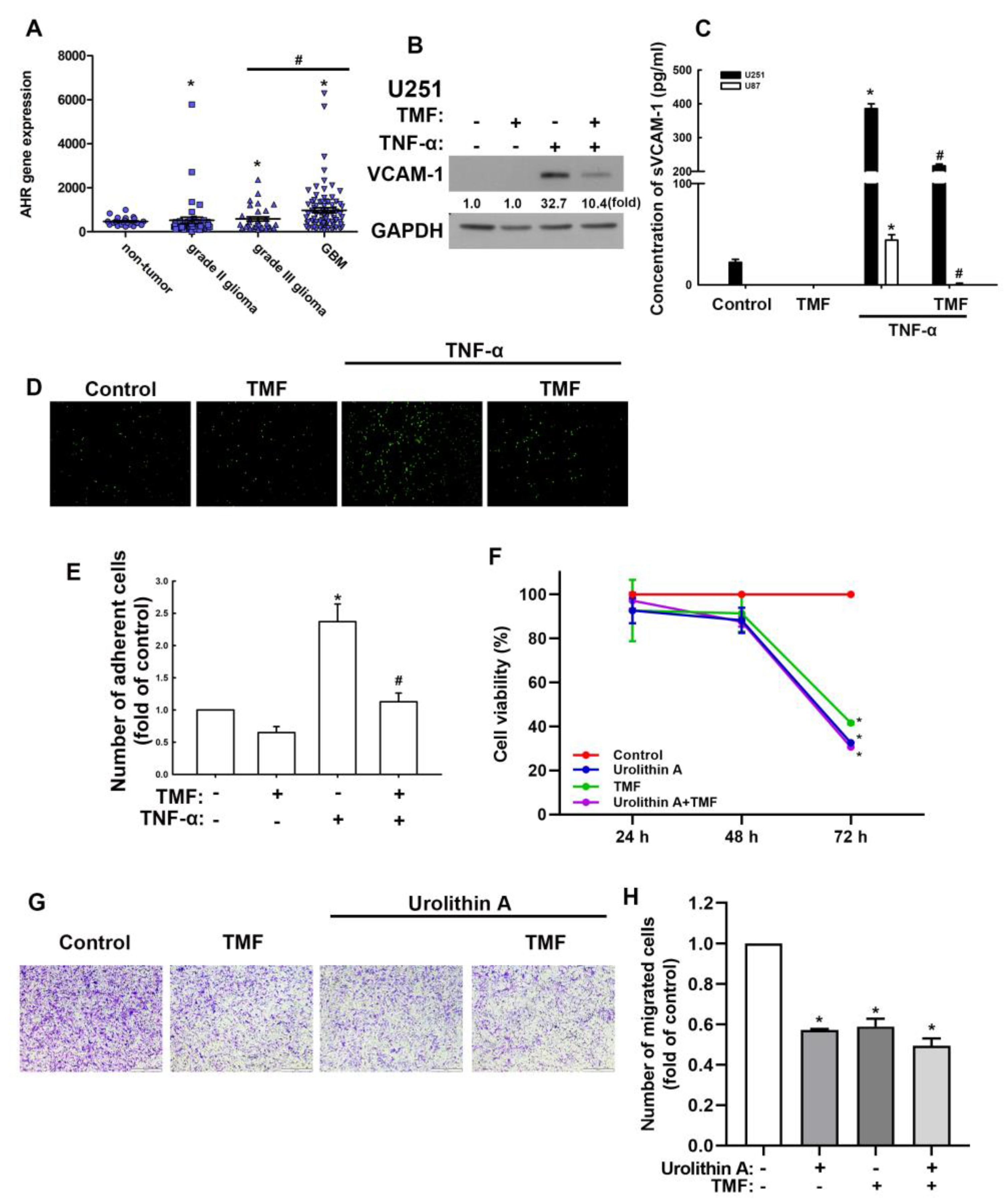
3.3. Involvement of Adhesion Molecules in AhR Regulation of GBM Progression
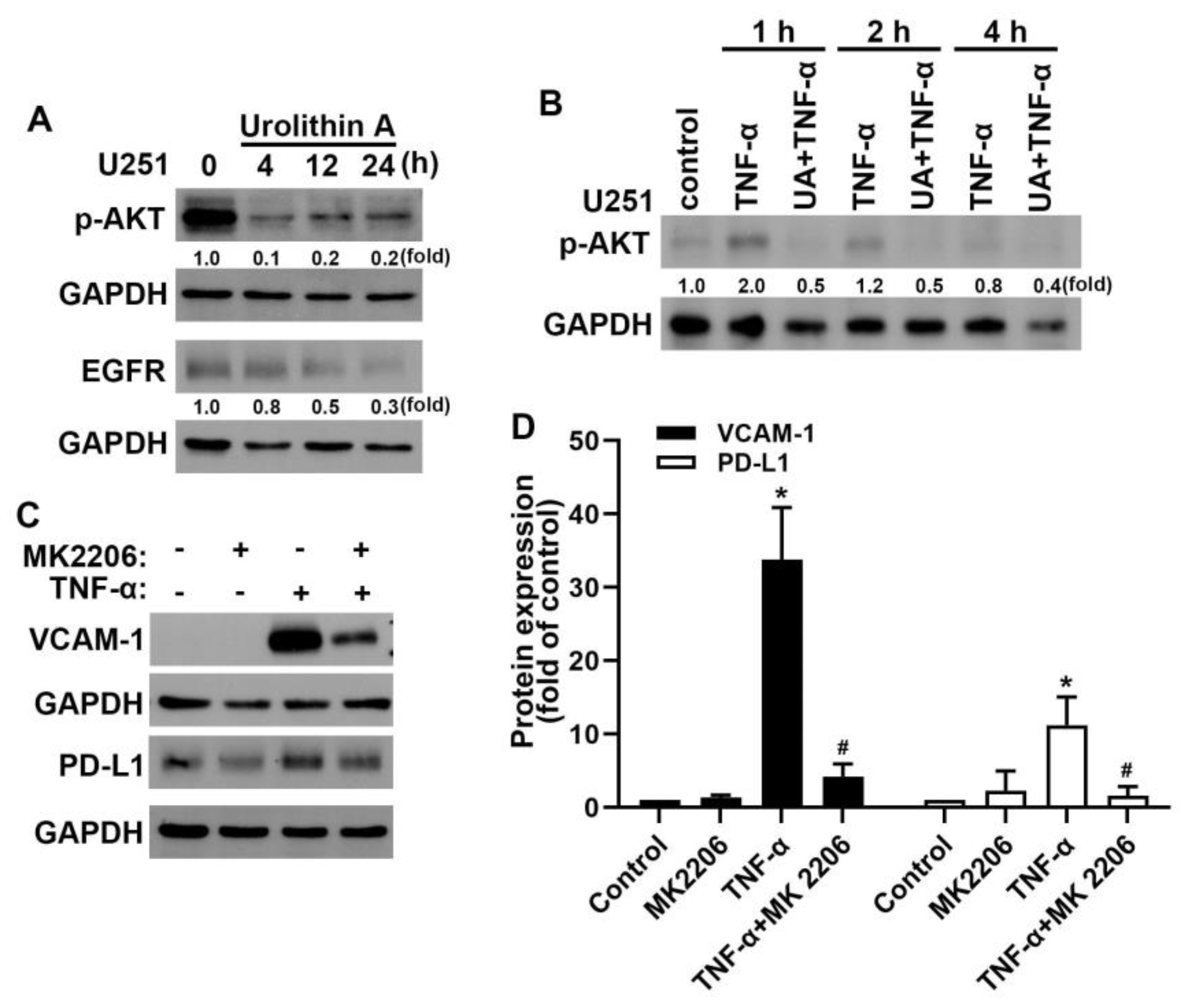
3.4. Akt and Epidermal Growth Factor Receptor Signaling Pathways Are Involved in Urolithin A Inhibition of GBM Progression
3.5. Treatment with Urolithins Suppress the GBM Growth in Xenograft Mouse Model
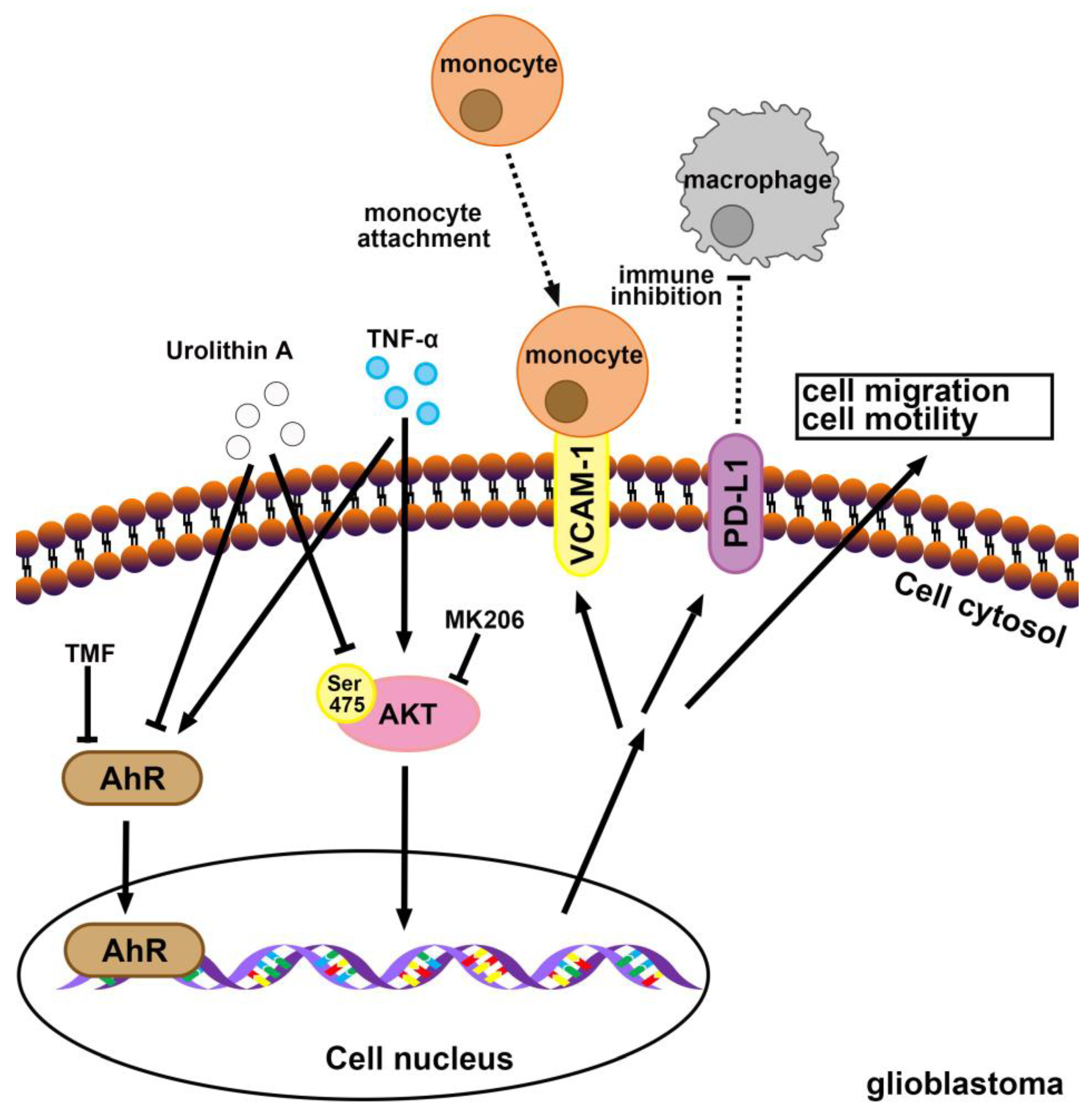
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [PubMed]
- Dapash, M.; Hou, D.; Castro, B.; Lee-Chang, C.; Lesniak, M.S. The Interplay between Glioblastoma and Its Microenvironment. Cells 2021, 10, 2257. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Jackson, E.; Giamas, G. Breaking through the glioblastoma micro-environment via extracellular vesicles. Oncogene 2020, 39, 4477–4490. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Morimura, T.; Neuchrist, C.; Kitz, K.; Budka, H.; Scheiner, O.; Kraft, D.; Lassmann, H. Monocyte subpopulations in human gliomas: Expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathol. 1990, 80, 287–294. [Google Scholar] [CrossRef]
- Antonios, J.P.; Soto, H.; Everson, R.G.; Moughon, D.; Orpilla, J.R.; Shin, N.P.; Sedighim, S.; Treger, J.; Odesa, S.; Tucker, A.; et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro-Oncology 2017, 19, 796–807. [Google Scholar] [CrossRef]
- Bloch, O.; Crane, C.A.; Kaur, R.; Safaee, M.; Rutkowski, M.J.; Parsa, A.T. Gliomas Promote Immunosuppression through Induction of B7-H1 Expression in Tumor-Associated Macrophages. Clin. Cancer Res. 2013, 19, 3165–3175. [Google Scholar] [CrossRef]
- Garber, S.T.; Hashimoto, Y.; Weathers, S.-P.; Xiu, J.; Gatalica, Z.; Verhaak, R.G.W.; Zhou, S.; Fuller, G.N.; Khasraw, M.; de Groot, J.; et al. Immune checkpoint blockade as a potential therapeutic target: Surveying CNS malignancies. Neuro-Oncology 2016, 18, 1357–1366. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxidative Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef]
- Faddladdeen, K.A.; Ojaimi, A.A. Protective Effect of Pomegranate (Punica granatum) Extract against Diabetic Changes in Adult Male Rat Liver: Histological Study. J. Microsc. Ultrastruct. 2019, 7, 165–170. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Dellacqua, L.O.; Delgado, N.T.; Rouver, W.N.; Podratz, P.L.; Lima, L.C.; Piccin, M.P.; Meyrelles, S.S.; Mauad, H.; Graceli, J.B.; et al. Pomegranate peel extract attenuates oxidative stress by decreasing coronary angiotensin-converting enzyme (ACE) activity in hypertensive female rats. J. Toxicol. Environ. Health A 2016, 79, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Hao, W.; Yang, H.; Song, X.; Zhao, M.; Peng, S. Preparative isolation and purification of urolithins from the intestinal metabolites of pomegranate ellagitannins by high-speed counter-current chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 990, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, V.; Zagotto, G.; Pasquale, R.; Moro, S.; Gatto, B. Ellagic acid and polyhydroxylated urolithins are potent catalytic inhibitors of human topoisomerase II: An in vitro study. J. Agric. Food Chem. 2012, 60, 9162–9170. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 647582. [Google Scholar] [CrossRef]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflamm. 2019, 16, 62. [Google Scholar] [CrossRef]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuzniak, V.; Mikolajczak, P.L.; Teissedre, P.L.; et al. Neuroprotective Effects of Pomegranate Juice against Parkinson’s Disease and Presence of Ellagitannins-Derived Metabolite-Urolithin A-In the Brain. Int. J. Mol. Sci. 2019, 21, 202. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Li, Q.; Zhou, B. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates D-Galactose-Induced Brain Aging in Mice. Neurotherapeutics 2019, 16, 1269–1282. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Gut microbial metabolite urolithin B attenuates intestinal immunity function in vivo in aging mice and in vitro in HT29 cells by regulating oxidative stress and inflammatory signalling. Food Funct. 2021, 12, 11938–11955. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Wang, G.; Zhou, B. The Gut Microbiota Metabolite Urolithin B Improves Cognitive Deficits by Inhibiting Cyt C-Mediated Apoptosis and Promoting the Survival of Neurons Through the PI3K Pathway in Aging Mice. Front. Pharmacol. 2021, 12, 768097. [Google Scholar] [CrossRef]
- Norden, E.; Heiss, E.H. Urolithin A gains in antiproliferative capacity by reducing the glycolytic potential via the p53/TIGAR axis in colon cancer cells. Carcinogenesis 2019, 40, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.H.; Aguilera-Barrantes, I.; Shiau, C.W.; Sheng, X.; Wang, L.S.; Stoner, G.D.; Huang, Y.W. Urolithin A suppresses the proliferation of endometrial cancer cells by mediating estrogen receptor-alpha-dependent gene expression. Mol. Nutr. Food Res. 2016, 60, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Totiger, T.M.; Srinivasan, S.; Jala, V.R.; Lamichhane, P.; Dosch, A.R.; Gaidarski, A.A., 3rd; Joshi, C.; Rangappa, S.; Castellanos, J.; Vemula, P.K.; et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zhou, J.; Zhang, C.; Cheng, Y.; Hu, J.; Zheng, G. Antiproliferative effect of urolithin A, the ellagic acid-derived colonic metabolite, on hepatocellular carcinoma HepG2.2.15 cells by targeting Lin28a/let-7a axis. Braz. J. Med. Biol. Res. 2018, 51, e7220. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Gramatzki, D.; Pantazis, G.; Schittenhelm, J.; Tabatabai, G.; Köhle, C.; Wick, W.; Schwarz, M.; Weller, M.; Tritschler, I. Aryl hydrocarbon receptor inhibition downregulates the TGF-β/Smad pathway in human glioblastoma cells. Oncogene 2009, 28, 2593–2605. [Google Scholar] [CrossRef]
- Harper, P.A.; Riddick, D.S.; Okey, A.B. Regulating the regulator: Factors that control levels and activity of the aryl hydrocarbon receptor. Biochem. Pharmacol. 2006, 72, 267–279. [Google Scholar] [CrossRef]
- Casado, F.L.; Singh, K.P.; Gasiewicz, T.A. The aryl hydrocarbon receptor: Regulation of hematopoiesis and involvement in the progression of blood diseases. Blood Cells Mol. Dis. 2010, 44, 199–206. [Google Scholar] [CrossRef]
- Perepechaeva, M.L.; Grishanova, A.Y. The Role of Aryl Hydrocarbon Receptor (AhR) in Brain Tumors. Int. J. Mol. Sci. 2020, 21, 2863. [Google Scholar] [CrossRef]
- Kubli, S.P.; Bassi, C.; Roux, C.; Wakeham, A.; Gobl, C.; Zhou, W.; Jafari, S.M.; Snow, B.; Jones, L.; Palomero, L.; et al. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Muku, G.E.; Murray, I.A.; Espín, J.C.; Perdew, G.H. Urolithin A Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites 2018, 8, 86. [Google Scholar] [CrossRef]
- Ghosh, S.; Moorthy, B.; Haribabu, B.; Jala, V.R. Cytochrome P450 1A1 is essential for the microbial metabolite, Urolithin A-mediated protection against colitis. Front. Immunol. 2022, 13, 1004603. [Google Scholar] [CrossRef]
- Shen, P.X.; Li, X.; Deng, S.Y.; Zhao, L.; Zhang, Y.Y.; Deng, X.; Han, B.; Yu, J.; Li, Y.; Wang, Z.Z.; et al. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine 2021, 64, 103227. [Google Scholar] [CrossRef] [PubMed]
- Rogovskii, V.S. The Therapeutic Potential of Urolithin A for Cancer Treatment and Prevention. Curr. Cancer Drug Targets 2022, 22, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.; Lin, H.J.; Liu, Y.S.; Yang, L.Y.; Lu, D.Y. Monocarboxylate Transporter 4 Regulates Glioblastoma Motility and Monocyte Binding Ability. Cancers 2020, 12, 380. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, Y.C.; Chin, Y.T.; Wang, C.C.; Hwang, L.L.; Yang, L.Y.; Lu, D.Y. 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside protects against neuronal cell death and traumatic brain injury-induced pathophysiology. Aging 2022, 14, 2607–2627. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.K.; Huang, B.R.; Yeh, W.L.; Chen, C.W.; Liu, Y.S.; Lai, S.W.; Tseng, W.P.; Lu, D.Y.; Tsai, C.F. Regulatory effects of IL-1beta in the interaction of GBM and tumor-associated monocyte through VCAM-1 and ICAM-1. Eur. J. Pharmacol. 2021, 905, 174216. [Google Scholar] [CrossRef]
- Setlai, B.P.; Hull, R.; Reis, R.M.; Agbor, C.; Ambele, M.A.; Mulaudzi, T.V.; Dlamini, Z. MicroRNA Interrelated Epithelial Mesenchymal Transition (EMT) in Glioblastoma. Genes 2022, 13, 244. [Google Scholar] [CrossRef]
- Liu, Y.S.; Lin, H.Y.; Lai, S.W.; Huang, C.Y.; Huang, B.R.; Chen, P.Y.; Wei, K.C.; Lu, D.Y. MiR-181b modulates EGFR-dependent VCAM-1 expression and monocyte adhesion in glioblastoma. Oncogene 2017, 36, 5006–5022. [Google Scholar] [CrossRef]
- Liu, Y.S.; Huang, B.R.; Lin, C.J.; Shen, C.K.; Lai, S.W.; Chen, C.W.; Lin, H.J.; Lin, C.H.; Hsieh, Y.C.; Lu, D.Y. Paliperidone Inhibits Glioblastoma Growth in Mouse Brain Tumor Model and Reduces PD-L1 Expression. Cancers 2021, 13, 4357. [Google Scholar] [CrossRef] [PubMed]
- Kelker, H.C.; Oppenheim, J.D.; Stone-Wolff, D.; Henriksen-DeStefano, D.; Aggarwal, B.B.; Stevenson, H.C.; Vilcek, J. Characterization of human tumor necrosis factor produced by peripheral blood monocytes and its separation from lymphotoxin. Int. J. Cancer 1985, 36, 69–73. [Google Scholar] [CrossRef]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.S.; Zhou, B.H.; Li, C.L.; Zhang, F.; Wang, X.F.; Zhang, G.; Bu, X.Z.; Cai, S.H.; Du, J. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef]
- Wagener, F.A.; Feldman, E.; de Witte, T.; Abraham, N.G. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc. Soc. Exp. Biol. Med. 1997, 216, 456–463. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.; Espín, J.C.; García-Conesa, M.-T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef]
- Hao, C.; Chen, G.; Zhao, H.; Li, Y.; Chen, J.; Zhang, H.; Li, S.; Zhao, Y.; Chen, F.; Li, W.; et al. PD-L1 Expression in Glioblastoma, the Clinical and Prognostic Significance: A Systematic Literature Review and Meta-Analysis. Front. Oncol. 2020, 10, 1015. [Google Scholar] [CrossRef]
- Maghrouni, A.; Givari, M.; Jalili-Nik, M.; Mollazadeh, H.; Bibak, B.; Sadeghi, M.M.; Afshari, A.R.; Johnston, T.P.; Sahebkar, A. Targeting the PD-1/PD-L1 pathway in glioblastoma multiforme: Preclinical evidence and clinical interventions. Int. Immunopharmacol. 2021, 93, 107403. [Google Scholar] [CrossRef]
- Denk, D.; Petrocelli, V.; Conche, C.; Drachsler, M.; Ziegler, P.K.; Braun, A.; Kress, A.; Nicolas, A.M.; Mohs, K.; Becker, C.; et al. Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy. Immunity 2022, 55, 2059–2073.e2058. [Google Scholar] [CrossRef]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Iser, I.C.; Pereira, M.B.; Lenz, G.; Wink, M.R. The Epithelial-to-Mesenchymal Transition-Like Process in Glioblastoma: An Updated Systematic Review and In Silico Investigation. Med. Res. Rev. 2017, 37, 271–313. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, K.C.; Huang, X.; Sime, W.; Mirkov, A.; Munksgaard Thorén, M.; Massoumi, R.; Lundgren-Åkerlund, E. Integrin α10-Antibodies Reduce Glioblastoma Tumor Growth and Cell Migration. Cancers 2021, 13, 1184. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Elkamhawy, A.; Choi, Y.H.; Lee, C.H.; Lee, K.; Cho, J. Suppression of Tumor Growth and Cell Migration by Indole-Based Benzenesulfonamides and Their Synergistic Effects in Combination with Doxorubicin. Int. J. Mol. Sci. 2022, 23, 9903. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, L.; Zeng, S.; Feng, T. Clinically relevant concentration of sevoflurane suppresses cervical cancer growth and migration through targeting multiple oncogenic pathways. Biochem. Biophys. Res. Commun. 2019, 514, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Lv, M.Y.; Shi, C.J.; Pan, F.F.; Shao, J.; Feng, L.; Chen, G.; Ou, C.; Zhang, J.F.; Fu, W.M. Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/beta-catenin signaling. J. Cell. Biochem. 2019, 120, 17273–17282. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P. The Multifarious Link between Cytochrome P450s and Cancer. Oxidative Med. Cell. Longev. 2020, 2020, 3028387. [Google Scholar] [CrossRef]
- Kolluri, S.K.; Jin, U.-H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Sha, R.; Li, Y.; Xu, T.; Hu, X.; Xu, L.; Xie, Q.; Zhao, B. A new insight into the role of aryl hydrocarbon receptor (AhR) in the migration of glioblastoma by AhR-IL24 axis regulation. Environ. Int. 2021, 154, 106658. [Google Scholar] [CrossRef]
- Yang, T.; Feng, Y.-L.; Chen, L.; Vaziri, N.D.; Zhao, Y.-Y. Dietary natural flavonoids treating cancer by targeting aryl hydrocarbon receptor. Crit. Rev. Toxicol. 2019, 49, 445–460. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Potential of the ellagic acid-derived gut microbiota metabolite—Urolithin A in gastrointestinal protection. World J. Gastroenterol. 2020, 26, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Zhao, D.; Li, J.J.; Liu, S.; An, J.J.; Wang, D.; Hu, F.A.; Qiu, C.Y.; Cui, M.H. Inhibition of glioblastoma progression by Urolithin A in vitro and in vivo by regulating Sirt1-FOXO1 axis via ERK/AKT signaling pathways. Neoplasma 2022, 69, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Hurtt, M.R.; Moossy, J.; Donovan-Peluso, M.; Locker, J. Amplification of epidermal growth factor receptor gene in gliomas: Histopathology and prognosis. J. Neuropathol. Exp. Neurol. 1992, 51, 84–90. [Google Scholar] [CrossRef]
- Zahonero, C.; Sanchez-Gomez, P. EGFR-dependent mechanisms in glioblastoma: Towards a better therapeutic strategy. Cell. Mol. Life Sci. 2014, 71, 3465–3488. [Google Scholar] [CrossRef]
- Talasila, K.M.; Soentgerath, A.; Euskirchen, P.; Rosland, G.V.; Wang, J.; Huszthy, P.C.; Prestegarden, L.; Skaftnesmo, K.O.; Sakariassen, P.O.; Eskilsson, E.; et al. EGFR wild-type amplification and activation promote invasion and development of glioblastoma independent of angiogenesis. Acta Neuropathol. 2013, 125, 683–698. [Google Scholar] [CrossRef]
- Nicholas, M.K.; Lukas, R.V.; Jafri, N.F.; Faoro, L.; Salgia, R. Epidermal growth factor receptor—Mediated signal transduction in the development and therapy of gliomas. Clin. Cancer Res. 2006, 12, 7261–7270. [Google Scholar] [CrossRef]
- Ruano, Y.; Ribalta, T.; de Lope, A.R.; Campos-Martin, Y.; Fiano, C.; Perez-Magan, E.; Hernandez-Moneo, J.L.; Mollejo, M.; Melendez, B. Worse outcome in primary glioblastoma multiforme with concurrent epidermal growth factor receptor and p53 alteration. Am. J. Clin. Pathol. 2009, 131, 257–263. [Google Scholar] [CrossRef]
- Shinojima, N.; Tada, K.; Shiraishi, S.; Kamiryo, T.; Kochi, M.; Nakamura, H.; Makino, K.; Saya, H.; Hirano, H.; Kuratsu, J.; et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003, 63, 6962–6970. [Google Scholar]
- Halatsch, M.E.; Schmidt, U.; Behnke-Mursch, J.; Unterberg, A.; Wirtz, C.R. Epidermal growth factor receptor inhibition for the treatment of glioblastoma multiforme and other malignant brain tumours. Cancer Treat. Rev. 2006, 32, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, W.; Aldape, K.; He, J.; Lu, Z. Epidermal growth factor (EGF)-enhanced vascular cell adhesion molecule-1 (VCAM-1) expression promotes macrophage and glioblastoma cell interaction and tumor cell invasion. J. Biol. Chem. 2013, 288, 31488–31495. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.P.; Wu, M.L.; Li, H.L.; Zhou, Y.; Xian, M.H.; Huang, W.Z.; Piao, X.H.; Ge, Y.W. Combined Metabolite Analysis and Network Pharmacology to Elucidate the Mechanisms of Therapeutic Effect of Melastoma dodecandrum Ellagitannins on Abnormal Uterine Bleeding. Chem. Biodivers. 2023, 20, e202300646. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-K.; Huang, B.-R.; Charoensaensuk, V.; Yang, L.-Y.; Tsai, C.-F.; Liu, Y.-S.; Lai, S.-W.; Lu, D.-Y.; Yeh, W.-L.; Lin, C. Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression. Nutrients 2023, 15, 4854. https://doi.org/10.3390/nu15234854
Shen C-K, Huang B-R, Charoensaensuk V, Yang L-Y, Tsai C-F, Liu Y-S, Lai S-W, Lu D-Y, Yeh W-L, Lin C. Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression. Nutrients. 2023; 15(23):4854. https://doi.org/10.3390/nu15234854
Chicago/Turabian StyleShen, Ching-Kai, Bor-Ren Huang, Vichuda Charoensaensuk, Liang-Yo Yang, Cheng-Fang Tsai, Yu-Shu Liu, Sheng-Wei Lai, Dah-Yuu Lu, Wei-Lan Yeh, and Chingju Lin. 2023. "Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression" Nutrients 15, no. 23: 4854. https://doi.org/10.3390/nu15234854
APA StyleShen, C.-K., Huang, B.-R., Charoensaensuk, V., Yang, L.-Y., Tsai, C.-F., Liu, Y.-S., Lai, S.-W., Lu, D.-Y., Yeh, W.-L., & Lin, C. (2023). Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression. Nutrients, 15(23), 4854. https://doi.org/10.3390/nu15234854




