Brassica oleracea Var italica by-Products Prevent Lipid Accumulation and Cell Death in a Liver Cell Model of Lipid Toxicity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Broccoli Leaf Extracts Possess the Highest Contents of Phenolic Compounds and Antioxidant Capacity
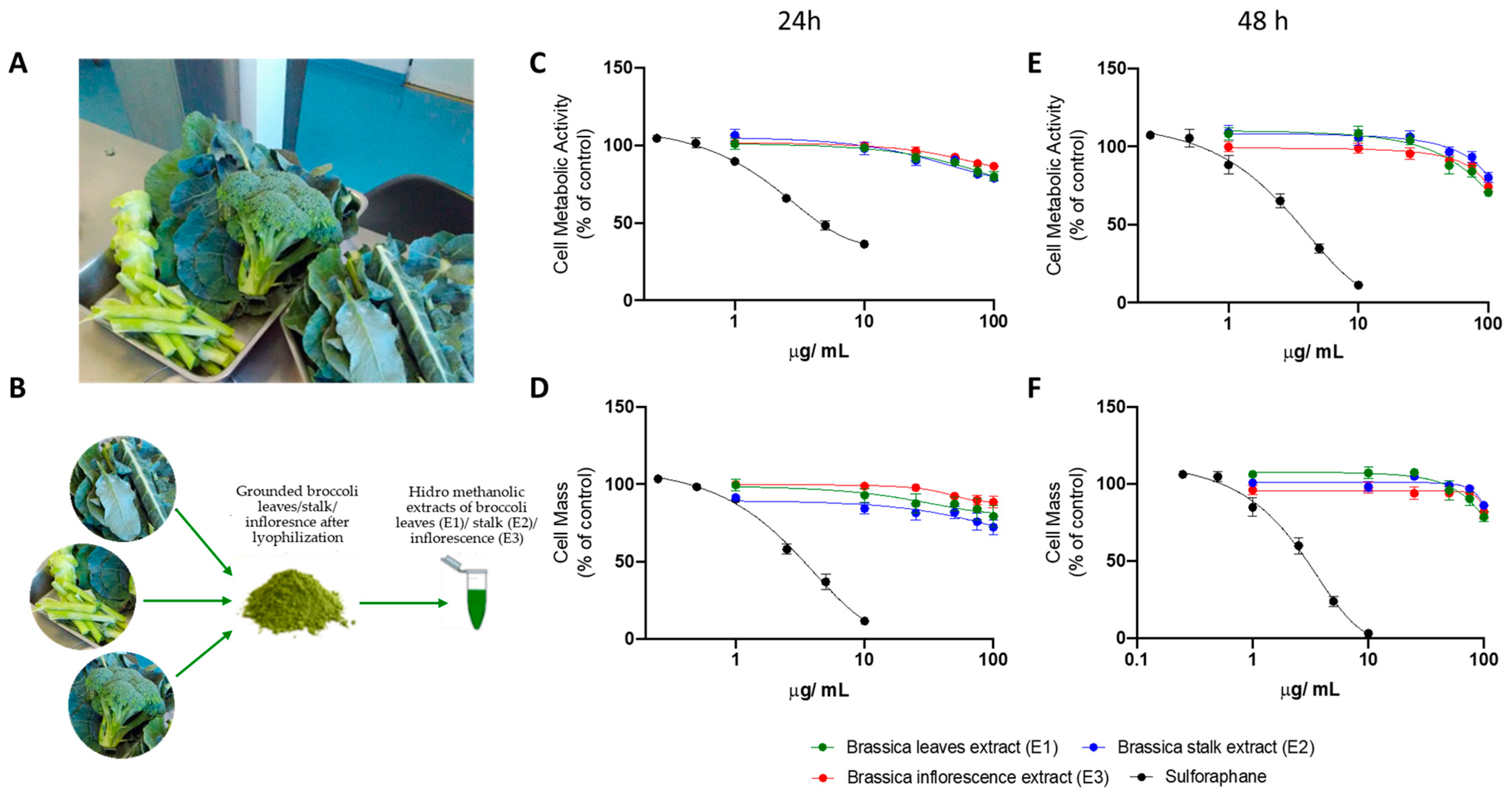
3.2. Broccoli Extracts Reduce the Viability of Human Hepatocyte (HepG2) Cells in a Dose-Dependent Manner
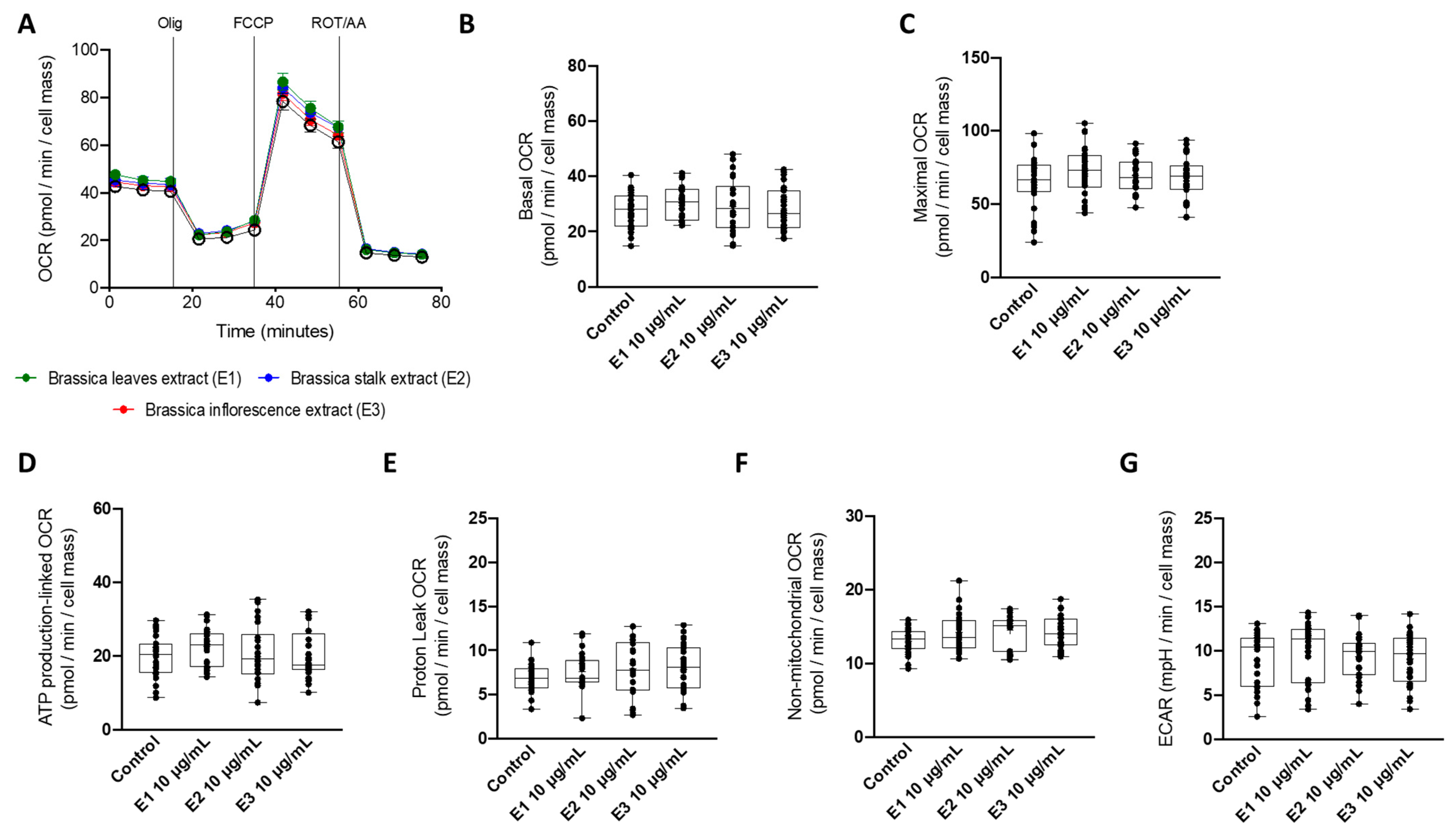
3.3. A Non-Toxic Concentration of Brassica Oleracea Extracts Had No Effect on Mitochondrial Oxygen Consumption Rates in HepG2 Cells
3.4. A Non-Toxic Concentration of Broccoli Extracts Was Ineffective to Prevent Tert-Butyl Hydroperoxide (t-BHP)-Induced Cell Damage
3.5. Broccoli Extracts Decreased the Accumulation of Lipid Droplets under Supra-Physiological Concentrations of FA in Human HepG2 Cells
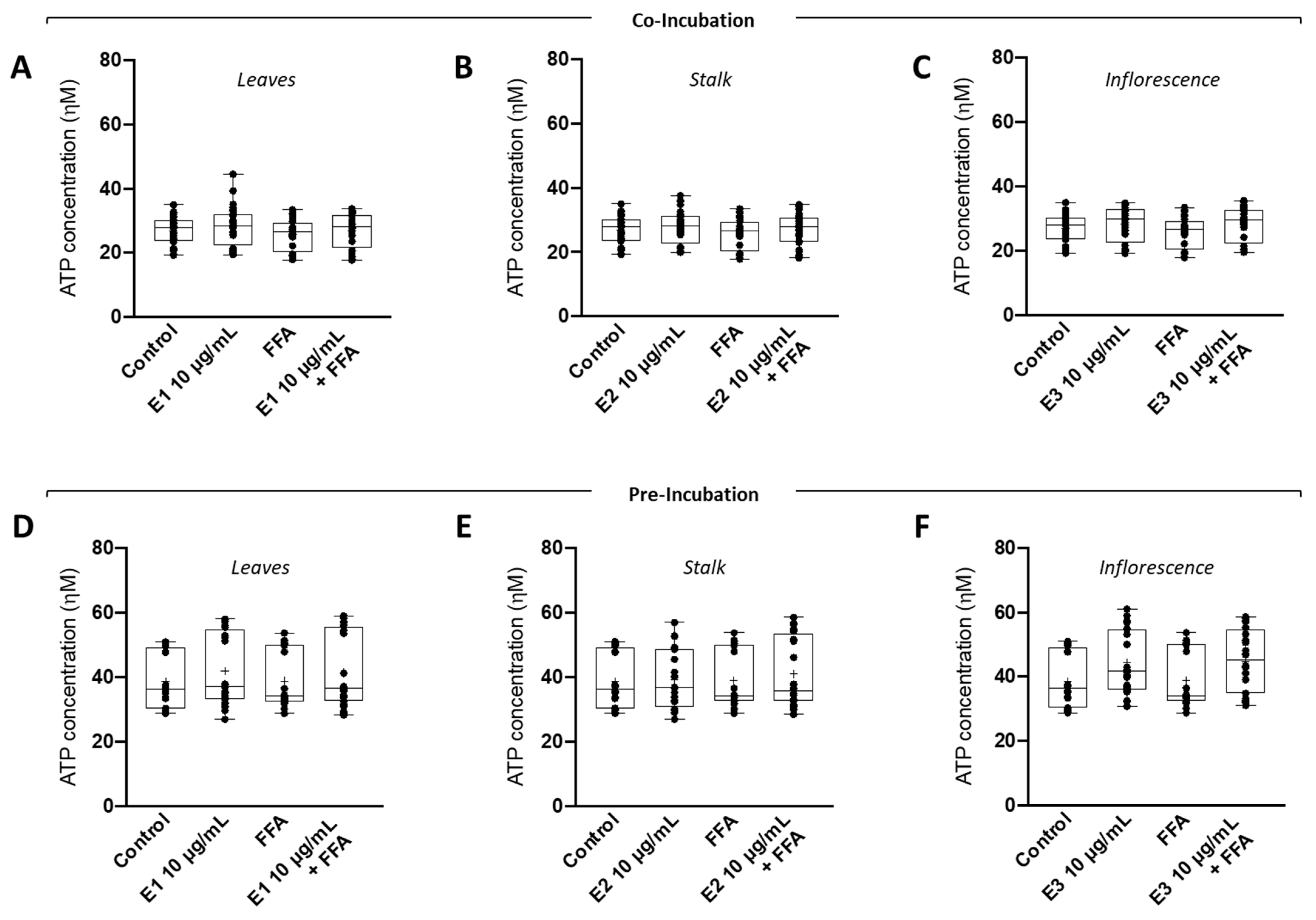
3.6. Supra-Physiological Concentrations of Fatty Acids (FA) Had No Impact on Intracellular ATP Levels
3.7. Broccoli Extracts Prevented the FFA-Induced Decrease in Catalase Activity in HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tremmel, M.; Gerdtham, U.; Nilsson, P.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Obesity-and-Overweight. Available online: www.Who.Int (accessed on 27 November 2022).
- Wright, S.M.; Aronne, L.J. Causes of obesity. Abdom. Radiol. 2012, 37, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.; Spyrou, N.; Mantzoros, C.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Zámbó, V.; Simon-Szabó, L.; Szelényi, P.; Kereszturi, E.; Bánhegyi, G.; Csala, M. Lipotoxicity in the liver. World J. Hepatol. 2013, 5, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Basaranoglu, M.; Ormeci, N. Nonalcoholic fatty liver disease: Diagnosis, pathogenesis, and management. Turk. J. Gastroenterol. 2014, 25, 127–132. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Wong, R.J.; Harrison, S.A. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin. Gastroenterol. Hepatol. 2015, 13, 2062–2070. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, G.C.; Tan, H.Y.; Hao, F.B.; Hu, J.J. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis. Sci. Rep. 2019, 9, 11124. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Bautista, M.; Esquivel-soto, J.; Esquivel-chirino, C.; Durante-montiel, I.; Valadez-vega, C. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 6, 3117–3132. [Google Scholar] [CrossRef]
- Legaki, A.I.; Moustakas, I.I.; Sikorska, M.; Papadopoulos, G.; Velliou, R.I.; Chatzigeorgiou, A. Hepatocyte Mitochondrial Dynamics and Bioenergetics in Obesity-Related Non-Alcoholic Fatty Liver Disease. Curr. Obes. Rep. 2022, 11, 126–143. [Google Scholar] [CrossRef]
- Nordgren, M.; Fransen, M. Peroxisomal metabolism and oxidative stress. Biochimie 2014, 98, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Balsano, C.; Santini, S.J.; Brienza, G.; Clemente, I.; Cosimini, B.; Sinatti, G. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int. J. Mol. Sci. 2021, 22, 13424. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Santoyo, S. The Health Benefits of the Bioactive Compounds in Foods. Foods 2021, 10, 325. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef]
- Castelão-Baptista, J.P.; Barros, A.; Martins, T.; Rosa, E.; Sardão, V.A. Three in One: The Potential of Brassica By-Products against Economic Waste, Environmental Hazard, and Metabolic Disruption in Obesity. Nutrients 2021, 13, 4194. [Google Scholar] [CrossRef]
- Martins, T.; Leite, R.; Matos, A.F.; Soares, J.; Pires, M.J.; de Lurdes Pinto, M.; Neuparth, M.J.; Sequeira, A.R.; Félix, L.; Venâncio, C.; et al. Beneficial Effects of Broccoli (Brassica oleracea var italica) By-products in Diet-induced Obese Mice. In Vivo 2022, 36, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Colaço, B.; Venâncio, C.; Pires, M.; Oliveira, P.; Rosa, E.; Antunes, L. Potential effects of sulforaphane to fight obesity. J. Sci. Food Agric. 2018, 98, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Tian, S.; Teng, C.; Huang, L.; Liu, X.; Wang, J.; Zhang, Y.; Li, B.; Shan, Y. Sulforaphane Improves Lipid Metabolism by Enhancing Mitochondrial Function and Biogenesis In Vivo and In Vitro. Mol. Nutr. Food Res. 2019, 63, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Joshi, V.; Devrajan, A. Ethanol recovery from solid state fermented apple pomace and evaluation of physico-chemical characteristics of the residue. Nat. Prod. Radiance 2008, 7, 127–132. [Google Scholar]
- Fink, M.; Feller, C.; Scharpf, H.-C.; Weier, U.; Maync, A.; Ziegler, J.; Paschold, P.-J.; Strohmeyer, K. Nitrogen, phosphorus, potassium and magnesium contents of field vegetables—Recent data for fertiliser recommendations and nutrient balances. J. Plant Nutr. Soil Sci. 1999, 162, 71–73. [Google Scholar] [CrossRef]
- Mahro, B.; Timm, M. Potential of Biowaste from the Food Industry as a Biomass Resource. Eng. Life Sci. 2007, 7, 457–468. [Google Scholar] [CrossRef]
- Jameel, F.; Alexeenko, A.; Bhambhani, A.; Sacha, G.; Zhu, T.; Tchessalov, S.; Kumar, L.; Sharma, P.; Moussa, E.; Iyer, L.; et al. Recommended Best Practices for Lyophilization Validation—2021 Part I: Process Design and Modeling. AAPS PharmSciTech 2021, 22, 221. [Google Scholar] [CrossRef]
- Jameel, F.; Alexeenko, A.; Bhambhani, A.; Sacha, G.; Zhu, T.; Tchessalov, S.; Sharma, P.; Moussa, E.; Iyer, L.; Luthra, S.; et al. Recommended Best Practices for Lyophilization Validation 2021 Part II: Process Qualification and Continued Process Verification. AAPS PharmSciTech 2021, 22, 266. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Guedes, A.; Queiroz, M.; Silva, A.M.; Barros, A.I.R.N.A. Oxidative stress prevention and anti-apoptosis activity of grape (Vitis vinifera L.) stems in human keratinocytes. Food Res. Int. 2016, 87, 92–102. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Texeira, A.; Baenas, N.; Domínguez-Perles, R. Grape stems as a source of bioactive compounds: Application towards added-value commodities and significance for human health. Phytochem. Rev. 2015, 14, 921–931. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Einer, C.; Hohenester, S.; Wimmer, R.; Wottke, L.; Artmann, R.; Schulz, S.; Gosmann, C.; Simmons, A.; Leitzinger, C.; Eberhagen, C.; et al. Data on chow, liver tissue and mitochondrial fatty acid compositions as well as mitochondrial proteome changes after feeding mice a western diet for 6–24 weeks. Data Br. 2017, 15, 163–169. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Starostina, I.G.; Ivanova, V.V.; Rizvanov, A.A.; Oliveira, P.J.; Pereira, S.P. Determination of Metabolic Viability and Cell Mass Using a Tandem Resazurin/Sulforhodamine B Assay. Curr. Protoc. Toxicol. 2016, 68, 2.24.1–2.24.15. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Promega CellTiter-Glo Luminescent Cell Viability Assay Technical Bulletin. 2020. Available online: https://worldwide.promega.com/resources/protocols/technical-bulletins/0/celltiter-glo-luminescent-cell-viability-assay-protocol/ (accessed on 27 November 2022).
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Grilo, L.F.; Martins, J.D.; Cavallaro, C.H.; Nathanielsz, P.W.; Oliveira, P.J.; Pereira, S.P. Development of a 96-well based assay for kinetic determination of catalase enzymatic-activity in biological samples. Toxicol. In Vitro 2020, 69, 104996. [Google Scholar] [CrossRef]
- Salvoza, N.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Natural Compounds for Counteracting Nonalcoholic Fatty Liver Disease (NAFLD): Advantages and Limitations of the Suggested Candidates. Int. J. Mol. Sci. 2022, 23, 2764. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lim, S.B. Antioxidant and anticancer activities of broccoli by-products from different cultivars and maturity stages at harvest. Prev. Nutr. food Sci. 2015, 20, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem. J. 1989, 257, 603–606. [Google Scholar] [CrossRef]
- Crane, D.; Hüussinger, D.; Graf, P.; Sies, H. Decreased Flux through Pyruvate Dehydrogenase by Thiol Oxidation during t-Butyl Hydroperoxide Metabolism in Perfused Rat Liver. Hoppe. Seylers. Z. Physiol. Chem. 1983, 364, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Simões, I.C.M.; Veloso, C.; Carvalho, A.; Simões, R.F.; Pereira, F.B.; Thiel, T.; Normann, A.; Morais, C.; Jurado, A.S.; et al. Exploratory data analysis of cell and mitochondrial high-fat, high-sugar toxicity on human hepg2 cells. Nutrients 2021, 13, 1723. [Google Scholar] [CrossRef]
- Charron, C.S.; Sams, C.E. Glucosinolate Content and Myrosinase Activity in Rapid-cycling Brassica oleracea Grown in a Controlled Environment. J. Am. Soc. Hortic. Sci. 2004, 129, 321–330. [Google Scholar] [CrossRef]
- Yen, G.-C.; Wei, Q.-K. Myrosinase activity and total glucosinolate content of cruciferous vegetables, and some properties of cabbage myrosinase in Taiwan. J. Sci. Food Agric. 1993, 61, 471–475. [Google Scholar] [CrossRef]
- Miranda Rossetto, M.R.; Shiga, T.M.; Vianello, F.; Pereira Lima, G.P. Analysis of total glucosinolates and chromatographically purified benzylglucosinolate in organic and conventional vegetables. LWT—Food Sci. Technol. 2013, 50, 247–252. [Google Scholar] [CrossRef]
- Pihakashi, K.; Pihakaski, S. Myrosinase in Brassicaceae (Cruciferae): II. Myrosinase Activity in Different Organs of Sinapis Alba L. J. Exp. Bot. 1978, 29, 335–345. [Google Scholar] [CrossRef]
- Zandani, G.; Anavi-Cohen, S.; Tsybina-Shimshilashvili, N.; Sela, N.; Nyska, A.; Madar, Z. Broccoli Florets Supplementation Improves Insulin Sensitivity and Alters Gut Microbiome Population-A Steatosis Mice Model Induced by High-Fat Diet. Front. Nutr. 2021, 8, 680241. [Google Scholar] [CrossRef]
- Chen, Y.J.; Myracle, A.D.; Wallig, M.A.; Jeffery, E.H. Dietary broccoli protects against fatty liver development but not against progression of liver cancer in mice pretreated with diethylnitrosamine. J. Funct. Foods 2016, 24, 57–62. [Google Scholar] [CrossRef] [Green Version]


| Phenolic Content | Antioxidant Activity | ||||
|---|---|---|---|---|---|
| Broccoli Part | Total Phenols | Ortho-Diphenols | Flavonoids | ABTS | DPPH |
| Leaf | 12.35 ± 1.27 c | 54.54 ± 6.51 b | 8.32 ± 1.25 b | 0.0192 ± 0.0089 c | 0.0269 ± 0.0067 c |
| Stalk | 3.24 ± 0.51 a | 4.34 ± 0.81 a | 0.84 ± 0.19 a | 0.0035 ± 0.0005 a | 0.0061 ± 0.0014 a |
| Inflorescence | 6.65 ± 1.73 b | 7.97 ± 2.17 a | 1.30 ± 0.36 a | 0.0070 ± 0.0010 b | 0.0123 ± 0.0025 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelão-Baptista, J.P.; Valente, S.A.; Canário, S.; Oppolzer, D.; Barros, A.; Venâncio, C.; Martins, T.; Antunes, L.; Sardão, V.A.; Rosa, E.; et al. Brassica oleracea Var italica by-Products Prevent Lipid Accumulation and Cell Death in a Liver Cell Model of Lipid Toxicity. Nutrients 2023, 15, 924. https://doi.org/10.3390/nu15040924
Castelão-Baptista JP, Valente SA, Canário S, Oppolzer D, Barros A, Venâncio C, Martins T, Antunes L, Sardão VA, Rosa E, et al. Brassica oleracea Var italica by-Products Prevent Lipid Accumulation and Cell Death in a Liver Cell Model of Lipid Toxicity. Nutrients. 2023; 15(4):924. https://doi.org/10.3390/nu15040924
Chicago/Turabian StyleCastelão-Baptista, José P., Sara A. Valente, Sara Canário, David Oppolzer, Ana Barros, Carlos Venâncio, Tânia Martins, Luís Antunes, Vilma A. Sardão, Eduardo Rosa, and et al. 2023. "Brassica oleracea Var italica by-Products Prevent Lipid Accumulation and Cell Death in a Liver Cell Model of Lipid Toxicity" Nutrients 15, no. 4: 924. https://doi.org/10.3390/nu15040924
APA StyleCastelão-Baptista, J. P., Valente, S. A., Canário, S., Oppolzer, D., Barros, A., Venâncio, C., Martins, T., Antunes, L., Sardão, V. A., Rosa, E., & Oliveira, P. J. (2023). Brassica oleracea Var italica by-Products Prevent Lipid Accumulation and Cell Death in a Liver Cell Model of Lipid Toxicity. Nutrients, 15(4), 924. https://doi.org/10.3390/nu15040924









