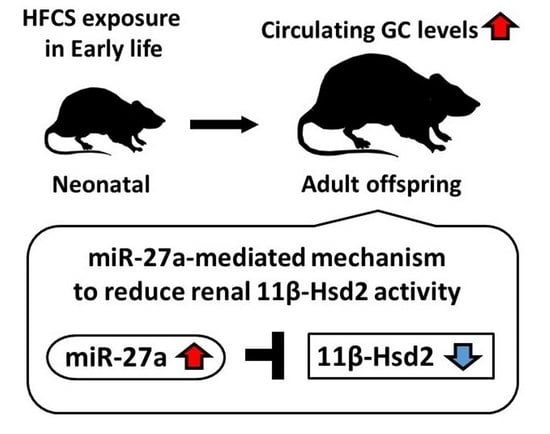
Maternal High-Fructose Corn Syrup Intake Impairs Corticosterone Clearance by Reducing Renal 11β-Hsd2 Activity via miR-27a-Mediated Mechanism in Rat Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. General Anesthesia
2.3. Quantification of Circulating Corticosterone Levels
2.4. Quantification of mRNA Expression
2.5. Western Blotting
2.6. 11β-Hsd1 and 11β-Hsd2 Activity
2.7. Analysis of Hsd11b2 DNA Methylation Levels in the Kidney
2.8. Quantification of miRNA Expression
2.9. Transfection with miRNA Mimic
2.10. Luciferase Assay
2.11. Statistical Analyses
3. Results
3.1. Comparison of Body Weight and Caloric Intake of Offspring
3.2. Comparison of Circulating GC Levels in Offspring
3.3. Comparison of 11β-Hsd1 and 11β-Hsd2 Activities in the Liver, Kidney, Adrenal Glands, Muscle, and WAT in Offspring
3.4. Comparison of the mRNA and Protein Expression of 11β-Hsd2 in Offspring Kidney
3.5. Comparison of DNA Methylation Levels in Renal Hsd11b2 Promoter Region in Offspring
3.6. Comparison of miRNA Expression Levels That Are Predicted to Be Biological Targets of Hsd11b2 mRNA in Offspring
3.7. Analysis of the miRNA Suppression of Hsd11b2 mRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOHaD | developmental origins of health and disease |
| GC | glucocorticoid |
| HFCS | high-fructose corn syrup |
| miRNA | microRNA |
| WAT | white adipose tissue |
| 11β-Hsd1 | 11 beta-hydroxysteroid dehydrogenases 1 |
| 11β-Hsd2 | 11 beta-hydroxysteroid dehydrogenases 2 |
References
- De Rooij, S.R.; Painter, R.C.; Phillips, D.I.W.; Osmond, C.; Michels, R.P.J.; Godsland, I.F.; Bossuyt, P.M.M.; Bleker, O.P.; Roseboom, T.J. Impaired Insulin Secretion after Prenatal Exposure to the Dutch Famine. Diabetes Care 2006, 29, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, G.-P.; Stein, Z.A.; Susser, M.W. Obesity in Young Men after Famine Exposure in Utero and Early Infancy. N. Engl. J. Med. 1976, 295, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Witek, K.; Wydra, K.; Filip, M. A High-Sugar Diet Consumption, Metabolism and Health Impacts with a Focus on the Development of Substance Use Disorder: A Narrative Review. Nutrients 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A Highly Lipogenic Nutrient Implicated in Insulin Resistance, Hepatic Steatosis, and the Metabolic Syndrome. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.C.; de Lima, W.G.; Pedrosa, M.L.; Silva, M.E. Health Implications of High-Fructose Intake and Current Research. Adv. Nutr. 2015, 6, 729–737. [Google Scholar] [CrossRef]
- Thompson, M.D.; Debosch, B.J. Maternal Fructose Diet-Induced Developmental Programming. Nutrients 2021, 13, 3278. [Google Scholar] [CrossRef]
- Yamazaki, M.; Yamada, H.; Munetsuna, E.; Ishikawa, H.; Mizuno, G.; Mukuda, T.; Mouri, A.; Nabeshima, T.; Saito, K.; Suzuki, K.; et al. Excess Maternal Fructose Consumption Impairs Hippocampal Function in Offspring via Epigenetic Modification of BDNF Promoter. FASEB J. 2018, 32, 2549–2562. [Google Scholar] [CrossRef]
- Yamazaki, M.; Munetsuna, E.; Yamada, H.; Ando, Y.; Mizuno, G.; Fujii, R.; Nouchi, Y.; Kageyama, I.; Teshigawara, A.; Ishikawa, H.; et al. Maternal Fructose Consumption Down-Regulates Lxra Expression via MiR-206-Mediated Regulation. J. Nutr. Biochem. 2020, 82, 108386. [Google Scholar] [CrossRef]
- Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Ota, T.; Hattori, Y.; Sadamoto, N.; Suzuki, K.; Ishikawa, H.; et al. Maternal Fructose Intake Disturbs Ovarian Estradiol Synthesis in Rats. Life Sci. 2018, 202, 117–123. [Google Scholar] [CrossRef]
- Ando, Y.; Yamada, H.; Munetsuna, E.; Yamazaki, M.; Kageyama, I.; Teshigawara, A.; Nouchi, Y.; Fujii, R.; Mizuno, G.; Sadamoto, N.; et al. Maternal High-Fructose Corn Syrup Consumption Causes Insulin Resistance and Hyperlipidemia in Offspring via DNA Methylation of the Pparα Promoter Region. J. Nutr. Biochem. 2022, 103, 108951. [Google Scholar] [CrossRef]
- Gillman, M.W.; Rifas-Shiman, S.L.; Fernandez-Barres, S.; Kleinman, K.; Taveras, E.M.; Oken, E. Beverage Intake During Pregnancy and Childhood Adiposity. Pediatrics 2017, 140, e20170031. [Google Scholar] [CrossRef] [PubMed]
- Jen, V.; Erler, N.S.; Tielemans, M.J.; Braun, K.V.E.; Jaddoe, V.W.V.; Franco, O.H.; Voortman, T. Mothers’ Intake of Sugar-Containing Beverages during Pregnancy and Body Composition of Their Children during Childhood: The Generation R Study. Am. J. Clin. Nutr. 2017, 105, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Moraitis, A.G.; Block, T.; Nguyen, D.; Belanoff, J.K. The Role of Glucocorticoid Receptors in Metabolic Syndrome and Psychiatric Illness. J. Steroid Biochem. Mol. Biol. 2017, 165, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. The Role of Glucocorticoid Action in the Pathophysiology of the Metabolic Syndrome. Nutr. Metab. 2005, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and Fatty Acid Metabolism in Humans: Fuelling Fat Redistribution in the Metabolic Syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Castrogiovanni, D.; Gaillard, R.C.; Spinedi, E.; Giovambattista, A. Increased Male Offspring’s Risk of Metabolic-Neuroendocrine Dysfunction and Overweight after Fructose-Rich Diet Intake by the Lactating Mother. Endocrinology 2010, 151, 4214–4223. [Google Scholar] [CrossRef]
- Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Hattori, Y.; Sadamoto, N.; Ishikawa, H.; Ohta, Y.; Fujii, R.; et al. Maternal High-Fructose Intake Increases Circulating Corticosterone Levels via Decreased Adrenal Corticosterone Clearance in Adult Offspring. J. Nutr. Biochem. 2019, 67, 44–50. [Google Scholar] [CrossRef]
- Ohashi, K.; Munetsuna, E.; Yamada, H.; Ando, Y.; Yamazaki, M.; Taromaru, N.; Nagura, A.; Ishikawa, H.; Suzuki, K.; Teradaira, R.; et al. High Fructose Consumption Induces DNA Methylation at PPARα and CPT1A Promoter Regions in the Rat Liver. Biochem. Biophys. Res. Commun. 2015, 468, 185–189. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Hattori, Y.; Kageyama, I.; Teshigawara, A.; Nouchi, Y.; Ishikawa, H.; et al. Maternal Fructose Intake Predisposes Rat Offspring to Metabolic Disorders via Abnormal Hepatic Programming. FASEB J. 2021, 35, e22030. [Google Scholar] [CrossRef]
- Mizuno, G.; Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Hattori, Y.; Kageyama, I.; Teshigawara, A.; Nouchi, Y.; Fujii, R.; et al. Maternal Fructose Consumption Downregulates Hippocampal Catalase Expression via DNA Methylation in Rat Offspring. Nutr. Res. 2021, 92, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Munetsuna, E.; Yamada, H.; Ando, Y.; Mizuno, G.; Murase, Y.; Kondo, K.; Ishikawa, H.; Teradaira, R.; Suzuki, K.; et al. Fructose Consumption Induces Hypomethylation of Hepatic Mitochondrial DNA in Rats. Life Sci. 2016, 149, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, G.; Yamada, H.; Munetsuna, E.; Ando, Y.; Teshigawara, A.; Ito, M.; Kageyama, I.; Nouchi, Y.; Wakasugi, T.; Sakakibara, T.; et al. High-Fructose Corn Syrup Intake Has Stronger Effects on the Transcription Level of Hepatic Lipid Metabolism-Related Genes, via DNA Methylation Modification, in Childhood and Adolescence than in Other Generations. Life Sci. 2022, 301, 120638. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, I.; Yamada, H.; Munetsuna, E.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Fujii, R.; Nouchi, Y.; Wakasugi, T.; Sakakibara, T.; et al. Differential Effects of Excess High-Fructose Corn Syrup on the DNA Methylation of Hippocampal Neurotrophic Factor in Childhood and Adolescence. PLoS ONE 2022, 17, e0270144. [Google Scholar] [CrossRef]
- Nouchi, Y.; Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Fujii, R.; Kageyama, I.; Wakasugi, T.; Sakakibara, T.; et al. Effects of High-Fructose Corn Syrup Intake on Glucocorticoid Metabolism in Rats During Childhood, Adolescence and Adulthood. Exp. Clin. Endocrinol. Diabetes 2022, 130, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Suzuki, K.; Fujii, R.; Kawado, M.; Hashimoto, S.; Watanabe, Y.; Iso, H.; Fujino, Y.; Wakai, K.; Tamakoshi, A. Circulating MiR-21, MiR-29a, and MiR-126 Are Associated with Premature Death Risk Due to Cancer and Cardiovascular Disease: The JACC Study. Sci. Rep. 2021, 11, 5298. [Google Scholar] [CrossRef]
- Kwekel, J.C.; Vijay, V.; Desai, V.G.; Moland, C.L.; Fuscoe, J.C. Age and sex differences in kidney microRNA expression during the life span of F344 rats. Biol. Sex Differ. 2015, 6, 1. [Google Scholar] [CrossRef]
- Hiratsuka, I.; Yamada, H.; Munetsuna, E.; Hashimoto, S.; Itoh, M. Circulating MicroRNAs in Graves’ Disease in Relation to Clinical Activity. Thyroid 2016, 26, 1431–1440. [Google Scholar] [CrossRef]
- Peña, C.J.; Monk, C.; Champagne, F.A. Epigenetic Effects of Prenatal Stress on 11β-Hydroxysteroid Dehydrogenase-2 in the Placenta and Fetal Brain. PLoS ONE 2012, 7, e39791. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Liu, P.; Xia, B.; Zhu, Q.; Wang, X.; Song, Q.; Kan, H.; Zhang, Y. Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci. Total. Environ. 2017, 607–608, 1103–1108. [Google Scholar] [CrossRef]
- Rezaei, M.; Andrieu, T.; Neuenschwander, S.; Bruggmann, R.; Mordasini, D.; Frey, F.J.; Vogt, B.; Frey, B.M. Regulation of 11β-Hydroxysteroid Dehydrogenase Type 2 by MicroRNA. Hypertension 2014, 64, 860–866. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, Q.; Zhang, Q.; Wang, T.; Xiao, X. Early Life Fructose Exposure and Its Implications for Long-Term Cardiometabolic Health in Offspring. Nutrients 2016, 8, 685. [Google Scholar] [CrossRef]
- Spiga, F.; Liu, Y.; Aguilera, G.; Lightman, S.L. Temporal Effect of Adrenocorticotrophic Hormone on Adrenal Glucocorticoid Steroidogenesis: Involvement of the Transducer of Regulated Cyclic AMP-Response Element-Binding Protein Activity. J. Neuroendocrinol. 2011, 23, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Mcclennen, S.J.; Cortright, D.N.; Seasholtz, A.F. Regulation of Pituitary Corticotropin-Releasing Hormone-Binding Protein Messenger Ribonucleic Acid Levels by Restraint Stress and Adrenalectomy. Endocrinology 1998, 139, 4435–4441. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Kirschbaum, C.; Steptoe, A. Hair Cortisol and Adiposity in a Population-Based Sample of 2,527 Men and Women Aged 54 to 87 Years. Obesity 2017, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.; Daimon, M.; Murakami, H.; Otaka, H.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; Matsuhashi, Y.; Yanagimachi, M.; et al. Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population. PLoS ONE 2016, 11, e0166077. [Google Scholar] [CrossRef]
- Nakayama, T.; Kosugi, T.; Gersch, M.; Connor, T.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Roncal, C.; Perez-Pozo, S.E.; Johnson, R.J.; Nakagawa, T. Dietary Fructose Causes Tubulointerstitial Injury in the Normal Rat Kidney. Am. J. Physiol. Ren. Physiol. 2010, 298, F712–F720. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sanchez-Lozada, L.G.; Andres-Hernando, A.; Kojima, H.; Kasahara, M.; Rodriguez-Iturbe, B.; Bjornstad, P.; Lanaspa, M.A.; Johnson, R.J. Endogenous Fructose Metabolism Could Explain the Warburg Effect and the Protection of SGLT2 Inhibitors in Chronic Kidney Disease. Front. Immunol. 2021, 12, 694457. [Google Scholar] [CrossRef]
- Björkman, O.; Felig, P. Role of the Kidney in the Metabolism of Fructose in 60-Hour Fasted Humans. Diabetes 1982, 31, 516–520. [Google Scholar] [CrossRef]
- Zhou, Z.; Wan, J.; Hou, X.; Geng, J.; Li, X.; Bai, X. MicroRNA-27a Promotes Podocyte Injury via PPARγ-Mediated β-Catenin Activation in Diabetic Nephropathy. Cell Death Dis. 2017, 8, e2658. [Google Scholar] [CrossRef]
- Hanousková, B.; Neprašová, B.; Skálová, L.; Maletínská, L.; Zemanová, K.; Ambrož, M.; Matoušková, P. High-Fructose Drinks Affect MicroRNAs Expression Differently in Lean and Obese Mice. J. Nutr. Biochem. 2019, 68, 42–50. [Google Scholar] [CrossRef]
- Bayati, P.; Kalantari, M.; Assarehzadegan, M.A.; Poormoghim, H.; Mojtabavi, N. MiR-27a as a Diagnostic Biomarker and Potential Therapeutic Target in Systemic Sclerosis. Sci. Rep. 2022, 12, 18932. [Google Scholar] [CrossRef] [PubMed]
- Mock, K.; Lateef, S.; Benedito, V.A.; Tou, J.C. High-Fructose Corn Syrup-55 Consumption Alters Hepatic Lipid Metabolism and Promotes Triglyceride Accumulation. J. Nutr. Biochem. 2017, 39, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bocarsly, M.E.; Powell, E.S.; Avena, N.M.; Hoebel, B.G. High-Fructose Corn Syrup Causes Characteristics of Obesity in Rats: Increased Body Weight, Body Fat and Triglyceride Levels. Pharmacol. Biochem. Behav. 2010, 97, 101–106. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouchi, Y.; Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Ikeya, M.; Kageyama, I.; Wakasugi, T.; Teshigawara, A.; et al. Maternal High-Fructose Corn Syrup Intake Impairs Corticosterone Clearance by Reducing Renal 11β-Hsd2 Activity via miR-27a-Mediated Mechanism in Rat Offspring. Nutrients 2023, 15, 2122. https://doi.org/10.3390/nu15092122
Nouchi Y, Munetsuna E, Yamada H, Yamazaki M, Ando Y, Mizuno G, Ikeya M, Kageyama I, Wakasugi T, Teshigawara A, et al. Maternal High-Fructose Corn Syrup Intake Impairs Corticosterone Clearance by Reducing Renal 11β-Hsd2 Activity via miR-27a-Mediated Mechanism in Rat Offspring. Nutrients. 2023; 15(9):2122. https://doi.org/10.3390/nu15092122
Chicago/Turabian StyleNouchi, Yuki, Eiji Munetsuna, Hiroya Yamada, Mirai Yamazaki, Yoshitaka Ando, Genki Mizuno, Miyuki Ikeya, Itsuki Kageyama, Takuya Wakasugi, Atsushi Teshigawara, and et al. 2023. "Maternal High-Fructose Corn Syrup Intake Impairs Corticosterone Clearance by Reducing Renal 11β-Hsd2 Activity via miR-27a-Mediated Mechanism in Rat Offspring" Nutrients 15, no. 9: 2122. https://doi.org/10.3390/nu15092122
APA StyleNouchi, Y., Munetsuna, E., Yamada, H., Yamazaki, M., Ando, Y., Mizuno, G., Ikeya, M., Kageyama, I., Wakasugi, T., Teshigawara, A., Hattori, Y., Tsuboi, Y., Ishikawa, H., Suzuki, K., & Ohashi, K. (2023). Maternal High-Fructose Corn Syrup Intake Impairs Corticosterone Clearance by Reducing Renal 11β-Hsd2 Activity via miR-27a-Mediated Mechanism in Rat Offspring. Nutrients, 15(9), 2122. https://doi.org/10.3390/nu15092122