Effect of Intermittent vs. Continuous Energy Restriction on Visceral Fat: Protocol for The Healthy Diet and Lifestyle Study 2 (HDLS2)
Abstract
:1. Introduction
2. Specific Aims/Outcomes
2.1. Aim 1: Effect of Intervention on Visceral and Ectopic Fat
- Intra-Abdominal VAT Volume: Assessed via abdominal MRI to detect changes attributable to the dietary interventions. Three-dimensional (3D) volumetric assessment of VAT over the abdominal region is used to improve the robustness of the VAT endpoint [34]. The approach is based on 3D volumetric Dixon fat images and automated software that performs localization and segmentation to produce VAT, subcutaneous adipose tissue (SAT), and Abdominal Adipose Tissue (AAT) volume outputs over the abdominal region of interest [35]. VAT refers to the fat deep within the abdominal cavity that surrounds organs, whereas SAT accumulates under the skin. AAT encompasses both VAT and SAT within the abdominal region.
- Liver Fat Percentage: This is quantified using MRI proton density fat fraction (PDFF) and magnetic resonance spectroscopy (MRS) to measure alterations in liver fat content. MRI-PDFF and MRS are MR-based noninvasive quantitative imaging modalities enabling liver fat assessment with high accuracy, repeatability, and reproducibility. They have emerged as a surrogate for liver biopsy in clinical trials [36].
- Relative VAT: Adjustments in VAT measures made in relation to total fat mass as determined by whole-body DXA.
- Total Fat Mass (FM): Comprehensive assessment via whole-body DXA to gauge overall changes in total adiposity.
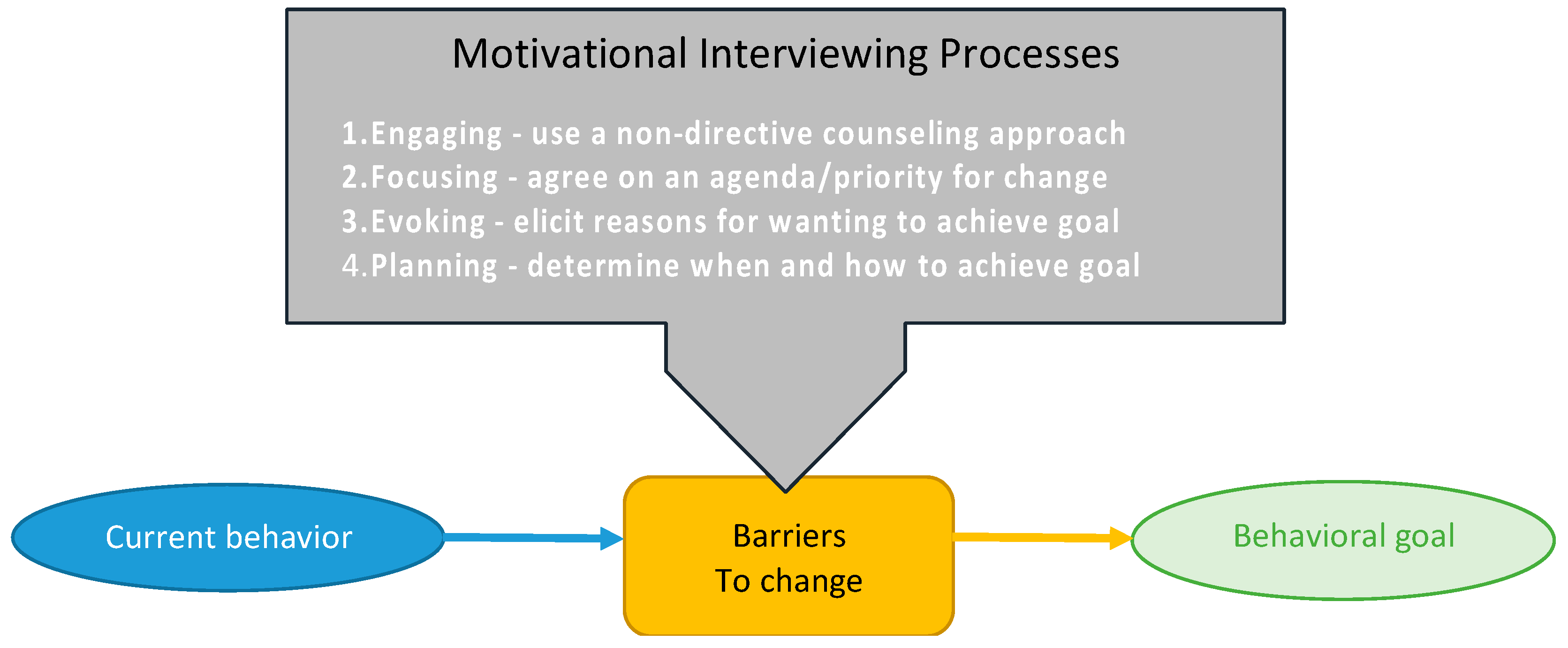
2.2. Aim 2: Effect of Behavioral Factors on Adherence to Intervention
3. Materials and Methods
3.1. Study Design and Participants
3.2. Randomization and Masking
3.3. Timing of Outcome Measurements
3.4. Diet and Physical Activity Interventions
3.5. Adherence to the Intervention
3.6. Data Analysis
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Adult Obesity Prevalence Remains High; Support for Prevention and Treatment Needed. Available online: https://www.cdc.gov/media/releases/2023/p0922-adult-obesity.html (accessed on 12 October 2023).
- Calle, E.E.; Thun, M.J. Obesity and cancer: Cancer Epidemiology. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Dubey, P.; Cistola, D.P.; Reddy, S.Y. Association Between Obesity and Cardiovascular Outcomes: Updated Evidence from Meta-analysis Studies. Curr. Cardiol. Rep. 2020, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Rapp, K.; Schroeder, J.; Klenk, J.; Stoehr, S.; Ulmer, H.; Concin, H.; Diem, G.; Oberaigner, W.; Weiland, S.K. Obesity and incidence of cancer: A large cohort study of over 145 000 adults in Austria. Br. J. Cancer 2005, 93, 1062–1067. [Google Scholar] [CrossRef]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association Between Obesity and Cardiovascular Outcomes A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw. Open 2018, 1, e183788. [Google Scholar] [CrossRef]
- Lim, U.; Monroe, K.R.; Buchthal, S.; Fan, B.; Cheng, I.; Kristal, B.S.; Lampe, J.W.; Hullar, M.A.; Franke, A.A.; Stram, D.O.; et al. Propensity for Intra-abdominal and Hepatic Adiposity Varies Among Ethnic Groups. Gastroenterology 2019, 156, 966–975.e910. [Google Scholar] [CrossRef]
- Le Marchand, L.; Wilkens, L.R.; Castelfranco, A.M.; Monroe, K.R.; Kristal, B.S.; Cheng, I.; Maskarinec, G.; Hullar, M.A.; Lampe, J.W.; Shepherd, J.A.; et al. Circulating Biomarker Score for Visceral Fat and Risks of Incident Colorectal and Postmenopausal Breast Cancer: The Multiethnic Cohort Adiposity Phenotype Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 966–973. [Google Scholar] [CrossRef]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [PubMed]
- Carceller, V.; Tell, M.; Diaz, N. European association for the study of the liver (EASL)-51st international liver congress Barcelona, Spain—April 13–17, 2016. Drugs Future 2016, 41, 321–324. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999. [Google Scholar] [CrossRef]
- Mirza, M.S. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN Gastroenterol. 2011, 2011, 592404–592411. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Hairston, K.G.; Hanley, A.J.G.; Tooze, J.A.; Norris, J.M.; Palmer, N.D.; Bowden, D.W.; Lorenzo, C.; Chen, Y.D.I.; Wagenknecht, L.E. Association of Visceral Adipose Tissue and Insulin Resistance with Incident Metabolic Syndrome Independent of Obesity Status: The IRAS Family Study. Obesity 2021, 29, 1195–1202. [Google Scholar] [CrossRef]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Elands, R.J.J.; Simons, C.C.J.M.; van Dongen, M.; Schouten, L.J.; Verhage, B.A.J.; van den Brandt, P.A.; Weijenberg, M.P. A Systematic Literature Review and Meta-Regression Analysis on Early-Life Energy Restriction and Cancer Risk in Humans. PLoS ONE 2016, 11, e0158003. [Google Scholar] [CrossRef] [PubMed]
- Middleton, K.R.; Anton, S.D.; Perri, M.G. Long-Term Adherence to Health Behavior Change. Am. J. Lifestyle Med. 2013, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. J. Lab. Clin. Med. 2014, 164, 302–311. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss. Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Chew, H.S.J.; Ang, W.H.D.; Tan, Z.Y.A.; Ang, W.W.; Chan, K.S.; Lau, Y. Umbrella review of time-restricted eating on weight loss, fasting blood glucose, and lipid profile. Nutr. Rev. 2023, 81, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Przulj, D.; Pesola, F.; McRobbie, H.; Peerbux, S.; Phillips-Waller, A.; Bisal, N.; Smith, K.M. A randomised controlled trial of the 5:2 diet. PLoS ONE 2021, 16, e0258853. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhang, J.; Xu, J. Intermittent Versus Continuous Energy Restriction for Weight Loss and Metabolic Improvement: A Meta-Analysis and Systematic Review. Obesity 2021, 29, 108–115. [Google Scholar] [CrossRef] [PubMed]
- EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Dernini, S.; Berry, E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015, 2, 15. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Howell, T. Could Intermittent Energy Restriction and Intermittent Fasting Reduce Rates of Cancer in Obese, Overweight, and Normal-Weight Subjects? A Summary of Evidence. Adv. Nutr. 2016, 7, 690–705. [Google Scholar] [CrossRef]
- Hsu, C.C.; Ness, E.; Kowdley, K.V. Nutritional Approaches to Achieve Weight Loss in Nonalcoholic Fatty Liver Disease. Adv. Nutr. 2017, 8, 253–265. [Google Scholar] [CrossRef]
- Lee, H.A.; Moon, H.; Kim, Y.; Lee, H.A.; Kim, H.Y. Effect of 12-week intermittent calorie restriction compared to standard of care in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Curr. Control. Trials Cardiovasc. Med. 2023, 24, 490. [Google Scholar] [CrossRef] [PubMed]
- Panizza, C.E.; Lim, U.; Yonemori, K.M.; Cassel, K.D.; Wilkens, L.R.; Harvie, M.N.; Maskarinec, G.; Delp, E.J.; Lampe, J.W.; Shepherd, J.A.; et al. Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients 2019, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Maislin, G.; Ahmed, M.M.; Gooneratne, N.; Thorne-Fitzgerald, M.; Kim, C.; Teff, K.; Arnardottir, E.S.; Benediktsdottir, B.; Einarsdottir, H.; Juliusson, S.; et al. Single Slice vs. Volumetric MR Assessment of Visceral Adipose Tissue: Reliability and Validity Among the Overweight and Obese. Obesity 2012, 20, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Estrada, S.; Lu, R.; Conjeti, S.; Orozco-Ruiz, X.; Panos-Willuhn, J.; Breteler, M.M.B.; Reuter, M. FatSegNet: A fully automated deep learning pipeline for adipose tissue segmentation on abdominal dixon MRI. Magn. Reson. Med. 2020, 83, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Shvetsov, Y.B.; Wong, M.C.; Garber, A.; Monroe, K.; Ernst, T.M.; Buchthal, S.D.; Lim, U.; Le Marchand, L.; Heymsfield, S.B.; et al. Subcutaneous and visceral fat assessment by DXA and MRI in older adults and children. Obesity 2022, 30, 920–930. [Google Scholar]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, S.; Zanjani, S.; Gjellan, S.; Leinhard, O.D.; Kihlberg, J.; Smedby, Ö.; Johansson, L.; Kullberg, J.; Ahlström, H.; Lindström, T.; et al. Effects of moderate red wine consumption on liver fat and blood lipids: A prospective randomized study. Ann. Med. 2011, 43, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Resnicow, K.; Jackson, A.; Blissett, D.; Wang, T.; McCarty, F.; Rahotep, S.; Periasamy, S. Results of the Healthy Body Healthy Spirit Trial. Health Psychol. 2005, 24, 339–348. [Google Scholar] [CrossRef]
- Resnicow, K.; Jackson, A.; Wang, T.; De, A.K.; McCarty, F.; Dudley, W.N.; Baranowski, T. A Motivational Interviewing Intervention to Increase Fruit and Vegetable Intake Through Black Churches: Results of the Eat for Life Trial. Am. J. Public Health 2001, 91, 1686–1693. [Google Scholar] [CrossRef]
- Resnicow, K.; Kramish Campbell, M.; Carr, C.; McCarty, F.; Wang, T.; Periasamy, S.; Rahotep, S.; Doyle, C.; Williams, A.; Stables, G. Body and soul: A dietary intervention conducted through African-American churches. Am. J. Prev. Med. 2004, 27, 97–105. [Google Scholar] [CrossRef]
- Miller, W.; Rollnick, S. Motivational Interviewing: Preparing People for Change, 2nd ed. J. Healthc. Qual. 2003, 25, 46. [Google Scholar] [CrossRef]
- Nouwen, A.; Ford, T.; Balan, A.T.; Twisk, J.W.; Ruggiero, L.; White, D. Longitudinal motivational predictors of dietary self-care and diabetes control in adults with newly diagnosed type 2 diabetes mellitus. Health Psychol. 2011, 30, 771–779. [Google Scholar] [CrossRef]
- Sallis, J.F.; Grossman, R.M.; Pinski, R.B.; Patterson, T.L.; Nader, P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987, 16, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Rieger, E.; Sellbom, M.; Murray, K.; Caterson, I. Measuring social support for healthy eating and physical activity in obesity. Br. J. Health Psychol. 2018, 23, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Boushey, C.J.; Spoden, M.; Zhu, F.M.; Delp, E.J.; Kerr, D.A. New mobile methods for dietary assessment: Review of image-assisted and image-based dietary assessment methods. Proc. Nutr. Soc. 2017, 76, 283–294. [Google Scholar] [CrossRef]
- Fengqing, Z.; Bosch, M.; Insoo, W.; SungYe, K.; Boushey, C.J.; Ebert, D.S.; Delp, E.J. The Use of Mobile Devices in Aiding Dietary Assessment and Evaluation. IEEE J. Sel. Top. Signal Process. 2010, 4, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Neishabouri, A.; Nguyen, J.; Samuelsson, J.; Guthrie, T.; Biggs, M.; Wyatt, J.; Cross, D.; Karas, M.; Migueles, J.H.; Khan, S.; et al. Quantification of acceleration as activity counts in ActiGraph wearable. Sci. Rep. 2022, 12, 11958. [Google Scholar] [CrossRef]
- Daw, J.R.; Hatfield, L.A. Matching and Regression to the Mean in Difference-in-Differences Analysis. Health Serv. Res. 2018, 53, 4138–4156. [Google Scholar] [CrossRef]
- Lindner, R.; Cohen, J. Statistical Power Analysis for the Behavioral Sciences, rev ed.; Erlbaum: Hillsdale, NJ, USA, 1988; Volume 67, p. 1007. [Google Scholar]
- Wei, X.; Cooper, A.; Lee, I.; Cernoch, C.A.; Huntoon, G.; Hodek, B.; Christian, H.; Chao, A.M. Intermittent Energy Restriction for Weight Loss: A Systematic Review of Cardiometabolic, Inflammatory and Appetite Outcomes. Biol. Res. Nurs. 2022, 24, 410–428. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.; Reeves, M.M.; Yeo, S.; Morrison, G.; Carey, D.; Capra, S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: A randomised trial. Int. J. Obes. 2003, 27, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Clarke, R.E.; Coulter, S.N.; Rounsefell, K.N.; Walker, R.E.; Rauch, C.E.; Huggins, C.E.; Ryan, L. Intermittent energy restriction and weight loss: A systematic review. Eur. J. Clin. Nutr. 2016, 70, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Arguin, H.; Dionne, I.J.; Sénéchal, M.; Bouchard, D.R.; Carpentier, A.C.; Ardilouze, J.-L.; Tremblay, A.; Leblanc, C.; Brochu, M. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: A pilot study. Menopause 2012, 19, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.V.; Mullen, M.L.; Kelley, D.E.; Wing, R.R. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998, 21, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hutchison, A.T.; Thompson, C.H.; Lange, K.; Heilbronn, L.K. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 2019, 13, 408–415. [Google Scholar] [CrossRef]
- Bowen, J.; Brindal, E.; James-Martin, G.; Noakes, M. Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes. Nutrients 2018, 10, 1145. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion or Deferral Criteria |
|---|---|
| Age 35 to 69 years | Pregnancy Contraindications to MRI (e.g., pacemaker, metal implants, claustrophobia) |
| BMI between 25 and 40 kg/m2 | Surgical history involving the rectum, colon, small intestine, or limb amputation |
| Residence within a 20-mile radius of the UHCC | Inability to engage in at least one hour of daily exercise (walking) |
| Of Native Hawaiian, Other Pacific Islander, Japanese, Chinese, Korean, Filipino, or European/white ancestry | Individuals receiving insulin for Type 1 or Type 2 diabetes |
| Non-smoker | Receiving medication or hormones for thyroid treatment |
| No serious underlying health issues | |
| Non-drinker or low habitual alcohol intake (<15 drinks/week for men, <10 drinks/week for women) | Men taking anti-androgen medications |
| Fully vaccinated against COVID-19 | Substantial recent weight change (≥20 pounds/10 kg in <6 months) |
| Normal blood chemistry profile | Received chemotherapy or radiation therapy in the past six months |
| VAT area at L4/L5 ≥90 cm2 for men, ≥80 cm2 for women (estimated by DXA) | |
| Using steroid hormones (Cortisone, Prednisone, Methylprednisone) or weight-loss prescriptions (Lorcaserin, Orlistat, Phentermine, Qsymia) in the past six months |
| Week | Activity | Outcome Assessments | Mobile Diet Records (mFR™) | Dietary Counseling Sessions |
|---|---|---|---|---|
| Week 0 | Baseline Visit | Blood draw, DXA, MRI, Questionnaires, Physical Exam, Clinical Measurements | 4 days | 1 in-person session |
| Weeks 1–11 | Remote Counseling | 4 days (Weeks 5, 9, and 11) | 7 by phone or video | |
| Week 12 | Mid-Study Visit 2 (Clinic Visit) | Blood draw, DXA, MRI, Questionnaires, Physical Exam, Clinical Measurements | 1 in-person session | |
| Weeks 13–23 | Remote Counseling | 4 days (Weeks 17, 21, and 23) | 5 by phone or video | |
| Week 24 | End of Intervention Visit (Clinic Visit) | Blood draw, DXA, MRI Questionnaires, Physical Exam, Clinical Measurements | 1 in-person session | |
| Weeks 25–47 | Maintenance (No Specific Activity) | Clinical Follow-up, Questionnaires | 4 days (Week 47) | |
| Week 48 | Follow-up Visit (Clinic Visit) | Blood draw, DXA, Questionnaires, Physical Exam, Clinical Measurements | 1 in-person follow-up session |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, M.Y.; Yonemori, K.; Ross, A.; Wilkens, L.R.; Shepherd, J.; Cassel, K.; Stenger, A.; Rettenmeier, C.; Lim, U.; Boushey, C.; et al. Effect of Intermittent vs. Continuous Energy Restriction on Visceral Fat: Protocol for The Healthy Diet and Lifestyle Study 2 (HDLS2). Nutrients 2024, 16, 1478. https://doi.org/10.3390/nu16101478
Lewis MY, Yonemori K, Ross A, Wilkens LR, Shepherd J, Cassel K, Stenger A, Rettenmeier C, Lim U, Boushey C, et al. Effect of Intermittent vs. Continuous Energy Restriction on Visceral Fat: Protocol for The Healthy Diet and Lifestyle Study 2 (HDLS2). Nutrients. 2024; 16(10):1478. https://doi.org/10.3390/nu16101478
Chicago/Turabian StyleLewis, Michelle Y., Kim Yonemori, Alison Ross, Lynne R. Wilkens, John Shepherd, Kevin Cassel, Andrew Stenger, Christoph Rettenmeier, Unhee Lim, Carol Boushey, and et al. 2024. "Effect of Intermittent vs. Continuous Energy Restriction on Visceral Fat: Protocol for The Healthy Diet and Lifestyle Study 2 (HDLS2)" Nutrients 16, no. 10: 1478. https://doi.org/10.3390/nu16101478
APA StyleLewis, M. Y., Yonemori, K., Ross, A., Wilkens, L. R., Shepherd, J., Cassel, K., Stenger, A., Rettenmeier, C., Lim, U., Boushey, C., & Le Marchand, L. (2024). Effect of Intermittent vs. Continuous Energy Restriction on Visceral Fat: Protocol for The Healthy Diet and Lifestyle Study 2 (HDLS2). Nutrients, 16(10), 1478. https://doi.org/10.3390/nu16101478







