Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Designs
3.2. Glucose Metabolism
3.3. Energy Intake and Body Weight
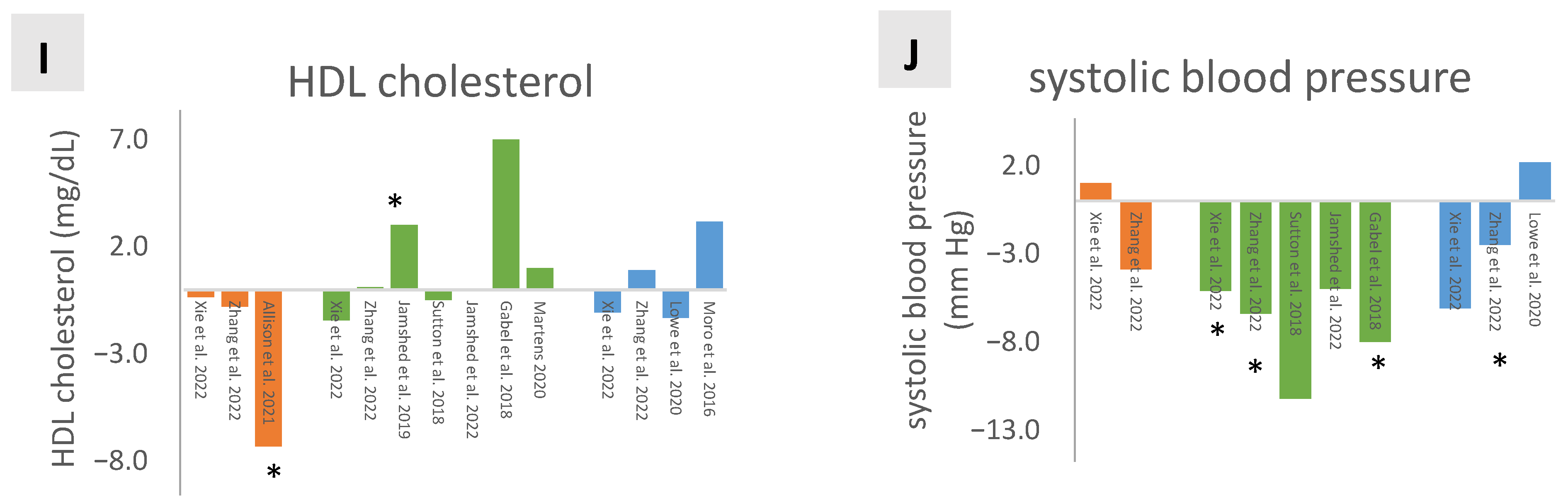
3.4. Lipid Profile
3.5. Systolic Blood Pressure (SBP)
3.6. Hunger Sensation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMAL1 | Basic helix-loop-helix ARNT like 1 |
| BW | Body weight |
| CLOCK | Circadian locomotor output cycles kaput |
| CVD | Cardiovascular disease |
| EI | Energy intake |
| eTRF | Early time-restricted feeding |
| GIP | Gastric inhibitory polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| HDL | High density lipoprotein |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| iAUC | Incremental area under the curve |
| IF | Intermittent fasting |
| LDL | Low density lipoprotein |
| lTRF | Late time-restricted feeding |
| MC4R | Melanocortin receptor 4 |
| PYY | Peptide YY |
| RCT | Randomized controlled trial |
| SBP | Systolic blood pressure |
| SCN | Suprachiasmatic nuclei |
| SNP | Single nucleotide polymorphism |
| T2D | Type 2 diabetes mellitus |
| TG | Triglycerides |
References
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Hall, J.; Reynolds, A.; Griffin, S.J.; Combet, E.; Lean, M.E.J. Diets for Weight Management in Adults with Type 2 Diabetes: An Umbrella Review of Published Meta-Analyses and Systematic Review of Trials of Diets for Diabetes Remission. Diabetologia 2022, 65, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, M.K. Chrononutrition—When We Eat Is of the Essence in Tackling Obesity. Nutrients 2022, 14, 5080. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Sassone-Corsi, P.; Zierath, J.R. Chrono-Nutrition for the Prevention and Treatment of Obesity and Type 2 Diabetes: From Mice to Men. Diabetologia 2020, 63, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-Nutrition: From Molecular and Neuronal Mechanisms to Human Epidemiology and Timed Feeding Patterns. J. Neurochem. 2021, 157, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Finger, A.M.; Dibner, C.; Kramer, A. Coupled Network of the Circadian Clocks: A Driving Force of Rhythmic Physiology. FEBS Lett. 2020, 594, 2734–2769. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, R.; Jouffe, C.; Oster, H.; Uhlenhaut, N.H.; Meyhöfer, S.M. When Should I Eat: A Circadian View on Food Intake and Metabolic Regulation. Acta Physiol. 2023, 237, e13936. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Tsameret, S.; Landau, Z. Role of High Energy Breakfast “Big Breakfast Diet” in Clock Gene Regulation of Postprandial Hyperglycemia and Weight Loss in Type 2 Diabetes. Nutrients 2021, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- van Amelsvoort, L.G.; Schouten, E.G.; Kok, F.J. Duration of Shiftwork Related to Body Mass Index and Waist to Hip Ratio. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Yang, C.; Tong, X.; Sun, H.; Cong, Y.; Yin, X.; Li, L.; Cao, S.; Dong, X.; Gong, Y.; et al. Shift Work and Diabetes Mellitus: A Meta-Analysis of Observational Studies. Occup. Environ. Med. 2015, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wefers, J.; Van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; Van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian Misalignment Induces Fatty Acid Metabolism Gene Profiles and Compromises Insulin Sensitivity in Human Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian Misalignment Augments Markers of Insulin Resistance and Inflammation, Independently of Sleep Loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Wicherski, J.; Schlesinger, S.; Fischer, F. Association between Breakfast Skipping and Body Weight—A Systematic Review and Meta-Analysis of Observational Longitudinal Studies. Nutrients 2021, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Q.; Pu, Y.; Guo, M.; Jiang, Z.; Huang, W.; Long, Y.; Xu, Y. Skipping Breakfast Is Associated with Overweight and Obesity: A Systematic Review and Meta-Analysis. Obes. Res. Clin. Pract. 2020, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Pilla, S.J.; Michalski, K.; Laferrère, B.; Clark, J.M.; Maruthur, N.M. Eating Breakfast Is Associated with Weight Loss during an Intensive Lifestyle Intervention for Overweight/Obesity. Obesity 2022, 30, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric Intake at Breakfast vs. Dinner Differentially Influences Weight Loss of Overweight and Obese Women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.P.; Cardel, M.I.; Cellini, J.; Hu, F.B.; Guasch-Ferré, M. Breakfast Skipping, Body Composition, and Cardiometabolic Risk: A Systematic Review and Meta-Analysis of Randomized Trials. Obesity 2020, 28, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Morgan, P.J.; Fyfe, C.L.; Filipe, J.A.N.; Horgan, G.W.; Westerterp, K.R.; Johnston, J.D.; Johnstone, A.M. Timing of Daily Calorie Loading Affects Appetite and Hunger Responses without Changes in Energy Metabolism in Healthy Subjects with Obesity. Cell Metab. 2022, 34, 1472–1485.e6. [Google Scholar] [CrossRef] [PubMed]
- Maria, P.; Carrasco-Benso, M.P.; Rivero-Gutierrez, B.; Lopez-Minguez, J.; Anzola, A.; Diez-Noguera, A.; Madrid, J.A.; Lujan, J.A.; Martínez-Augustin, O.; Scheer, F.A.J.L.; et al. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016, 30, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Morris, C.J.; Caputo, R.; Garaulet, M.; Scheer, F.A.J.L. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. 2019, 43, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Al Abdi, T.; Andreou, E.; Papageorgiou, A.; Heraclides, A.; Philippou, E. Personality, Chrono-Nutrition and Cardiometabolic Health: A Narrative Review of the Evidence. Adv. Nutr. 2020, 11, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.A.; Sandesara, P.B.; Dhindsa, D.S.; Mehta, A.; Arneson, L.C.; Dollar, A.L.; Taub, P.R.; Sperling, L.S. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am. J. Med. 2020, 133, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Crupi, A.N.; Haase, J.; Brandhorst, S.; Longo, V.D. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr. Diabetes Rep. 2020, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657.e3. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized Controlled Trial for Time-Restricted Eating in Healthy Volunteers without Obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M.; Liu, Z.; Wang, J.-Q.; Li, R.-Q.; Ren, J.-Y.; Gao, X.; Lv, S.-S.; Liang, L.-Y.; Zhang, F.; Yin, B.-W.; et al. Randomized Controlled Trial for Time-Restricted Eating in Overweight and Obese Young Adults. iScience 2022, 25, 104870. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of Eight Weeks of Time-Restricted Feeding (16/8) on Basal Metabolism, Maximal Strength, Body Composition, Inflammation, and Cardiovascular Risk Factors in Resistance-Trained Males. J. Transl. Med. 2016, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Hong, N.; Kim, K.W.; Cho, S.J.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.; Johnston, J.D.; Robertson, M.D. Early versus Late Time-Restricted Feeding in Adults at Increased Risk of Developing Type 2 Diabetes: Is There an Optimal Time to Eat for Metabolic Health? Nutr. Bull. 2021, 46, 69–76. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-Term Time-Restricted Feeding Is Safe and Feasible in Non-Obese Healthy Midlife and Older Adults. Geroscience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults with Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Pabla, P.; Mallinson, J.; Nixon, A.; Taylor, T.; Bennett, A.; Tsintzas, K. Two Weeks of Early Time-Restricted Feeding (ETRF) Improves Skeletal Muscle Insulin and Anabolic Sensitivity in Healthy Men. Am. J. Clin. Nutr. 2020, 112, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-Hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ezpeleta, M.; Cienfuegos, S.; Lin, S.; Pavlou, V.; Gabel, K.; Tussing-Humphreys, L.; Varady, K.A. Time-Restricted Eating: Watching the Clock to Treat Obesity. Cell Metab. 2024, 36, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do Ketogenic Diets Really Suppress Appetite? A Systematic Review and Meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.R.; With, E.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. The Impact of Rate of Weight Loss on Body Composition and Compensatory Mechanisms during Weight Reduction: A Randomized Control Trial. Clin. Nutr. 2018, 37, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Nymo, S.; Coutinho, S.R.; Jørgensen, J.; Rehfeld, J.F.; Truby, H.; Kulseng, B.; Martins, C. Timeline of Changes in Appetite during Weight Loss with a Ketogenic Diet. Int. J. Obes. 2017, 41, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Lyngstad, A.; Nymo, S.; Coutinho, S.R.; Rehfeld, J.F.; Truby, H.; Kulseng, B.; Martins, C. Investigating the Effect of Sex and Ketosis on Weight-Loss-Induced Changes in Appetite. Am. J. Clin. Nutr. 2019, 109, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and Appetite-Mediating Nutrients and Hormones after Weight Loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, Ketogenic Diet and Food Intake Control: A Complex Relationship. Front. Psychol. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Deemer, S.E.; Plaisance, E.P.; Martins, C. Impact of Ketosis on Appetite Regulation—A Review. Nutr. Res. 2020, 77, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.J.; James, L.J. The Effect of Breakfast on Appetite Regulation, Energy Balance and Exercise Performance. Proc. Nutr. Soc. 2016, 75, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Lesani, A.; Barkhidarian, B.; Jafarzadeh, M.; Akbarzade, Z.; Djafarian, K.; Shab-Bidar, S. Time-Related Meal Patterns and Breakfast Quality in a Sample of Iranian Adults. BMC Nutr. 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.; Das, S.K.; Lichtenstein, A.H.; Dallal, G.E.; Corrales, A.; Schaefer, E.J.; Greenberg, A.S.; Roberts, S.B. An Oat-Containing Hypocaloric Diet Reduces Systolic Blood Pressure and Improves Lipid Profile beyond Effects of Weight Loss in Men and Women. J. Nutr. 2001, 131, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Kohara, K.; Katoh, T.; Kusunoki, T.; Ohtsuka, N.; Abe, M.; Kumagi, T.; Miki, T. Effect of Weight Loss on Central Systolic Blood Pressure in Elderly Community-Dwelling Persons. Hypertens. Res. 2014, 37, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Flanagan, A.; Johnston, J.D.; Morgan, P.J.; Johnstone, A.M. Circadian Rhythms in Resting Metabolic Rate Account for Apparent Daily Rhythms in the Thermic Effect of Food. J. Clin. Endocrinol. Metab. 2022, 107, E708–E715. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Joo, Y.; Kim, M.S.; Choe, H.K.; Tong, Q.; Kwon, O. Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones. Endocrinol. Metab. 2021, 36, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Zitting, K.M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human Resting Energy Expenditure Varies with Circadian Phase. Curr. Biol. 2018, 28, 3685–3690.e3. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Morgan, P.J.; Johnstone, A.M. Mealtime: A Circadian Disruptor and Determinant of Energy Balance? J. Neuroendocrinol. 2020, 32, 12886. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, Q.; Wu, C. The Role of Adiponectin in Cardiovascular Disease. Cardiovasc. Pathol. 2023, 64, 107514. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Yamamoto, Y.; Takasugi, Y.; Ishii, H.; Uchiyama, T.; Saitoh, K.; Suzuki, M.; Uchiyama, M.; Yoshitane, H.; Fukada, Y.; et al. Adiponectin Regulates the Circadian Rhythm of Glucose and Lipid Metabolism. J. Endocrinol. 2022, 254, 121–133. [Google Scholar] [CrossRef] [PubMed]
- BaHammam, A.S.; Pirzada, A. Timing Matters: The Interplay between Early Mealtime, Circadian Rhythms, Gene Expression, Circadian Hormones, and Metabolism—A Narrative Review. Clocks Sleep 2023, 5, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Bitsanis, D.; Giannakou, K.; Hadjimbei, E.; Chrysostomou, S. The Effect of Early Time-Restricted Feeding on Glycemic Profile in Adults: A Systematic Review of Interventional Studies. Rev. Diabet. Stud. 2022, 18, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Skow, S.L.; Jha, R.K. A Ketogenic Diet Is Effective in Improving Insulin Sensitivity in Individuals with Type 2 Diabetes. Curr. Diabetes Rev. 2023, 19, e250422203985. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Chang, A.M.; Bjonnes, A.C.; Aeschbach, D.; Anderson, C.; Cade, B.E.; Cain, S.W.; Czeisler, C.A.; Gharib, S.A.; Gooley, J.J.; et al. Impact of Common Diabetes Risk Variant in MTNR1B on Sleep, Circadian, and Melatonin Physiology. Diabetes 2016, 65, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, A.; Gerrard, J.; Taylor, R. The Second-Meal Phenomenon in Type 2 Diabetes. Diabetes Care 2009, 32, 1199–1201. [Google Scholar] [CrossRef]
- Xiao, K.; Furutani, A.; Sasaki, H.; Takahashi, M.; Shibata, S. Effect of a High Protein Diet at Breakfast on Postprandial Glucose Level at Dinner Time in Healthy Adults. Nutrients 2023, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty Acids, Obesity, and Insulin Resistance: Time for a Reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. The Impact of Fasting on Adipose Tissue Metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159262. [Google Scholar] [CrossRef] [PubMed]
- Mend, H.; Zhu, L.; Kord-Varkaneh, H.; Santos, H.; Tinsley, G.; Fu, P. Effects of Intermittent Fasting and Energy-Restricted Diets on Lipid Profile: A Systematic Review and Meta-Analysis. Nutrition 2020, 77, 110801. [Google Scholar]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-Restricted Feeding and Risk of Metabolic Disease: A Review of Human and Animal Studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Maranhão Pureza, I.R.; de Lima Macena, M.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; Bezerra Bueno, N. Effect of Early Time-Restricted Feeding on the Metabolic Profile of Adults with Excess Weight: A Systematic Review with Meta-Analysis. Clin. Nutr. 2021, 40, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Lin, S.; Varady, K.A. Changes in Body Weight and Metabolic Risk during Time Restricted Feeding in Premenopausal versus Postmenopausal Women. Exp. Gerontol. 2021, 154, 111545. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Wang, Y.-T.; Chan, L.-C.; Chu, N.-F. Effect of Time-Restricted Feeding on Body Composition and Cardio-Metabolic Risk in Middle-Aged Women in Taiwan. Nutrition 2022, 93, 111504. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Garaulet, M.; Scheer, F.A.J.L. Meal timing and obesity; interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.S.G.; Cañavate, R.; Hernández, C.M.; Cara-Salmerón, V.; Morante, J.J.H. The association among chronotype, timing of food intake and food preferences depends on body mass status. Eur. J. Clin. Nutr. 2017, 71, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.D.; Ordovás, J.M.; Scheer, F.A.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv. Nutr. 2016, 7, 399–406. [Google Scholar] [CrossRef] [PubMed]


| Total number of studies (n) | 13 |
| Number of participants per study (n) | 8 to 90 |
| Ethnicity | All ethnicities (usually >1 in each study) |
| Age range (years) | 22.1 to 68 |
| BMI range (kg/m2) | 21.4 to 39.6 |
| Fasting glucose baseline (mg/dL) | 73.4 to 110 |
| Intervention groups (arms) | 2× eTRF vs. lTRF [28,29] 2× eTRF vs. lTRF vs. control [30,31] 7× eTRF vs. control [34,38,39,40,41,42,43] 2× lTRF vs. control [32,33] |
| Caloric intake | 5× isocaloric or energy resricted [33,38,39,41,42] 6× ad libitum/habitual diets [29,30,31,32,40,43] 2× prescribed, isocaloric diets [28,34] |
| Feeding window (h) | 6 to 11 h for TRF and >8 to 15 h for control |
| Intervention duration | 4 days to 14 weeks |
| Macronutrient composition (%) | Carbohydrates: 45–55% Fat: 23.9–35% Protein: 15–21.4% |
| Meal frequency | IF predetermined: 3 to 5 meals/day |
| Breakfast calories (in TRF arms) | 33–40% of energy intake |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petridi, F.; Geurts, J.M.W.; Nyakayiru, J.; Schaafsma, A.; Schaafsma, D.; Meex, R.C.R.; Singh-Povel, C.M. Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment. Nutrients 2024, 16, 1721. https://doi.org/10.3390/nu16111721
Petridi F, Geurts JMW, Nyakayiru J, Schaafsma A, Schaafsma D, Meex RCR, Singh-Povel CM. Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment. Nutrients. 2024; 16(11):1721. https://doi.org/10.3390/nu16111721
Chicago/Turabian StylePetridi, Froso, Jan M. W. Geurts, Jean Nyakayiru, Anne Schaafsma, Dedmer Schaafsma, Ruth C. R. Meex, and Cécile M. Singh-Povel. 2024. "Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment" Nutrients 16, no. 11: 1721. https://doi.org/10.3390/nu16111721
APA StylePetridi, F., Geurts, J. M. W., Nyakayiru, J., Schaafsma, A., Schaafsma, D., Meex, R. C. R., & Singh-Povel, C. M. (2024). Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment. Nutrients, 16(11), 1721. https://doi.org/10.3390/nu16111721









