Unveiling the Threat of Maternal Advanced Glycation End Products to Fetal Muscle: Palmitoleic Acid to the Rescue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Preparation of AGEs
2.4. Preparation of BSA-Conjugated Palmitoleic Acid
2.5. Cell Viability Assay
2.6. Measurement of Intracellular ROS Generation
2.7. Immunofluorescence Staining
2.8. RNA Extraction and RT-qPCR
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
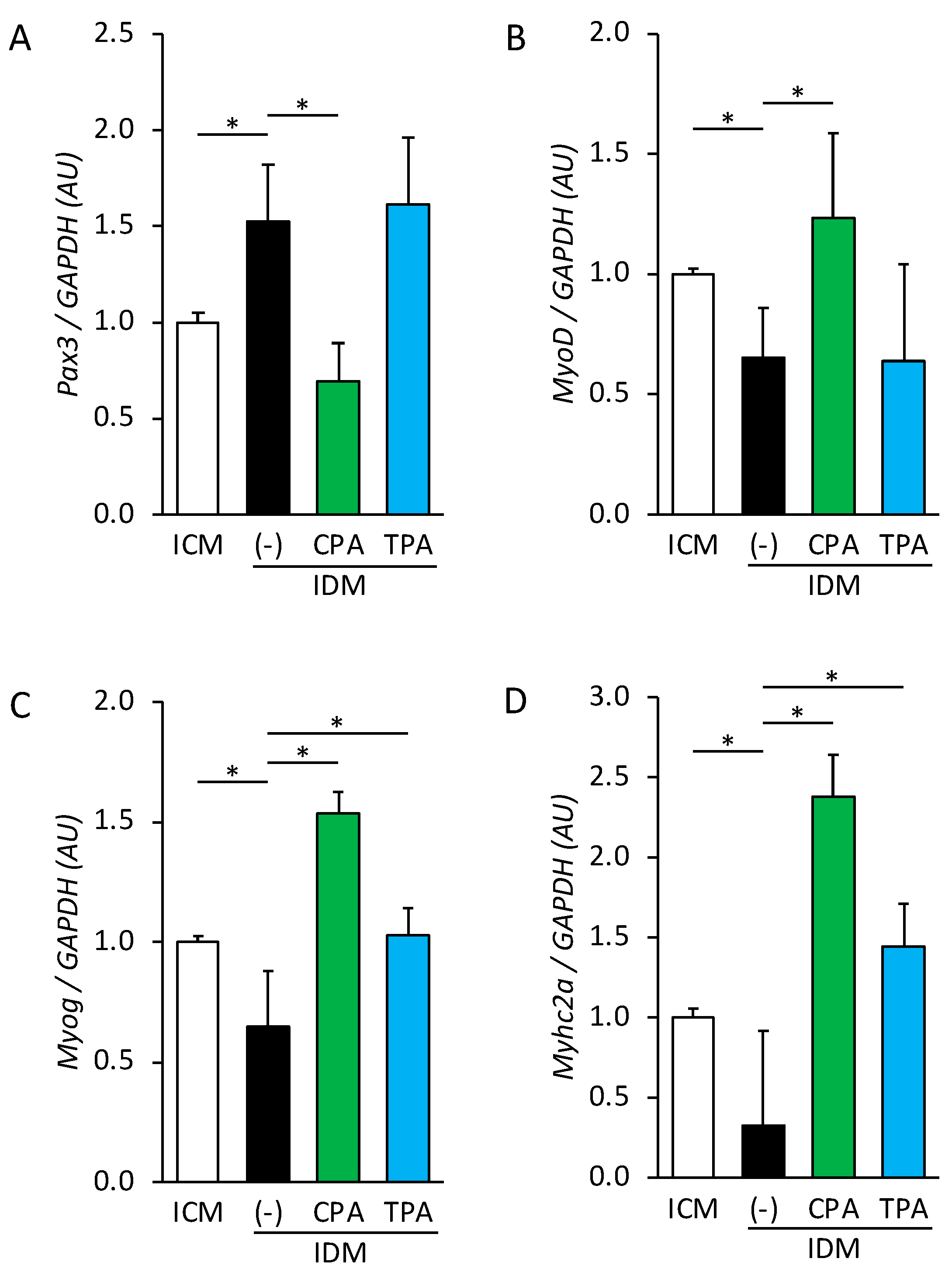
3.1. Effect of Palmitoleic Acid Intake on Gene Expression in Fetal Skeletal Muscle in Hyperglycemic Intrauterine Environments
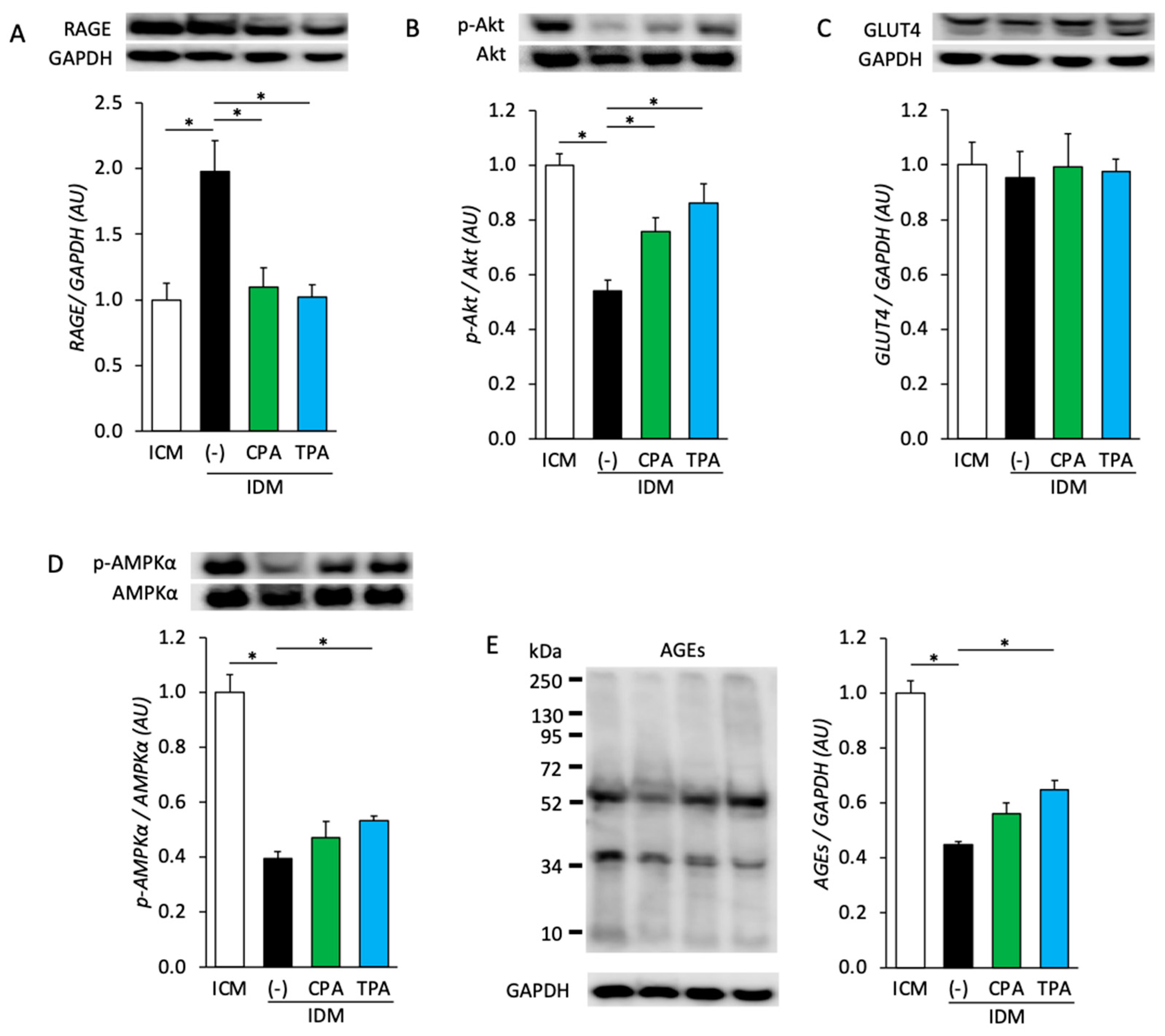
3.2. Effects of Maternal Hyperglycemia and Intake of Palmitoleic Acid on Protein Expression and Phosphorylation in Fetal Skeletal Muscle
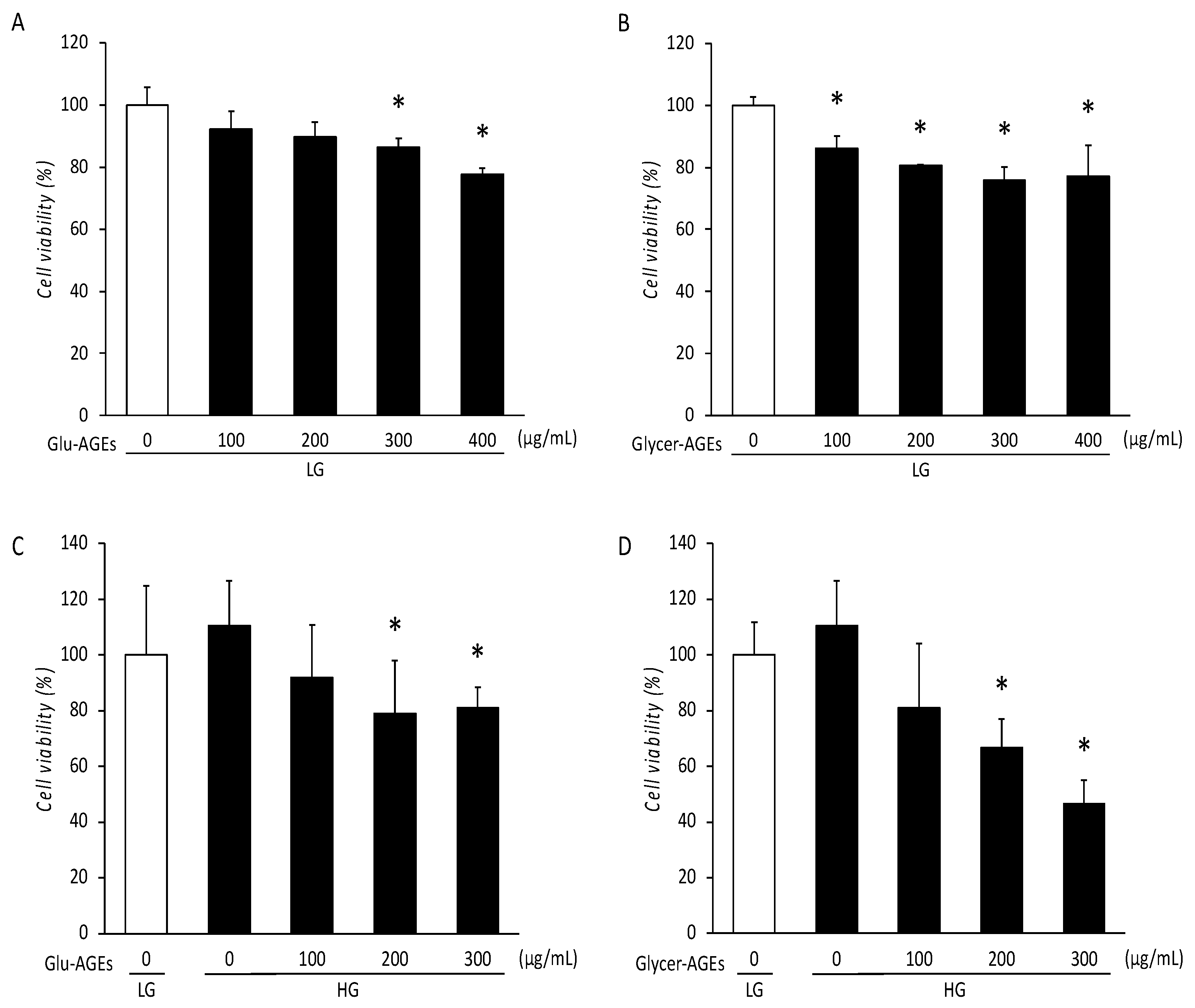
3.3. Viability of C2C12 Cells Treated with AGEs
3.4. Intracellular ROS Generation Level in AGE-Treated C2C12 Cells
3.5. Myotube Formation in AGE-Treated C2C12 Cells
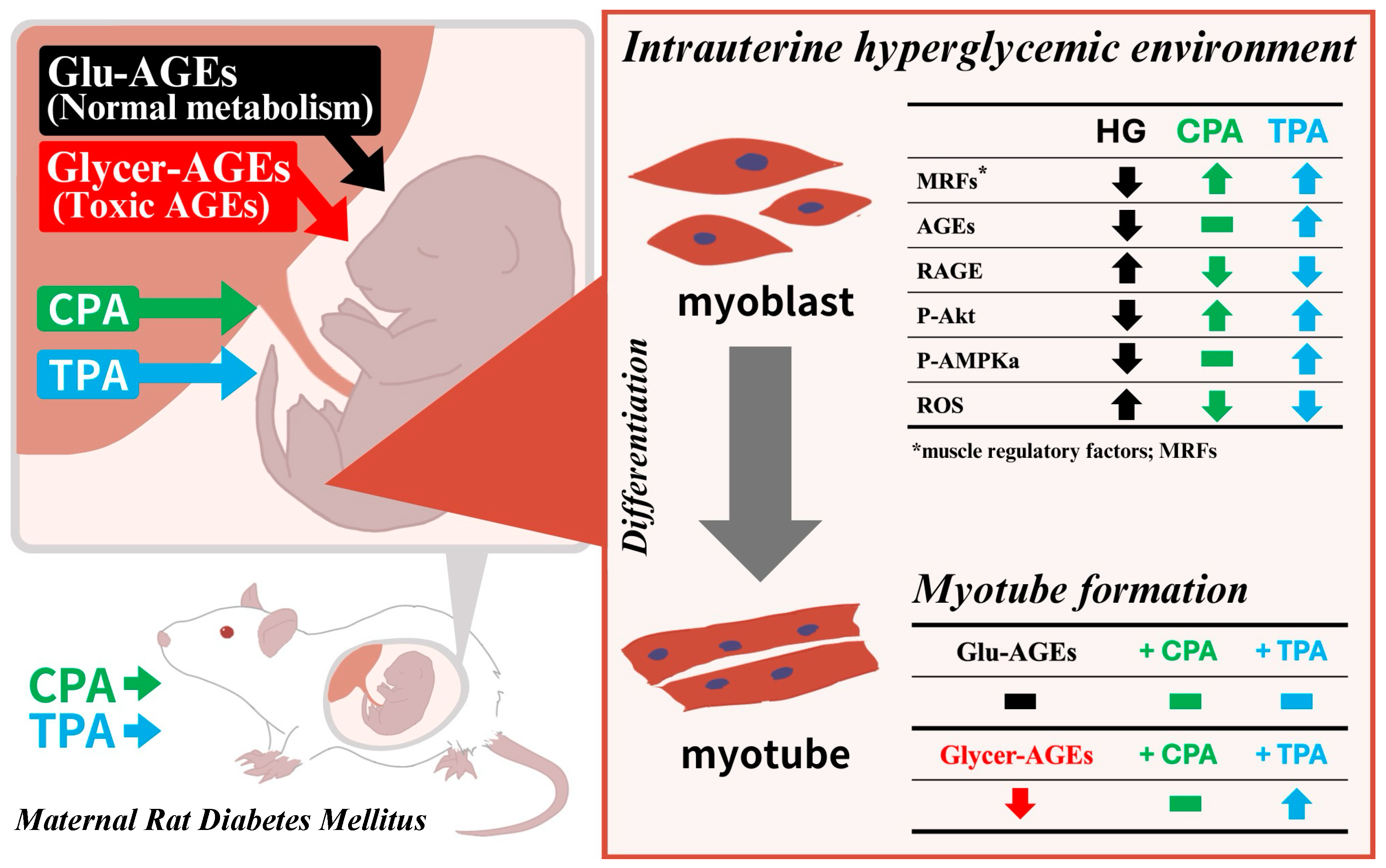
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhao, Z.; Xu, J.; Chen, Y.; Zhu, Q.; Zhou, L.; Cai, J.; Ji, L. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front. Endocrinol. 2022, 13, 960877. [Google Scholar] [CrossRef] [PubMed]
- Tinker, S.C.; Gilboa, S.M.; Moore, C.A.; Waller, D.K.; Simeone, R.M.; Kim, S.Y.; Jamieson, D.J.; Botto, L.D.; Reefhuis, J.; National Birth Defects Prevention Study. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am. J. Obstet. Gynecol. 2020, 222, 176.e1–176.e11. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Nilsson, I.A.K.; Gissler, M.; Lavebratt, C. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA Pediatr. 2019, 173, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Q.; Qiu, X.; Wang, L.; Li, X.; Huo, X.Y. Left atrial shortening fraction to predict fetal cardiac abnormalities and dysfunction in gestational diabetes mellitus. Front. Cardiovasc. Med. 2022, 9, 1026587. [Google Scholar] [CrossRef]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Edessa, D.; Ali, T.; Mekuria, A.N.; Gebrie, A. The relationship between advanced glycation end products and gestational diabetes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0240382. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Takeuchi, M.; Iwaki, M.; Takino, J.; Shirai, H.; Kawakami, M.; Bucala, R.; Yamagishi, S. Immunological detection of fructose-derived advanced glycation end-products. Lab. Investig. 2010, 90, 1117–1127. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamagishi, S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med. Hypotheses 2004, 63, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Sakasai-Sakai, A.; Ueda, T.; Takeuchi, M. Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease. Sci. Rep. 2019, 9, 2121. [Google Scholar] [CrossRef] [PubMed]
- Hyogo, H.; Yamagishi, S.; Iwamoto, K.; Arihiro, K.; Takeuchi, M.; Sato, T.; Ochi, H.; Nonaka, M.; Nabeshima, Y.; Inoue, M.; et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sato, T.; Takino, J.; Kobayashi, Y.; Furuno, S.; Kikuchi, S.; Yamagishi, S. Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer’s disease. Med. Hypotheses 2007, 69, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Takino, J.I.; Yamagishi, S.I.; Takeuchi, M. Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J. Oncol. 2010, 2010, 739852. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative stress in health and disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Kawaharada, R.; Masuda, H.; Chen, Z.; Blough, E.; Kohama, T.; Nakamura, A. Intrauterine hyperglycemia-induced inflammatory signalling via the receptor for advanced glycation end products in the cardiac muscle of the infants of diabetic mother rats. Eur. J. Nutr. 2018, 57, 2701–2712. [Google Scholar] [CrossRef]
- Okami, H.; Kawaharada, R.; Yoshizaki, H.; Toriumi, A.; Tsutsumi, S.; Nakamura, A. Maternal n-7 Unsaturated Fatty Acids Protect the Fetal Brain from Neuronal Degeneration in an intrauterine hyperglycemic Animal Model. Nutrients 2023, 15, 3434. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef]
- Cervelli, M.; Leonetti, A.; Duranti, G.; Sabatini, S.; Ceci, R.; Mariottini, P. Skeletal muscle pathophysiology: The emerging role of spermine oxidase and spermidine. Med. Sci. 2018, 6, 14. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E949–E959. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.F.L.; Junqueira, R.L.; Gaspar, R.C.; Muñoz, V.R.; Pauli, J.R. Exercise activates AMPK signaling: Impact on glucose uptake in the skeletal muscle in aging. J. Rehab Ther. 2020, 2, 48–53. [Google Scholar]
- Tokunaga, Y.; Yoshizaki, H.; Toriumi, A.; Kawaharada, R.; Ishida, C.; Hori, M.; Nakamura, A. Effects of omega-7 palmitoleic acids on skeletal muscle differentiation in a hyperglycemic condition. J. Vet. Med. Sci. 2021, 83, 1369–1377. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Zhong, L.; Sun, C.; Zhang, Z. Simultaneous determination of cis- and trans-palmitoleic acid in rat serum by UPLC-MS/MS. Sci. Rep. 2022, 12, 16637. [Google Scholar] [CrossRef]
- Guillocheau, E.; Legrand, P.; Rioux, V. Trans-palmitoleic acid (trans-9-C16:1, or trans-C16:1 n-7): Nutritional impacts, metabolism, origin, compositional data, analytical methods and chemical synthesis. A review. Biochimie 2020, 169, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Willett, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef]
- Mozaffarian, D.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Hotamisligil, G.; Tsai, M.Y.; Siscovick, D.S.; Nettleton, J.A. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2013, 97, 854–861. [Google Scholar] [CrossRef]
- Takeuchi, M.; Bucala, R.; Suzuki, T.; Ohkubo, T.; Yamazaki, M.; Koike, T.; Kameda, Y.; Makita, Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol. 2000, 59, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Impact of intracellular toxic advanced glycation end-products (TAGE) on murine myoblast cell death. Diabetol. Metab. Syndr. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Lin, I.H.; Shieh, T.M.; Ko, H.A.; Chen, H.I.; Chi, T.C.; Chang, S.S.; Hsu, Y.C. Cell hypertrophy and MEK/ERK phosphorylation are regulated by glyceraldehyde-derived AGEs in cardiomyocyte H9c2 cells. Cell Biochem. Biophys. 2013, 66, 537–544. [Google Scholar] [CrossRef]
- Meng, H.Z.; Zhang, W.L.; Liu, F.; Yang, M.W. Advanced glycation end products affect osteoblast proliferation and function by modulating autophagy via the receptor of advanced glycation end products/Raf protein/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (RAGE/Raf/MEK/ERK) pathway. J. Biol. Chem. 2015, 290, 28189–28199. [Google Scholar] [CrossRef]
- Epstein, J.A.; Lam, P.; Jepeal, L.; Maas, R.L.; Shapiro, D.N. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J. Biol. Chem. 1995, 270, 11719–11722. [Google Scholar] [CrossRef]
- Yokoyama, S.; Asahara, H. The myogenic transcriptional network. Cell. Mol. Life Sci. 2011, 68, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Walke, P.B.; Bansode, S.B.; More, N.P.; Chaurasiya, A.H.; Joshi, R.S.; Kulkarni, M.J. Molecular investigation of glycated insulin-induced insulin resistance via insulin signaling and AGE-RAGE axis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166029. [Google Scholar] [CrossRef]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin signal transduction perturbations in insulin resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef]
- Abdelmoez, A.M.; Sardón Puig, L.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. [Google Scholar] [CrossRef]
- Lawson, M.A.; Purslow, P.P. Differentiation of myoblasts in serum-free media: Effects of modified media are cell line-specific. Cells Tissues Organs 2000, 167, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Yonei, Y. Glycative stress and antiaging: 10. Glycative stress and liver disease. Glycative Stress Res. 2018, 5, 177–180. [Google Scholar]
- Takeuchi, M. Toxic AGEs (TAGE) theory: A new concept for preventing the development of diseases related to lifestyle. Diabetol. Metab. Syndr. 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.I.; Koriyama, Y. Effects of toxic AGEs (TAGE) on human health. Cells 2022, 11, 2178. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the pathophysiology of skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-stimulated ROS Sensitive Signaling pathways in skeletal muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. C2C12 cell model: Its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. J. Pharm. Pharmacol. 2020, 72, 1667–1693. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Pax3 | CAGCCCACGTCTATTCCACA | CACGAAGCTGTCGGTGTAGC |
| MyoD | TGGATCAATCCCACTCTAATAGC | TTCGCTGGTAGGAAAGTGAAG |
| Myog | CTACAGGCCTTGCTCA | TGGGAGTTGCATTCAC |
| Myhc2a | TCCTCAGGCTTCAAGATTTG | TTAAATAGAATCACATGGGGAC |
| GAPDH | CTACCCACGGCAAGTTCAAC | CCAGTAGACTCCACGACATAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshizaki, H.; Kawaharada, R.; Tsutsumi, S.; Okami, H.; Toriumi, A.; Miyata, E.; Nakamura, A. Unveiling the Threat of Maternal Advanced Glycation End Products to Fetal Muscle: Palmitoleic Acid to the Rescue. Nutrients 2024, 16, 1898. https://doi.org/10.3390/nu16121898
Yoshizaki H, Kawaharada R, Tsutsumi S, Okami H, Toriumi A, Miyata E, Nakamura A. Unveiling the Threat of Maternal Advanced Glycation End Products to Fetal Muscle: Palmitoleic Acid to the Rescue. Nutrients. 2024; 16(12):1898. https://doi.org/10.3390/nu16121898
Chicago/Turabian StyleYoshizaki, Hitomi, Ritsuko Kawaharada, Saki Tsutsumi, Haruka Okami, Akiyo Toriumi, Eri Miyata, and Akio Nakamura. 2024. "Unveiling the Threat of Maternal Advanced Glycation End Products to Fetal Muscle: Palmitoleic Acid to the Rescue" Nutrients 16, no. 12: 1898. https://doi.org/10.3390/nu16121898
APA StyleYoshizaki, H., Kawaharada, R., Tsutsumi, S., Okami, H., Toriumi, A., Miyata, E., & Nakamura, A. (2024). Unveiling the Threat of Maternal Advanced Glycation End Products to Fetal Muscle: Palmitoleic Acid to the Rescue. Nutrients, 16(12), 1898. https://doi.org/10.3390/nu16121898






