Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Participants
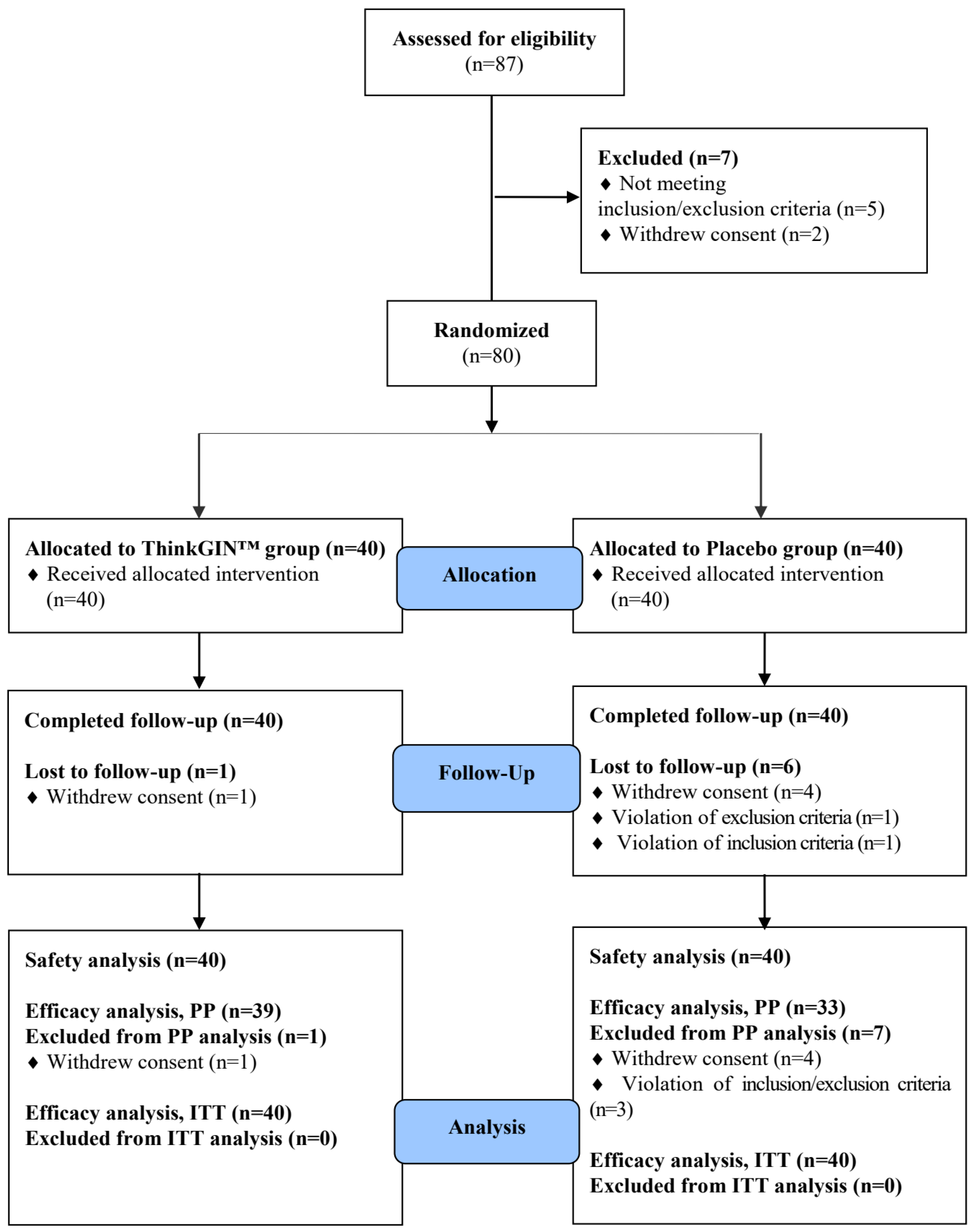
2.3. Study Design and Randomization
2.4. Study Products and Interventions
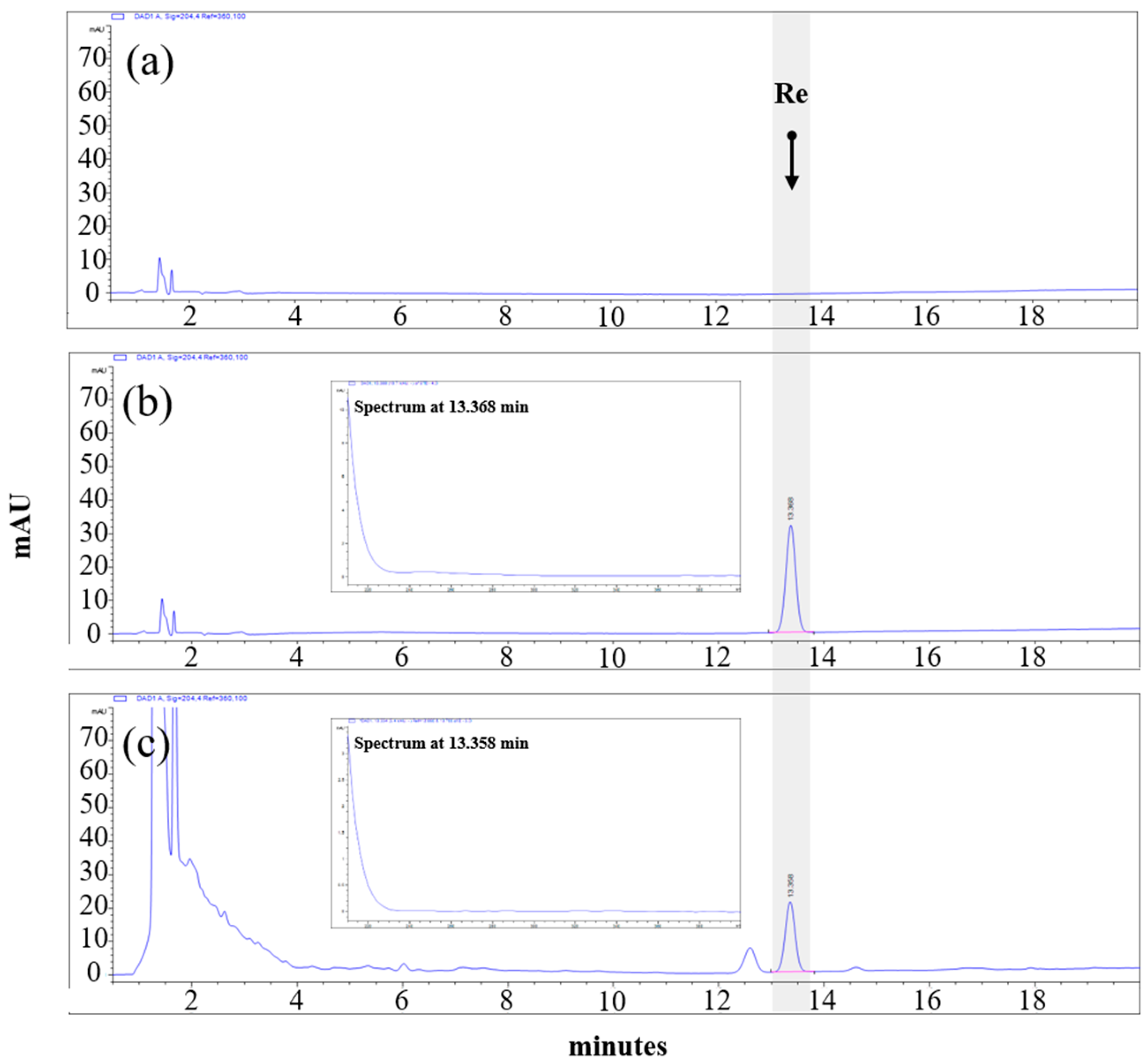
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Efficacy Outcome Measures
2.7. Safety Outcome Measures
2.8. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Dietary Intake and Physical Activity
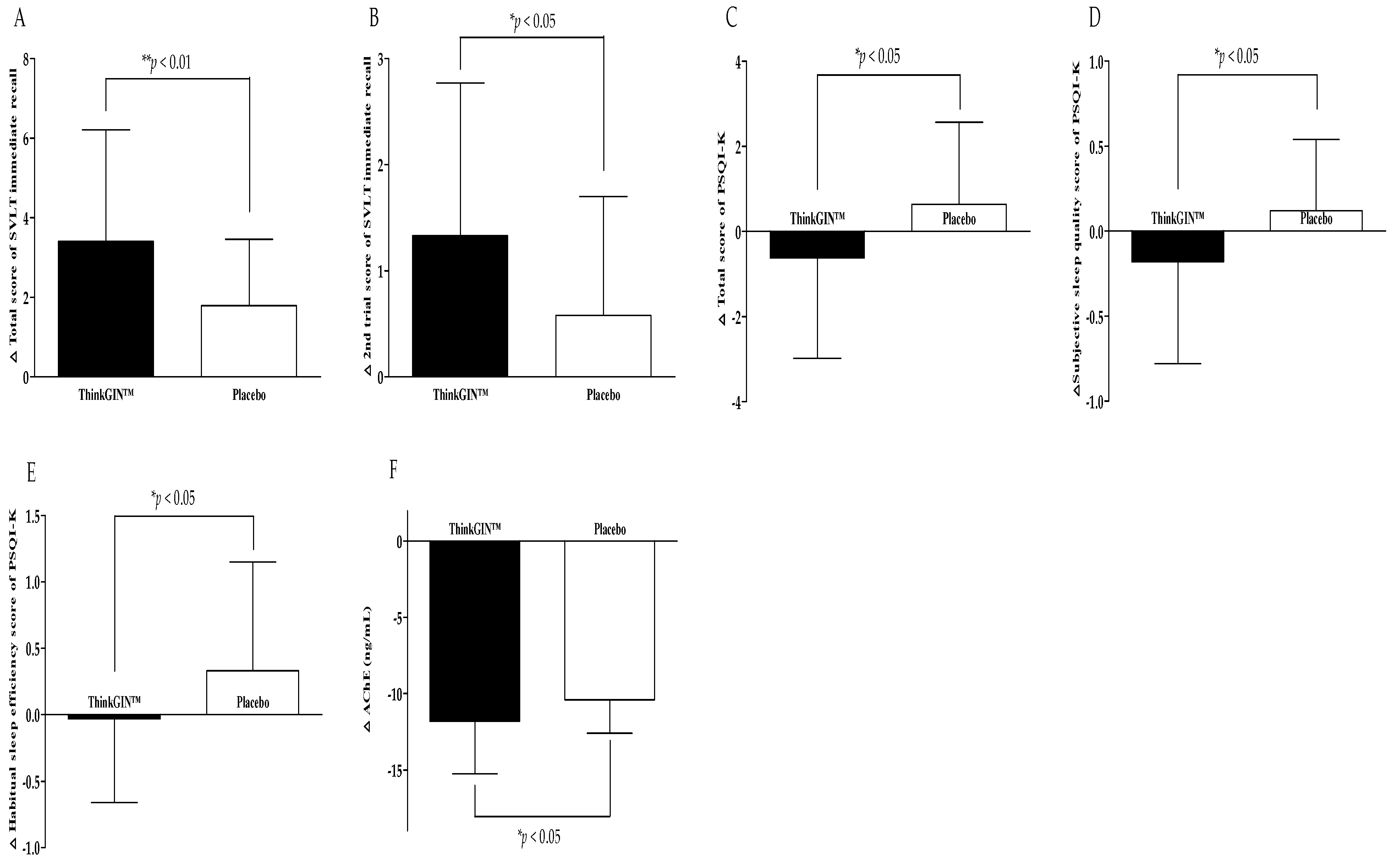
3.3. Efficacy Outcomes
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public Health 2021, 50, 2451–2460. [Google Scholar] [CrossRef]
- United Nations. Department of Economic and Social Affairs, Population Division. In World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Nakazaki, E.; Mah, E.; Sanoshy, K.; Citrolo, D.; Watanabe, F. Citicoline and Memory Function in Healthy Older Adults: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Nutr. 2021, 151, 2153–2160. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Su, K.-P.; Cheng, T.-C.; Liu, H.-C.; Chang, C.-J.; Dewey, M.E.; Stewart, R.; Huang, S.-Y. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: A preliminary randomized double-blind placebo-controlled study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1538–1544. [Google Scholar] [CrossRef]
- Burns, A. Diagnosis and management of Alzheimer’s disease. Dialogues Clin. Neurosci. 2000, 2, 129–138. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Weigand, S.D.; Wiste, H.J.; Vemuri, P.; Lowe, V.; Kantarci, K.; Gunter, J.L.; Senjem, M.L.; Ivnik, R.J. An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann. Neurol. 2012, 71, 765–775. [Google Scholar] [CrossRef]
- Lee, R.; Kim, J.-H.; Kim, W.-W.; Hwang, S.-H.; Choi, S.-H.; Kim, J.-H.; Cho, I.-H.; Kim, M.; Nah, S.-Y. Emerging evidence that ginseng components improve cognition in subjective memory impairment, mild cognitive impairment, and early Alzheimer’s disease dementia. J. Ginseng Res. 2024, 48, 245–252. [Google Scholar] [CrossRef]
- Massoud, F.; Léger, G.C. Pharmacological treatment of Alzheimer disease. Can. J. Psychiatry 2011, 56, 579–588. [Google Scholar] [CrossRef]
- Kim, J.-H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Ying, Z.; Awais, M.; Akter, R.; Xu, F.; Baik, S.; Jung, D.; Yang, D.C.; Kwak, G.-Y.; Wenying, Y. Discrimination of Panax ginseng from counterfeits using single nucleotide polymorphism: A focused review. Front. Plant Sci. 2022, 13, 903306. [Google Scholar] [CrossRef]
- Adil, M.; Jeong, B.R. In vitro cultivation of Panax ginseng C.A. Meyer. Ind. Crops Prod. 2018, 122, 239–251. [Google Scholar] [CrossRef]
- Morshed, M.N.; Ahn, J.C.; Mathiyalagan, R.; Rupa, E.J.; Akter, R.; Karim, M.R.; Jung, D.H.; Yang, D.U.; Yang, D.C.; Jung, S.K. Antioxidant Activity of Panax ginseng to Regulate ROS in Various Chronic Diseases. Appl. Sci. 2023, 13, 2893. [Google Scholar] [CrossRef]
- Cho, I.H. Effects of Panax ginseng in Neurodegenerative Diseases. J. Ginseng Res. 2012, 36, 342–353. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Liu, J.; Guo, M.; Li, H. Panax Ginseng in the treatment of Alzheimer’s disease and vascular dementia. J. Ginseng Res. 2023, 47, 506–514. [Google Scholar] [CrossRef]
- Huang, T.; Lee, S.; Lee, T.; Yun, S.; Kim, Y.; Yang, H. Smart Farming Enhances Bioactive Compounds Content of Panax ginseng on Moderating Scopolamine-Induced Memory Deficits and Neuroinflammation. Plants 2023, 12, 640. [Google Scholar] [CrossRef]
- Park, K.-W.; Kim, J.-H.; Jeong, B.-G.; Park, J.-K.; Jang, H.-Y.; Oh, Y.-S.; Kang, K.-Y. Increased Accumulation of Ginsenosides in Panax ginseng Sprouts Cultivated with Kelp Fermentates. Plants 2024, 13, 463. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, L.; Zhang, L.; Liu, C.; Han, M. Effects of cultivation ages and modes on microbial diversity in the rhizosphere soil of Panax ginseng. J. Ginseng Res. 2016, 40, 28–37. [Google Scholar] [CrossRef]
- Song, Y.-N.; Hong, H.-G.; Son, J.S.; Kwon, Y.O.; Lee, H.H.; Kim, H.J.; Park, J.H.; Son, M.J.; Oh, J.-G.; Yoon, M.-H. Investigation of ginsenosides and antioxidant activities in the roots, leaves, and stems of hydroponic-cultured ginseng (Panax ginseng Meyer). Prev. Nutr. Food Sci. 2019, 24, 283. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, J.Y.; Cho, Y.-J.; Kim, J.-E.; Kim, S.Y.; Park, J.H.Y.; Yang, H.; Lee, K.W. Optimization of the extraction process of high levels of chlorogenic acid and ginsenosides from short-term hydroponic-cultured ginseng and evaluation of the extract for the prevention of atopic dermatitis. J. Ginseng Res. 2022, 46, 367–375. [Google Scholar] [CrossRef]
- Kim, T.H.; Baek, S.; Kwon, K.H.; Oh, S.E. Hierarchical Machine Learning-Based Growth Prediction Model of Panax ginseng Sprouts in a Hydroponic Environment. Plants 2023, 12, 3867. [Google Scholar] [CrossRef]
- Balafoutis, A.T.; Beck, B.; Fountas, S.; Tsiropoulos, Z.; Vangeyte, J.; van der Wal, T.; Soto-Embodas, I.; Gómez-Barbero, M.; Pedersen, S.M. Smart farming technologies–description, taxonomy and economic impact. In Precision Agriculture: Technology and Economic Perspectives; Springer: Berlin/Heidelberg, Germany, 2017; pp. 21–77. [Google Scholar]
- O’Grady, M.; O’Hare, G. Modelling the smart farm. Inf. Process. Agric. 2017, 4, 179–187. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Nguyen, T.K.L.; Oh, M.-M. Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes. Agronomy 2020, 10, 1979. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Jang, S.-N.; Lee, G.O.; Sim, H.-S.; Kang, M.J.; et al. Comprehensive Comparison of Chemical Composition and Antioxidant Activity of Panax ginseng Sprouts by Different Cultivation Systems in a Plant Factory. Plants 2022, 11, 1818. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Growth and Photosynthetic Responses to Increased LED Light Intensity in Korean Ginseng (Panax ginseng C.A. Meyer) Sprouts. Agronomy 2023, 13, 2375. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, S.C.; Seong, J.A.; Lee, H.Y.; Cho, D.Y.; Kim, M.J.; Jung, J.G.; Jeong, E.H.; Son, K.-H.; Cho, K.M. Comparison of ginsenoside contents and antioxidant activity according to the size of ginseng sprout has produced in a plant factory. J. Appl. Biol. Chem. 2021, 64, 253–261. [Google Scholar] [CrossRef]
- Park, K.W.; Kim, E.-J.; Joo, H.; Jeon, S.-M.; Choi, S.-H.; Kwon, J.C.; Kim, B.G.; Kim, J.W. Cognitive profiles and subtypes of patients with mild cognitive impairment: Data from a clinical follow-up study. Int. J. Clin. Med. 2012, 3, 352–360. [Google Scholar] [CrossRef][Green Version]
- Kang, I.-W.; Beom, I.-G.; Cho, J.-Y.; Son, H.-R. Accuracy of Korean-mini-mental status examination based on Seoul neuro-psychological screening battery II results. Korean J. Fam. Med. 2016, 37, 177. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.; Park, S. Effects of ginseng sprout extract and modified Kyung-ok-Ko on scopolamine-induced cognitive impairment in mice. J. Agric. Life Environ. Sci. 2019, 31, 151–159. [Google Scholar]
- Kang, Y.; Na, D.; Hahn, S. Seoul Neuropsychological Screening Battery; Human Brain Research & Consulting, Co.: Incheon, Republic of Korea, 2003. [Google Scholar]
- Fastenau, P.S.; Denburg, N.L.; Hufford, B.J. Adult norms for the Rey-Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the Extended Complex Figure Test. Clin. Neuropsychol. 1999, 13, 30–47. [Google Scholar] [CrossRef]
- Lee, J.Y.; Dong Woo, L.; Cho, S.J.; Na, D.L.; Hong Jin, J.; Kim, S.K.; You Ra, L.; Youn, J.H.; Kwon, M.; Lee, J.H.; et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: Validation of the Korean version of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2008, 21, 104–110. [Google Scholar] [CrossRef]
- Youn, J.C.; Kim, K.W.; Lee, D.Y.; Jhoo, J.H.; Lee, S.B.; Park, J.H.; Choi, E.A.; Choe, J.Y.; Jeong, J.W.; Choo, I.H.; et al. Development of the Subjective Memory Complaints Questionnaire. Dement. Geriatr. Cogn. Disord. 2009, 27, 310–317. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef]
- Sakurai, K.; Shen, C.; Ezaki, Y.; Inamura, N.; Fukushima, Y.; Masuoka, N.; Hisatsune, T. Effects of Matcha Green Tea Powder on Cognitive Functions of Community-Dwelling Elderly Individuals. Nutrients 2020, 12, 3639. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Weimer, D.L.; Sager, M.A. Early identification and treatment of Alzheimer’s disease: Social and fiscal outcomes. Alzheimer’s Dement. 2009, 5, 215–226. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M.; et al. Prediction of Dementia by Subjective Memory Impairment: Effects of Severity and Temporal Association with Cognitive Impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef]
- Jonker, C.; Geerlings, M.I.; Schmand, B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int. J. Geriatr. Psychiatry 2000, 15, 983–991. [Google Scholar] [CrossRef]
- Reid, L.M.; MacLullich, A.M. Subjective memory complaints and cognitive impairment in older people. Dement. Geriatr. Cogn. Disord. 2006, 22, 471–485. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, R.; Chen, S.; Dai, L.; Shen, T.; Feng, Y.; Gu, P.; Shariff, M.; Nguyen, T.; Ye, Y.; et al. The Relieving Effects of BrainPower Advanced, a Dietary Supplement, in Older Adults with Subjective Memory Complaints: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2016, 2016, 7898093. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.; Sohn, S.W.; Kim, S.; Park, K.W. Memory performance on the story recall test and prediction of cognitive dysfunction progression in mild cognitive impairment and Alzheimer’s dementia. Geriatr. Gerontol. Int. 2017, 17, 1603–1609. [Google Scholar] [CrossRef]
- Park, K.-C.; Jin, H.; Zheng, R.; Kim, S.; Lee, S.-E.; Kim, B.-H.; Yim, S.-V. Cognition enhancing effect of panax ginseng in Korean volunteers with mild cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial. Transl. Clin. Pharmacol. 2019, 27, 92. [Google Scholar] [CrossRef]
- Feng, H.; Xue, M.; Deng, H.; Cheng, S.; Hu, Y.; Zhou, C. Ginsenoside and its therapeutic potential for cognitive impairment. Biomolecules 2022, 12, 1310. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.-p.; Fang, J.-y.; Wang, H.-c.; Wei, Y.; Cao, Y.; Liu, J.-g.; Liu, L.-t.; Li, H. Effect and safety of huannao yicong formula in patients with mild-to-moderate Alzheimer’s disease: A randomized, double-blinded, donepezil-controlled trial. Chin. J. Integr. Med. 2019, 25, 574–581. [Google Scholar] [CrossRef]
- Avidan, A.Y. Sleep disorders in the older patient. Prim. Care Clin. Off. Pract. 2005, 32, 563–586. [Google Scholar] [CrossRef]
- Hancock, P.; Larner, A.J. Diagnostic utility of the Pittsburgh Sleep Quality Index in memory clinics. Int. J. Geriatr. Psychiatry 2009, 24, 1237–1241. [Google Scholar] [CrossRef]
- Han, H.J.; Kim, H.Y.; Choi, J.J.; Ahn, S.-Y.; Lee, S.-H.; Oh, K.-W.; Kim, S.-Y. Effects of red ginseng extract on sleeping behaviors in human volunteers. J. Ethnopharmacol. 2013, 149, 597–599. [Google Scholar]
- Lee, S.-A.; Kang, S.-G.; Lee, H.-J.; Jung, K.-Y.; Kim, L. Effect of Korean red ginseng on sleep: A randomized, placebo-controlled trial. Sleep Med. Psychophysiol. 2010, 17, 85–90. [Google Scholar]
- Kitaoka, K.; Uchida, K.; Okamoto, N.; Chikahisa, S.; Miyazaki, T.; Takeda, E.; Séi, H. Fermented ginseng improves the first-night effect in humans. Sleep 2009, 32, 413–421. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Rogers, S.; Farlow, M.; Doody, R.; Mohs, R.; Friedhoff, L.; Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 1998, 50, 136–145. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Lilienfeld, S.; Gaens, E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: Multicentre randomised controlled trial. BMJ 2000, 321, 1445. [Google Scholar] [CrossRef]
- Rösler, M.; Bayer, T.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stähelin, H.B.; Hartman, R.; Gharabawi, M. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trialCommentary: Another piece of the Alzheimer’s jigsaw. BMJ 1999, 318, 633–640. [Google Scholar] [CrossRef]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar]
- Choi, Y.P.; Jang, Y.B.; Choi, G.S.; Kwon, M.H.; Kang, B.W.; Rhee, J.; Seo, B.S.; Kim, D.W.; Choung, J.J.; Lee, J.Y. Neuroprotective Effects of Extract of Panax ginseng Sprouts Cultivated by Hydroponics Using Desalinated Magma Seawater of Jeju Island (Korea) against Neurotoxicity In Vitro. J. Korean Soc. Food Sci. Nutr. 2020, 49, 925–939. [Google Scholar] [CrossRef]
- Lee, M.R.; Yun, B.S.; In, O.H.; Sung, C.K. Comparative study of korean white, red, and black ginseng extract on cholinesterase inhibitory activity and cholinergic function. J. Ginseng Res. 2011, 35, 421–428. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Shin, E.-J.; Jeong, J.H.; Sharma, N.; Nah, S.Y.; Ko, S.K.; Byun, J.K.; Lee, Y.; Lei, X.G.; Kim, D.-J.; et al. Ginsenoside Re attenuates memory impairments in aged Klotho deficient mice via interactive modulations of angiotensin II AT1 receptor, Nrf2 and GPx-1 gene. Free. Radic. Biol. Med. 2022, 189, 2–19. [Google Scholar] [CrossRef]
- Cao, G.; Su, P.; Zhang, S.; Guo, L.; Zhang, H.; Liang, Y.; Qin, C.; Zhang, W. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016, 793, 101–108. [Google Scholar] [CrossRef]
- Kim, M.S.; Yu, J.M.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Jang, S.K.; Choi, Y.W.; Lee, D.I.; Joo, S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol. Pharm. Bull. 2014, 37, 826–833. [Google Scholar] [CrossRef]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef]
- Lee, W.-J.; Shin, Y.-W.; Kim, D.-E.; Kweon, M.-H.; Kim, M. Effect of desalted Salicornia europaea L. ethanol extract (PM-EE) on the subjects complaining memory dysfunction without dementia: A 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2020, 10, 19914. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Woo, J.-Y.; Han, C.-K.; Chang, I.-M. Safety Analysis of Panax Ginseng in Randomized Clinical Trials: A Systematic Review. Medicines 2015, 2, 106–126. [Google Scholar] [CrossRef]
- Lee, N.-H.; Son, C.-G. Systematic Review of Randomized Controlled Trials Evaluating the Efficacy and Safety of Ginseng. J. Acupunct. Meridian Stud. 2011, 4, 85–97. [Google Scholar] [CrossRef]
- Jang, I.B.; Yu, J.; Suh, S.J.; Jang, I.B.; Kwon, K.B. Growth and ginsenoside content in different parts of ginseng sprouts depending on harvest time. Korean J. Med. Crop Sci. 2018, 26, 205–213. [Google Scholar] [CrossRef]
- Islam, M.J.; Ryu, B.R.; Azad, M.O.K.; Rahman, M.H.; Rana, M.S.; Lim, J.-D.; Lim, Y.-S. Exogenous Putrescine Enhances Salt Tolerance and Ginsenosides Content in Korean Ginseng (Panax ginseng Meyer) Sprouts. Plants 2021, 10, 1313. [Google Scholar] [CrossRef]
- Khalid, N.; Atkins, M.; Tredget, J.; Giles, M.; Champney-Smith, K.; Kirov, G. The effectiveness of electroconvulsive therapy in treatment-resistant depression: A naturalistic study. J. ECT 2008, 24, 141–145. [Google Scholar] [CrossRef]
- Kumar, S.; Mulsant, B.H.; Liu, A.Y.; Blumberger, D.M.; Daskalakis, Z.J.; Rajji, T.K. Systematic review of cognitive effects of electroconvulsive therapy in late-life depression. Am. J. Geriatr. Psychiatry 2016, 24, 547–565. [Google Scholar] [CrossRef]
- Han, K.-Y.; Wang, C.-M.; Du, C.-B.; Qiao, J.; Wang, Y.-L.; Lv, L.-Z. Treatment outcomes and cognitive function following electroconvulsive therapy in patients with severe depression. World J. Psychiatry 2023, 13, 949. [Google Scholar] [CrossRef]
- Porter, R.J.; Baune, B.T.; Morris, G.; Hamilton, A.; Bassett, D.; Boyce, P.; Hopwood, M.J.; Mulder, R.; Parker, G.; Singh, A.B. Cognitive side-effects of electroconvulsive therapy: What are they, how to monitor them and what to tell patients. BJPsych Open 2020, 6, e40. [Google Scholar] [CrossRef]
- Seo, M.W.; Han, Y.K.; Bae, Y.S.; Lee, S.H. The disease severity and related pathogens caused by root rot on 6 years old ginseng cultivation fields. Korean J. Plant Resour. 2019, 32, 144–152. [Google Scholar]
- Kim, G.; Hyun, D.; Kim, Y.; Lee, S.; Kwon, H.; Cha, S.; Park, C.; Kim, Y. Investigation of ginsenosides in different parts of Panax ginseng cultured by hydroponics. Korean J. Hortic. Sci. Technol. 2010, 28, 216–226. [Google Scholar]
- Lee, S.-T.; Chu, K.; Sim, J.-Y.; Heo, J.-H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Heo, J.-H.; Lee, S.-T.; Chu, K.; Oh, M.J.; Park, H.-J.; Shim, J.-Y.; Kim, M. Heat-processed ginseng enhances the cognitive function in patients with moderately severe Alzheimer’s disease. Nutr. Neurosci. 2012, 15, 278–282. [Google Scholar] [CrossRef]
- Ries, M.L.; Carlsson, C.M.; Rowley, H.A.; Sager, M.A.; Gleason, C.E.; Asthana, S.; Johnson, S.C. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: A review. J. Am. Geriatr. Soc. 2008, 56, 920–934. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Hu, X.; Li, K.; Sun, C. Simultaneous determination of ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and protopanaxatriol in human plasma and urine by LC–MS/MS and its application in a pharmacokinetics study of G-Re in volunteers. J. Chromatogr. B 2011, 879, 2011–2017. [Google Scholar] [CrossRef]
- Kryger, M.; Steljes, D.; Pouliot, Z.; Neufeld, H.; Odynski, T. Subjective versus objective evaluation of hypnotic efficacy: Experience with zolpidem. Sleep 1991, 14, 399–407. [Google Scholar] [CrossRef]
- Chung, S.; Youn, S.; Yi, K.; Park, B.; Lee, S. Sleeping pill administration time and patient subjective satisfaction. J. Clin. Sleep Med. 2016, 12, 57–62. [Google Scholar] [CrossRef]
- Bojar, I.; Stasiak, M.; Cyniak-Magierska, A.; Raczkiewicz, D.; Lewiński, A. Cognitive function, APOE gene polymorphisms, and thyroid status associations in postmenopausal women in Poland. Dement. Geriatr. Cogn. Disord. 2016, 42, 169–185. [Google Scholar] [CrossRef]
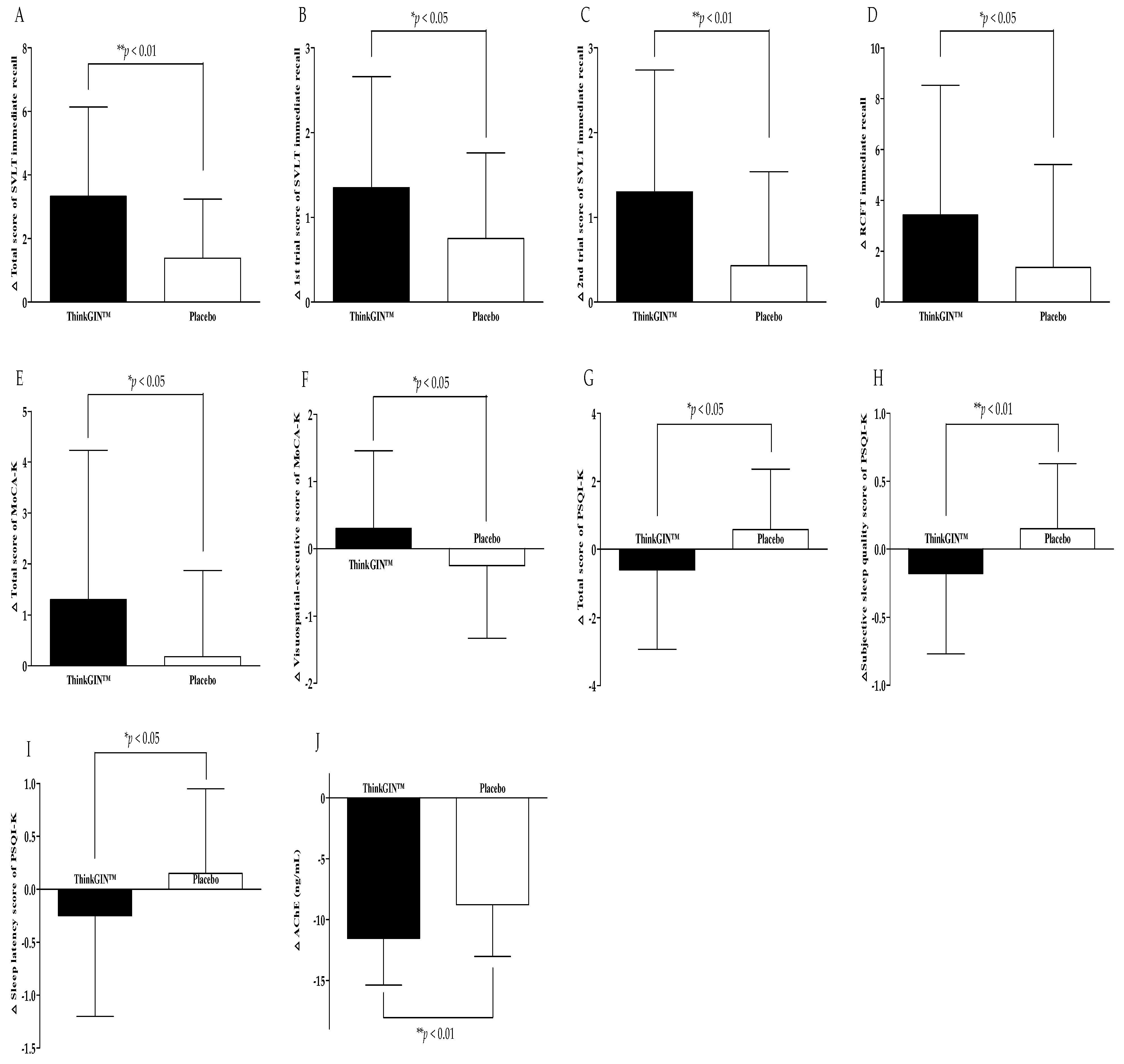
| ThinkGIN™ Group (n = 40) | Placebo Group (n = 40) | Total (n = 80) | p-Value 1 | |
|---|---|---|---|---|
| Sex (M/F) | 7/33 | 10/30 | 17/63 | 0.412 2 |
| Age (years) | 63.45 ± 5.05 | 62.95 ± 5.71 | 63.20 ± 5.36 | 0.679 |
| Education (years) | 12.63 ± 2.91 | 13.40 ± 3.18 | 13.01 ± 3.05 | 0.259 |
| Height (cm) | 157.93 ± 6.68 | 160.48 ± 8.52 | 159.20 ± 7.72 | 0.140 |
| Weight (kg) | 59.76 ± 9.70 | 63.54 ± 10.81 | 61.65 ± 10.38 | 0.104 |
| BMI (kg/m2) | 23.86 ± 2.72 | 24.55 ± 2.82 | 24.21 ± 2.78 | 0.267 |
| SBP (mmHg) | 118.93 ± 11.25 | 126.00 ± 17.37 | 122.46 ± 14.97 | 0.034 * |
| DBP (mmHg) | 74.53 ± 8.02 | 76.18 ± 8.99 | 75.35 ± 8.51 | 0.389 |
| Pulse (beats/minute) | 74.28 ± 7.63 | 74.23 ± 10.06 | 74.25 ± 8.87 | 0.980 |
| Alcohol (n, %) | 11 (27.50) | 13 (32.50) | 24 (30.00) | 0.626 2 |
| Alcohol (units/week) | 4.44 ± 6.45 | 4.31 ± 3.60 | 4.37 ± 4.98 | 0.951 |
| Smoking (n, %) | 2 (5.00) | 3 (7.50) | 5 (6.25) | 1.000 3 |
| ThinkGIN™ (n = 39) | Placebo (n = 33) | p-Value 1 | Adj.p-Value 2 | |||
|---|---|---|---|---|---|---|
| SVLT | immediate recall | total score | 3.41 ± 2.80 | 1.79 ± 1.67 | 0.004 ** | 0.005 ** |
| 1st trial | 1.38 ± 1.31 | 0.94 ± 1.00 | 0.115 | 0.115 | ||
| 2nd trial | 1.33 ± 1.44 | 0.58 ± 1.12 | 0.016 * | 0.016 * | ||
| 3rd trial | 0.69 ± 1.40 | 0.27 ± 1.18 | 0.178 | 0.178 | ||
| delayed recall | 0.92 ± 1.75 | 0.39 ± 1.32 | 0.159 | 0.159 | ||
| recognition | 0.90 ± 2.36 | 0.27 ± 1.94 | 0.229 | 0.229 | ||
| RCFT | Copy | copy score | −1.26 ± 2.60 | −1.45 ± 3.39 | 0.780 | 0.780 |
| copy time (s) | −15.03 ± 67.07 | −37.64 ± 83.91 | 0.208 | 0.208 | ||
| immediate recall | 3.51 ± 5.14 | 1.70 ± 4.39 | 0.115 | 0.115 | ||
| delayed recall | 2.46 ± 4.76 | 1.45 ± 4.38 | 0.357 | 0.357 | ||
| recognition | 1.33 ± 2.31 | 0.52 ± 2.00 | 0.116 | 0.116 | ||
| MoCA-K | total score | 1.33 ± 2.96 | 0.27 ± 1.82 | 0.068 | 0.078 | |
| visuospatial-executive | 0.31 ± 1.17 | −0.21 ± 1.08 | 0.056 | 0.056 | ||
| naming | 0.00 ± 0.65 | 0.06 ± 0.70 | 0.705 | 0.705 | ||
| attention | 0.05 ± 0.97 | 0.06 ± 0.70 | 0.964 | 0.964 | ||
| language | 0.13 ± 0.57 | −0.09 ± 0.52 | 0.096 | 0.068 3 | ||
| abstraction | −0.03 ± 0.71 | 0.09 ± 0.38 | 0.379 | 0.400 | ||
| delayed recall | 0.72 ± 1.62 | 0.30 ± 1.47 | 0.263 | 0.263 | ||
| orientation | 0.15 ± 0.84 | 0.06 ± 0.50 | 0.563 | 0.572 | ||
| SMCQ | −1.08 ± 2.07 | −0.61 ± 1.41 | 0.258 | 0.272 | ||
| PSQI-K | total score | −0.62 ± 2.36 | 0.64 ± 1.93 | 0.018 * | 0.018 * | |
| subjective sleep quality | −0.18 ± 0.60 | 0.12 ± 0.42 | 0.015 * | 0.018 * | ||
| sleep latency | −0.26 ± 0.97 | 0.15 ± 0.87 | 0.066 | 0.066 | ||
| sleep duration | −0.03 ± 0.74 | 0.24 ± 0.90 | 0.171 | 0.171 | ||
| habitual sleep efficiency | −0.03 ± 0.63 | 0.33 ± 0.82 | 0.039 * | 0.039 * | ||
| sleep disturbances | −0.05 ± 0.46 | −0.03 ± 0.39 | 0.837 | 0.837 | ||
| use of sleeping medication | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | ||
| daytime dysfunction | −0.08 ± 0.81 | −0.18 ± 0.88 | 0.600 | 0.600 | ||
| Blood biomarkers related to memory | AChE (ng/mL) | −11.83 ± 3.41 | −10.41 ± 2.19 | 0.037 * | 0.044 * | |
| BDNF (pg/mL) | 19,178.44 ± 10,716.94 | 21,133.00 ± 9689.28 | 0.423 | 0.423 | ||
| amyloid β 1–40 (pg/mL) | 4.82 ± 181.31 | 23.43 ± 37.56 | 0.535 | 0.565 | ||
| amyloid β 1–42 (pg/mL) | 3.71 ± 13.09 | 2.17 ± 2.25 | 0.476 | 0.509 | ||
| amyloid β 40/42 | −63.34 ± 126.53 | −64.77 ± 110.46 | 0.960 | 0.960 | ||
| ApoE4 (pg/mL) | 7858.05 ± 199,152.49 | −45,397.27 ± 173,563.04 | 0.235 | 0.235 | ||
| TAS (mmol/L) | −0.76 ± 0.27 | −0.85 ± 0.29 | 0.169 | 0.169 | ||
| hs-CRP (mg/L) | 0.50 ± 2.85 | 0.65 ± 1.39 | 0.767 | 0.778 | ||
| ThinkGIN™ (n = 40) | Placebo (n = 40) | p-Value 1 | Adj. p-Value 2 | |||
|---|---|---|---|---|---|---|
| SVLT | immediate recall | total score | 3.33 ± 2.81 | 1.38 ± 1.86 | 0.001 ** | 0.001 ** |
| 1st trial | 1.35 ± 1.31 | 0.75 ± 1.01 | 0.024 * | 0.024 * | ||
| 2nd trial | 1.30 ± 1.44 | 0.43 ± 1.11 | 0.003 ** | 0.003 ** | ||
| 3rd trial | 0.68 ± 1.38 | 0.20 ± 1.09 | 0.092 | 0.092 | ||
| delayed recall | 0.90 ± 1.74 | 0.35 ± 1.21 | 0.105 | 0.104 | ||
| recognition | 0.88 ± 2.33 | 0.20 ± 1.77 | 0.149 | 0.149 | ||
| RCFT | Copy | copy score | −1.23 ± 2.57 | −1.13 ± 3.19 | 0.878 | 0.878 |
| copy time (s) | −14.65 ± 66.25 | −31.65 ± 77.23 | 0.294 | 0.294 | ||
| immediate recall | 3.43 ± 5.10 | 1.36 ± 4.05 | 0.049 * | 0.049 * | ||
| delayed recall | 2.40 ± 4.72 | 1.23 ± 4.00 | 0.233 | 0.233 | ||
| recognition | 1.30 ± 2.29 | 0.43 ± 1.82 | 0.062 | 0.062 | ||
| MoCA-K | total score | 1.30 ± 2.93 | 0.18 ± 1.69 | 0.039 * | 0.021 *3 | |
| visuospatial-executive | 0.30 ± 1.16 | −0.25 ± 1.08 | 0.031 * | 0.031 * | ||
| naming | 0.00 ± 0.64 | 0.05 ± 0.64 | 0.728 | 0.728 | ||
| attention | 0.05 ± 0.96 | 0.05 ± 0.64 | 1.000 | 1.000 | ||
| language | 0.13 ± 0.56 | −0.08 ± 0.47 | 0.090 | 0.069 4 | ||
| abstraction | −0.03 ± 0.70 | 0.05 ± 0.39 | 0.555 | 0.554 | ||
| delayed recall | 0.70 ± 1.60 | 0.30 ± 1.36 | 0.233 | 0.233 | ||
| orientation | 0.15 ± 0.83 | 0.05 ± 0.45 | 0.507 | 0.506 | ||
| SMCQ | −1.05 ± 2.05 | −0.53 ± 1.30 | 0.176 | 0.175 | ||
| PSQI-K | total score | −0.60 ± 2.33 | 0.58 ± 1.78 | 0.013 * | 0.013 * | |
| subjective sleep quality | −0.18 ± 0.59 | 0.15 ± 0.48 | 0.009 ** | 0.009 ** | ||
| sleep latency | −0.25 ± 0.95 | 0.15 ± 0.80 | 0.046 * | 0.046 * | ||
| sleep duration | −0.03 ± 0.73 | 0.20 ± 0.82 | 0.201 | 0.201 | ||
| habitual sleep efficiency | −0.03 ± 0.62 | 0.28 ± 0.75 | 0.055 | 0.055 | ||
| sleep disturbances | −0.05 ± 0.45 | −0.03 ± 0.36 | 0.784 | 0.784 | ||
| use of sleeping medication | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | ||
| daytime dysfunction | −0.08 ± 0.80 | −0.18 ± 0.81 | 0.580 | 0.580 | ||
| Blood biomarkers related to memory | AChE (ng/mL) | −11.53 ± 3.85 | −8.78 ± 4.25 | 0.003 ** | 0.003 ** | |
| BDNF (pg/mL) | 18,698.98 ± 11,004.69 | 18,317.18 ± 11,947.91 | 0.882 | 0.882 | ||
| amyloid β 1–40 (pg/mL) | 4.69 ± 178.97 | 16.60 ± 40.56 | 0.684 | 0.683 | ||
| amyloid β 1–42 (pg/mL) | 3.62 ± 12.94 | 1.79 ± 2.20 | 0.385 | 0.383 | ||
| amyloid β 40/42 | −61.76 ± 125.29 | −54.54 ± 102.76 | 0.779 | 0.779 | ||
| ApoE4 (pg/mL) | 7661.60 ± 196,586.60 | −37,401.80 ± 158,197.42 | 0.262 | 0.262 | ||
| TAS (mmol/L) | −0.75 ± 0.29 | −0.73 ± 0.40 | 0.824 | 0.824 | ||
| hs-CRP (mg/L) | 0.48 ± 2.81 | 0.53 ± 1.29 | 0.928 | 0.927 | ||
| ThinkGIN™ (n = 40) | Placebo (n = 40) | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Week | Change Value | p-Value 1 | Baseline | 12 Week | Change Value | p-Value 1 | |||
| CBC | Hemoglobin (g/dL) | 13.54 ± 1.17 | 13.36 ± 1.09 | −0.18 ± 0.67 | 0.103 | 13.90 ± 1.31 | 13.67 ± 1.24 | −0.23 ± 0.48 | 0.005 ** | 0.711 |
| Hematocrit (%) | 40.15 ± 3.54 | 39.43 ± 3.22 | −0.72 ± 2.18 | 0.043 * | 41.30 ± 4.01 | 40.51 ± 3.88 | −0.79 ± 1.72 | 0.006 ** | 0.883 | |
| WBC (K/UL) | 5.22 ± 1.16 | 5.15 ± 1.32 | −0.07 ± 1.05 | 0.690 | 5.56 ± 1.23 | 5.42 ± 1.19 | −0.13 ± 0.76 | 0.274 | 0.747 | |
| RBC (M/UL) | 4.31 ± 0.36 | 4.32 ± 0.36 | 0.01 ± 0.22 | 0.717 | 4.39 ± 0.43 | 4.40 ± 0.42 | 0.01 ± 0.19 | 0.729 | 0.970 | |
| Platelet (K/UL) | 231.48 ± 61.45 | 215.60 ± 56.88 | −15.88 ± 26.72 | 0.001 ** | 228.43 ± 38.36 | 220.13 ± 41.38 | −8.30 ± 34.46 | 0.136 | 0.275 | |
| Biochemistry | AST (U/L) | 25.03 ± 6.20 | 24.63 ± 6.50 | −0.40 ± 5.48 | 0.647 | 25.08 ± 6.64 | 24.53 ± 5.63 | −0.55 ± 4.42 | 0.436 | 0.893 |
| ALT (U/L) | 21.55 ± 10.19 | 21.65 ± 12.34 | 0.10 ± 8.96 | 0.944 | 24.20 ± 9.40 | 22.95 ± 8.61 | −1.25 ± 5.62 | 0.168 | 0.422 | |
| Gamma-GT (U/L) | 19.60 ± 13.36 | 19.80 ± 19.45 | 0.20 ± 10.85 | 0.908 | 28.55 ± 34.35 | 26.80 ± 27.55 | −1.75 ± 11.86 | 0.357 | 0.445 | |
| Total cholesterol (mg/dL) | 206.20 ± 48.85 | 199.65 ± 48.20 | −6.55 ± 30.31 | 0.180 | 203.15 ± 37.92 | 202.15 ± 35.22 | −1.00 ± 33.11 | 0.850 | 0.437 | |
| LDL-C (mg/dL) | 124.58 ± 36.16 | 123.68 ± 35.24 | −0.90 ± 24.13 | 0.815 | 123.53 ± 35.09 | 122.45 ± 31.00 | −1.08 ± 27.98 | 0.809 | 0.976 | |
| HDL-C (mg/dL) | 61.35 ± 19.18 | 58.08 ± 15.30 | −3.28 ± 10.55 | 0.057 | 59.58 ± 13.47 | 57.25 ± 13.64 | −2.33 ± 7.92 | 0.071 | 0.650 | |
| Albumin (g/dL) | 4.28 ± 0.27 | 4.26 ± 0.27 | −0.02 ± 0.27 | 0.641 | 4.22 ± 0.27 | 4.32 ± 0.19 | 0.10 ± 0.31 | 0.057 | 0.078 | |
| Total protein (g/dL) | 7.25 ± 0.36 | 7.19 ± 0.29 | −0.05 ± 0.33 | 0.316 | 7.16 ± 0.35 | 7.25 ± 0.31 | 0.09 ± 0.35 | 0.132 | 0.073 | |
| Total bilirubin (mg/dL) | 0.98 ± 0.26 | 0.94 ± 0.26 | −0.04 ± 0.20 | 0.248 | 0.97 ± 0.22 | 0.87 ± 0.24 | −0.10 ± 0.17 | 0.001 ** | 0.151 | |
| ALP (U/L) | 61.78 ± 14.99 | 63.80 ± 17.24 | 2.03 ± 12.19 | 0.300 | 61.98 ± 16.04 | 67.05 ± 14.64 | 5.08 ± 8.88 | 0.001 ** | 0.205 | |
| LD (U/L) | 171.00 ± 35.93 | 160.30 ± 28.61 | −10.70 ± 30.00 | 0.030 * | 169.05 ± 34.09 | 163.78 ± 27.33 | −5.28 ± 18.74 | 0.083 | 0.336 | |
| Glucose (mg/dL) | 101.85 ± 7.87 | 96.95 ± 11.08 | −4.90 ± 9.65 | 0.003 ** | 104.80 ± 11.11 | 99.68 ± 9.96 | −5.13 ± 8.49 | 0.001 ** | 0.912 | |
| BUN (mg/dL) | 16.45 ± 3.29 | 15.53 ± 3.02 | −0.92 ± 2.78 | 0.043 * | 14.97 ± 2.76 | 14.55 ± 3.12 | −0.42 ± 2.58 | 0.307 | 0.410 | |
| Triglyceride (mg/dL) | 117.45 ± 71.58 | 115.63 ± 70.09 | −1.83 ± 40.41 | 0.777 | 127.20 ± 56.69 | 130.50 ± 51.37 | 3.30 ± 46.37 | 0.655 | 0.600 | |
| Creatinine (mg/dL) | 0.85 ± 0.17 | 0.83 ± 0.15 | −0.02 ± 0.10 | 0.233 | 0.83 ± 0.15 | 0.84 ± 0.14 | 0.01 ± 0.09 | 0.458 | 0.168 | |
| CK (U/L) | 136.93 ± 69.62 | 137.55 ± 82.88 | 0.63 ± 72.48 | 0.957 | 120.58 ± 110.47 | 108.08 ± 65.52 | −12.50 ± 92.37 | 0.397 | 0.482 | |
| Urin-alysis | pH | 6.45 ± 1.07 | 6.44 ± 1.03 | −0.01 ± 0.92 | 0.932 | 6.54 ± 1.00 | 6.53 ± 0.94 | −0.01 ± 1.15 | 0.945 | 1.000 |
| Specific gravity | 1.02 ± 0.01 | 1.02 ± 0.01 | 0.00 ± 0.01 | 0.324 | 1.02 ± 0.01 | 1.02 ± 0.01 | 0.00 ± 0.01 | 0.529 | 0.262 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, H.-I.; Ha, K.-C.; Park, Y.-K.; Kim, T.-Y.; Park, S.-J. Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 1952. https://doi.org/10.3390/nu16121952
Baek H-I, Ha K-C, Park Y-K, Kim T-Y, Park S-J. Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2024; 16(12):1952. https://doi.org/10.3390/nu16121952
Chicago/Turabian StyleBaek, Hyang-Im, Ki-Chan Ha, Yu-Kyung Park, Tae-Young Kim, and Soo-Jung Park. 2024. "Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 16, no. 12: 1952. https://doi.org/10.3390/nu16121952
APA StyleBaek, H.-I., Ha, K.-C., Park, Y.-K., Kim, T.-Y., & Park, S.-J. (2024). Efficacy and Safety of Panax ginseng Sprout Extract in Subjective Memory Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 16(12), 1952. https://doi.org/10.3390/nu16121952






