The Effects of Almond Consumption on Cardiovascular Health and Gut Microbiome: A Comprehensive Review
Abstract
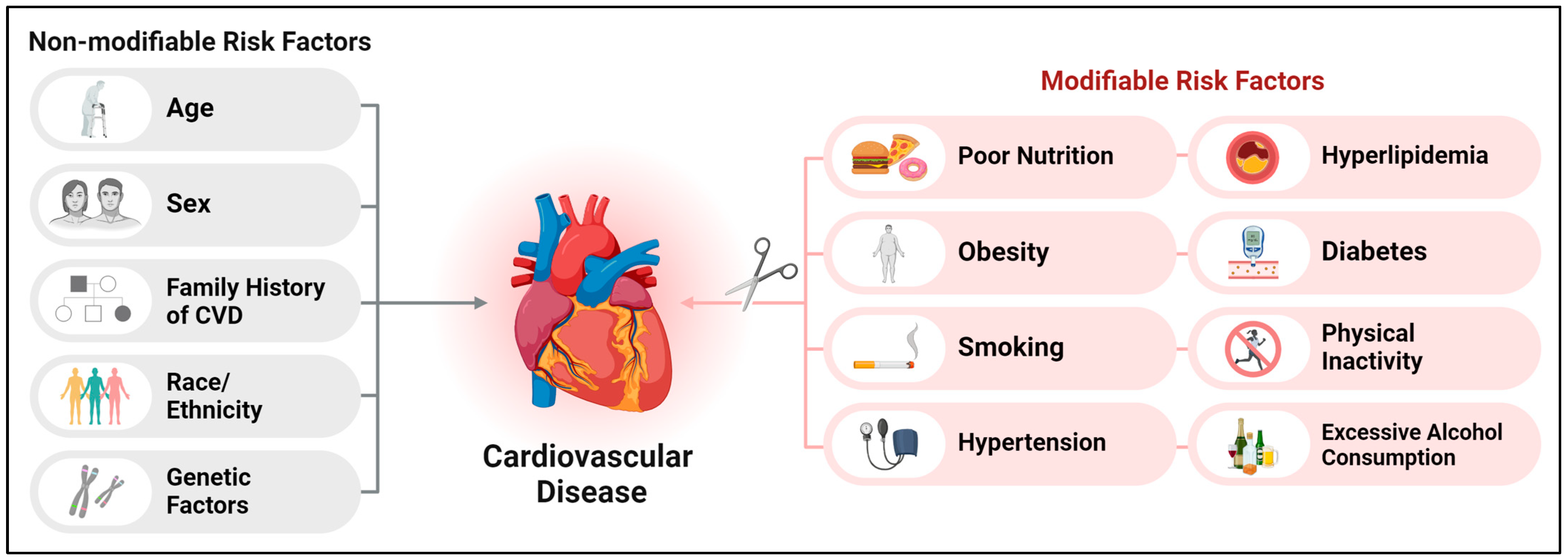
:1. Introduction
2. Nutrient Composition and Polyphenol Content of Almonds
3. Potential Roles of Almonds in Gut–Heart Axis Modulation
4. Impacts of Almond Consumption on Cardiovascular Health and Associated Risk Factors
4.1. Lipid Profile Improvement
4.2. Blood Sugar Regulation
4.3. Antioxidant Increase
4.4. Blood Pressure Reduction
4.5. Chronic Low-Grade Inflammation Amelioration
4.6. Endothelial Function Improvement
4.7. Body Composition Improvement
4.8. Appetite and Satiety Regulation
4.9. Gut Microbiome Modulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Kubik, M. Risk Assessment for Cardiovascular Disease with Nontraditional Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 272–280. [Google Scholar] [PubMed]
- Mohebi, R.; Chen, C.; Ibrahim, N.E.; McCarthy, C.P.; Gaggin, H.K.; Singer, D.E.; Hyle, E.P.; Wasfy, J.H.; Januzzi, J.L., Jr. Cardiovascular Disease Projections in the United States Based on the 2020 Census Estimates. J. Am. Coll. Cardiol. 2022, 80, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.; Fabian, D.; Farid, M.; Mahtani, A.; Sethi, Y.; Ralhan, T.; Das, M.; Chandi, S.; Johal, G. Social Determinants of Health and Its Impacts on Cardiovascular Disease in Underserved Populations: A Critical Review. Curr. Probl. Cardiol. 2024, 49, 102373. [Google Scholar] [CrossRef] [PubMed]
- Hypertension. Available online: https://medlineplus.gov/genetics/condition/hypertension/ (accessed on 20 February 2024).
- Aggarwal, R.; Yeh, R.W.; Maddox, K.E.J.; Wadhera, R.K. Cardiovascular Risk Factor Prevalence, Treatment, and Control in US Adults Aged 20 to 44 Years, 2009 to March 2020. JAMA 2023, 329, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.-Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes among US Adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef]
- Mayer, S.B.; Graybill, S.; Raffa, S.D.; Tracy, C.; Gaar, E.; Wisbach, G.; Goldstein, M.G.; Sall, J. Synopsis of the 2020 US VA/DoD Clinical Practice Guideline for the Management of Adult Overweight and Obesity. Mil. Med. 2021, 186, 884–896. [Google Scholar] [CrossRef]
- Maguire, L.; O’sullivan, S.; Galvin, K.; O’connor, T.; O’brien, N. Fatty Acid Profile, Tocopherol, Squalene and Phytosterol Content of Walnuts, Almonds, Peanuts, Hazelnuts and the Macadamia Nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.; Kendall, C.W.C.; Gascoyne, A.-M.; Bazinet, R.P.; Balachandran, B.; Lapsley, K.G.; Augustin, L.S.A.; Sievenpiper, J.L.; Jenkins, D.J.A. Effect of Almond Consumption on the Serum Fatty Acid Profile: A Dose–Response Study. Br. J. Nutr. 2014, 112, 1137–1146. [Google Scholar] [CrossRef]
- Özcan, M.M. A Review on Some Properties of Almond: Impact of Processing, Fatty Acids, Polyphenols, Nutrients, Bioactive Properties, and Health Aspects. J. Food Sci. Technol. 2023, 60, 1493–1504. [Google Scholar] [CrossRef]
- Zamany, A.J.; Samadi, G.R.; Kim, D.H.; Keum, Y.; Saini, R.K. Comparative Study of Tocopherol Contents and Fatty Acids Composition in Twenty Almond Cultivars of Afghanistan. J. Am. Oil Chem. Soc. 2017, 94, 805–817. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, S.-C.; Hu, C.-C.; Shyu, Y.-S.; Hsu, C.-Y.; Yang, D.-J. Effects of Roasting Temperature and Duration on Fatty Acid Composition, Phenolic Composition, Maillard Reaction Degree and Antioxidant Attribute of Almond (Prunus dulcis) Kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.; Bisignano, C.; Saija, A.; Dugo, P. Characterization of Polyphenols, Lipids and Dietary Fibre from Almond Skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Phenolic Compounds in Natural and Roasted Nuts and Their Skins: A Brief Review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol Content and Antioxidant Activity of California Almonds Depend on Cultivar and Harvest Year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree Nut Phytochemicals: Composition, Antioxidant Capacity, Bioactivity, Impact Factors. A Systematic Review of Almonds, Brazils, Cashews, Hazelnuts, Macadamias, Pecans, Pine Nuts, Pistachios and Walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic Content and Biological Properties of Avola Almond (Prunus dulcis Mill. DA Webb) Skin and Its Industrial Byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) DA Webb) Skins as a Potential Source of Bioactive Polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef]
- Wijeratne, S.S.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant Polyphenols in Almond and Its Coproducts. J. Agric. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The Importance of Almond (Prunus amygdalus L.) and Its by-Products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Barreira, J.C.; Ferreira, I.C.; Oliveira, M.B.P.; Pereira, J.A. Antioxidant Potential of Chestnut (Castanea sativa L.) and Almond (Prunus dulcis L.) by-Products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Boukid, F.; Angelis, D.D.; Summo, C. Use of Almond Skins to Improve Nutritional and Functional Properties of Biscuits: An Example of Upcycling. Foods 2020, 9, 1705. [Google Scholar] [CrossRef]
- Creedon, A.C.; Dimidi, E.; Hung, E.S.; Rossi, M.; Probert, C.; Grassby, T.; Miguens-Blanco, J.; Marchesi, J.R.; Scott, S.M.; Berry, S.E. The Impact of Almonds and Almond Processing on Gastrointestinal Physiology, Luminal Microbiology, and Gastrointestinal Symptoms: A Randomized Controlled Trial and Mastication Study. Am. J. Clin. Nutr. 2022, 116, 1790–1804. [Google Scholar] [CrossRef]
- Berryman, C.E.; West, S.G.; Fleming, J.A.; Bordi, P.L.; Kris-Etherton, P.M. Effects of Daily Almond Consumption on Cardiometabolic Risk and Abdominal Adiposity in Healthy Adults with Elevated LDL-cholesterol: A Randomized Controlled Trial. J. Am. Heart Assoc. 2015, 4, e000993. [Google Scholar] [CrossRef]
- Sabaté, J.; Haddad, E.; Tanzman, J.S.; Jambazian, P.; Rajaram, S. Serum Lipid Response to the Graduated Enrichment of a Step I Diet with Almonds: A Randomized Feeding Trial. Am. J. Clin. Nutr. 2003, 77, 1379–1384. [Google Scholar] [CrossRef]
- Berryman, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Inclusion of Almonds in a Cholesterol-Lowering Diet Improves Plasma HDL Subspecies and Cholesterol Efflux to Serum in Normal-Weight Individuals with Elevated LDL Cholesterol. J. Nutr. 2017, 147, 1517–1523. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Parker, T.L.; Connelly, P.W.; Qian, W.; Haight, J.S.; Faulkner, D.; Vidgen, E.; Lapsley, K.G. Dose Response of Almonds on Coronary Heart Disease Risk Factors: Blood Lipids, Oxidized Low-Density Lipoproteins, Lipoprotein (a), Homocysteine, and Pulmonary Nitric Oxide: A Randomized, Controlled, Crossover Trial. Circulation 2002, 106, 1327–1332. [Google Scholar] [CrossRef]
- Asbaghi, O.; Moodi, V.; Hadi, A.; Eslampour, E.; Shirinbakhshmasoleh, M.; Ghaedi, E.; Miraghajani, M. The Effect of Almond Intake on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Funct. 2021, 12, 1882–1896. [Google Scholar] [CrossRef]
- Ruisinger, J.F.; Gibson, C.A.; Backes, J.M.; Smith, B.K.; Sullivan, D.K.; Moriarty, P.M.; Kris-Etherton, P. Statins and Almonds to Lower Lipoproteins (the STALL Study). J. Clin. Lipidol. 2015, 9, 58–64. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Rahimlou, M.; Rezaei Kelishadi, M.; Moradi, S.; Jalili, C. Effects of Almond on Cardiometabolic Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Phytother. Res. 2022, 36, 1839–1853. [Google Scholar] [CrossRef]
- Berryman, C.E.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris-Etherton, P.M. Effects of Almond Consumption on the Reduction of LDL-Cholesterol: A Discussion of Potential Mechanisms and Future Research Directions. Nutr. Rev. 2011, 69, 171–185. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, H.-J.; Kim, H.-S.; Park, H. Time and Intervention Effects of Daily Almond Intake on the Changes of Lipid Profile and Body Composition among Free-Living Healthy Adults. J. Med. Food 2018, 21, 340–347. [Google Scholar] [CrossRef]
- Hyson, D.A.; Schneeman, B.O.; Davis, P.A. Almonds and Almond Oil Have Similar Effects on Plasma Lipids and LDL Oxidation in Healthy Men and Women. J. Nutr. 2002, 132, 703–707. [Google Scholar] [CrossRef]
- Jamshed, H.; Gilani, A.H. Almonds Inhibit Dyslipidemia and Vascular Dysfunction in Rats through Multiple Pathways. J. Nutr. 2014, 144, 1768–1774. [Google Scholar] [CrossRef]
- Josse, A.R.; Kendall, C.W.; Augustin, L.S.; Ellis, P.R.; Jenkins, D.J. Almonds and Postprandial Glycemia—A Dose-Response Study. Metabolism 2007, 56, 400–404. [Google Scholar] [CrossRef]
- Mori, A.M.; Considine, R.V.; Mattes, R.D. Acute and Second-Meal Effects of Almond Form in Impaired Glucose Tolerant Adults: A Randomized Crossover Trial. Nutr. Metab. 2011, 8, 1–8. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Tiwari, R.; Sharma, M.; Pandey, R.M.; Upadhyay, A.D.; Sati, H.C. Beneficial Effects of Premeal Almond Load on Glucose Profile on Oral Glucose Tolerance and Continuous Glucose Monitoring: Randomized Crossover Trials in Asian Indians with Prediabetes. Eur. J. Clin. Nutr. 2023, 77, 586–595. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mattes, R. Appetitive, Dietary and Health Effects of Almonds Consumed with Meals or as Snacks: A Randomized, Controlled Trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Liu, Y.-H.; Liu, J.-F.; Chang, W.-H.; Chen, C.-M.; Chen, C.-Y.O. Almond Consumption Improved Glycemic Control and Lipid Profiles in Patients with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Johnston, C.S. Almond Ingestion at Mealtime Reduces Postprandial Glycemia and Chronic Ingestion Reduces Hemoglobin A1c in Individuals with Well-Controlled Type 2 Diabetes Mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Gravesteijn, E.; Mensink, R.P.; Plat, J. The Effects of Long-Term Almond Consumption on Whole-Body Insulin Sensitivity, Postprandial Glucose Responses, and 48 h Continuous Glucose Concentrations in Males and Females with Prediabetes: A Randomized Controlled Trial. Eur. J. Nutr. 2023, 62, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Wang, X.-H.; Ojo, O.O.; Adegboye, A.R.A. The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 3377. [Google Scholar] [CrossRef] [PubMed]
- Bodnaruc, A.M.; Prud’homme, D.; Giroux, I. Acute Effects of an Isocaloric Macronutrient-Matched Breakfast Meal Containing Almonds on Glycemic, Hormonal, and Appetite Responses in Men with Type 2 Diabetes: A Randomized Crossover Study. Appl. Physiol. Nutr. Metab. 2020, 45, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Tan, S.-Y.; Mattes, R.D. Almond Consumption during Energy Restriction Lowers Truncal Fat and Blood Pressure in Compliant Overweight or Obese Adults. J. Nutr. 2016, 146, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Kendall, C.; Faulkner, D.; Kemp, T.; Marchie, A.; Nguyen, T.; Wong, J.; De Souza, R.; Emam, A.; Vidgen, E. Long-Term Effects of a Plant-Based Dietary Portfolio of Cholesterol-Lowering Foods on Blood Pressure. Eur. J. Clin. Nutr. 2008, 62, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Qorbani, M.; Shokati Eshkiki, Z.; Estêvão, M.D.; Mohammadi Ganjaroudi, N.; Toupchian, O.; Abdollahi, S.; Pizarro, A.B.; Abu-Zaid, A.; Zadro, J.R. The Effect of Almond Intake on Cardiometabolic Risk Factors, Inflammatory Markers, and Liver Enzymes: A Systematic Review and Meta-analysis. Phytother. Res. 2022, 36, 4325–4344. [Google Scholar] [CrossRef]
- Eslampour, E.; Asbaghi, O.; Hadi, A.; Abedi, S.; Ghaedi, E.; Lazaridi, A.-V.; Miraghajani, M. The Effect of Almond Intake on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 50, 102399. [Google Scholar] [CrossRef]
- Bae, Y.-J.; Kim, M.-H.; Choi, M.-K. Dietary Mineral Intake from Nuts and Its Relationship to Hypertension among Korean Adults. Biol. Trace Elem. Res. 2022, 200, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, Z.; Zhou, L.; Chen, M.; Zheng, X.; Yang, P.; Zhao, X.; Tian, Z. Effects of Almonds on Ameliorating Salt-induced Hypertension in Dahl Salt-sensitive Rats. J. Sci. Food Agric. 2022, 102, 2710–2722. [Google Scholar] [CrossRef] [PubMed]
- Lee-Bravatti, M.A.; Wang, J.; Avendano, E.E.; King, L.; Johnson, E.J.; Raman, G. Almond Consumption and Risk Factors for Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2019, 10, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.; Ojueromi, O.; Peace, O.; Oboh, G. Cardiomodulatory and Antioxidative Potentials of Almond–Citrus Peel Fortified Shortbread in High Fat Diet/L-NAME-Induced Hyperlipidemic–Hypertensive Rats. J. Med. Food 2023, 26, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Dikariyanto, V.; Smith, L.; Francis, L.; Robertson, M.; Kusaslan, E.; O’Callaghan-Latham, M.; Palanche, C.; D’Annibale, M.; Christodoulou, D.; Basty, N. Snacking on Whole Almonds for 6 Weeks Improves Endothelial Function and Lowers LDL Cholesterol but Does Not Affect Liver Fat and Other Cardiometabolic Risk Factors in Healthy Adults: The ATTIS Study, a Randomized Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.O.; Holbrook, M.; Duess, M.-A.; Dohadwala, M.M.; Hamburg, N.M.; Asztalos, B.F.; Milbury, P.E.; Blumberg, J.B.; Vita, J.A. Effect of Almond Consumption on Vascular Function in Patients with Coronary Artery Disease: A Randomized, Controlled, Cross-over Trial. Nutr. J. 2015, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.; Clark, J.; Griffiths, H. An Almond-Enriched Diet Increases Plasma α-Tocopherol and Improves Vascular Function but Does Not Affect Oxidative Stress Markers or Lipid Levels. Free Radic. Res. 2014, 48, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Ware, L.; Gray, A.R.; Tey, S.L.; Chisholm, A. Comparing the Effects of Consuming Almonds or Biscuits on Body Weight in Habitual Snackers: A 1-Year Randomized Controlled Trial. Am. J. Clin. Nutr. 2023, 118, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Hill, A.M.; Buckley, J.D.; Tan, S.-Y.; Rogers, G.B.; Coates, A.M. Acute Feeding with Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones with No Effect on Self-Reported Appetite Sensations: A Randomised Controlled Trial. Eur. J. Nutr. 2023, 62, 857–866. [Google Scholar] [CrossRef]
- Brown, R.; Ware, L.; Gray, A.R.; Chisholm, A.; Tey, S.L. Snacking on Almonds Lowers Glycaemia and Energy Intake Compared to a Popular High-Carbohydrate Snack Food: An Acute Randomised Crossover Study. Int. J. Environ. Res. Public Health 2021, 18, 10989. [Google Scholar] [CrossRef]
- Hollis, J.; Mattes, R. Effect of Chronic Consumption of Almonds on Body Weight in Healthy Humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.M.; Tran, C.D.; Luscombe-Marsh, N.D.; Stonehouse, W.; Bowen, J.; Johnson, N.; Thompson, C.H.; Watson, E.-J.; Brinkworth, G.D.; Rogers, G.B. Almond Consumption Affects Fecal Microbiota Composition, Stool pH, and Stool Moisture in Overweight and Obese Adults with Elevated Fasting Blood Glucose: A Randomized Controlled Trial. Nutr. Res. 2021, 85, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. A Comprehensive Review of Almond Clinical Trials on Weight Measures, Metabolic Health Biomarkers and Outcomes, and the Gut Microbiota. Nutrients 2021, 13, 1968. [Google Scholar] [CrossRef]
- Dikariyanto, V.; Berry, S.E.; Francis, L.; Smith, L.; Hall, W.L. Whole Almond Consumption Is Associated with Better Diet Quality and Cardiovascular Disease Risk Factors in the UK Adult Population: National Diet and Nutrition Survey (NDNS) 2008–2017. Eur. J. Nutr. 2021, 60, 643–654. [Google Scholar] [CrossRef]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S. A Mid-Morning Snack of Almonds Generates Satiety and Appropriate Adjustment of Subsequent Food Intake in Healthy Women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of Almonds: Effects of Lipid Bioaccessibility, Appetite, and Hormone Response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef]
- Appleton, K.M.; Newbury, A.; Almiron-Roig, E.; Yeomans, M.R.; Brunstrom, J.M.; de Graaf, K.; Geurts, L.; Kildegaard, H.; Vinoy, S. Sensory and Physical Characteristics of Foods That Impact Food Intake without Affecting Acceptability: Systematic Review and Meta-analyses. Obes. Rev. 2021, 22, e13234. [Google Scholar] [CrossRef]
- James, B. Oral Processing and Texture Perception Influences Satiation. Physiol. Behav. 2018, 193, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Grundy, M.M.-L.; Grassby, T.; Parker, M.L.; Cross, K.L.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Lagana, G. The Effects of Processing and Mastication on Almond Lipid Bioaccessibility Using Novel Methods of in Vitro Digestion Modelling and Micro-Structural Analysis. Br. J. Nutr. 2014, 112, 1521–1529. [Google Scholar] [CrossRef]
- Mandalari, G.; Parker, M.L.; Grundy, M.M.-L.; Grassby, T.; Smeriglio, A.; Bisignano, C.; Raciti, R.; Trombetta, D.; Baer, D.J.; Wilde, P.J. Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection. Nutrients 2018, 10, 213. [Google Scholar] [CrossRef]
- Trombetta, D.; Smeriglio, A.; Denaro, M.; Zagami, R.; Tomassetti, M.; Pilolli, R.; De Angelis, E.; Monaci, L.; Mandalari, G. Understanding the Fate of Almond (Prunus Dulcis (Mill.) DA Webb) Oleosomes during Simulated Digestion. Nutrients 2020, 12, 3397. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food Processing and Structure Impact the Metabolizable Energy of Almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia Spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J. Interactions between Roseburia Intestinalis and Diet Modulate Atherogenesis in a Murine Model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, R.; McCormick, K.L.; Zhang, Y.; Lin, X.; Yang, X. The Role of the Gut Microbiota on the Metabolic Status of Obese Children. Microb. Cell Factories 2021, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alcoholado, L.; Castellano-Castillo, D.; Jordán-Martínez, L.; Moreno-Indias, I.; Cardila-Cruz, P.; Elena, D.; Muñoz-Garcia, A.J.; Queipo-Ortuño, M.I.; Jimenez-Navarro, M. Role of Gut Microbiota on Cardio-Metabolic Parameters and Immunity in Coronary Artery Disease Patients with and without Type-2 Diabetes Mellitus. Front. Microbiol. 2017, 8, 1936. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Arce-Cordero, J.A.; Liu, T.; Ravelo, A.; Lobo, R.R.; Agustinho, B.C.; Monteiro, H.F.; Jeong, K.C.; Faciola, A.P. Effects of Calcium-Magnesium Carbonate and Calcium-Magnesium Hydroxide as Supplemental Sources of Magnesium on Ruminal Microbiome. Transl. Anim. Sci. 2022, 6, txac092. [Google Scholar] [CrossRef]
- Conte, G.; Dimauro, C.; Daghio, M.; Serra, A.; Mannelli, F.; McAmmond, B.M.; Van Hamme, J.D.; Buccioni, A.; Viti, C.; Mantino, A. Exploring the Relationship between Bacterial Genera and Lipid Metabolism in Bovine Rumen. Animal 2022, 16, 100520. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.; Narbad, A. Potential Prebiotic Properties of Almond (Amygdalus communis L.) Seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M.; Arioglu-Tuncil, S.; Ünver, A.; Deemer, D.; Lindemann, S.R.; Tunçil, Y.E. Dietary Fibers of Tree Nuts Differ in Composition and Distinctly Impact the Fecal Microbiota and Metabolic Outcomes In Vitro. J. Agric. Food Chem. 2023, 71, 9762–9771. [Google Scholar] [CrossRef] [PubMed]
- Poeker, S.A.; Geirnaert, A.; Berchtold, L.; Greppi, A.; Krych, L.; Steinert, R.E.; de Wouters, T.; Lacroix, C. Understanding the Prebiotic Potential of Different Dietary Fibers Using an in Vitro Continuous Adult Fermentation Model (PolyFermS). Sci. Rep. 2018, 8, 4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic Effects of Almonds and Almond Skins on Intestinal Microbiota in Healthy Adult Humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic Fermentation of Polyphenols: Potential Sources of Novel Functional Foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Lee, M.D.; Pedroso, A.A.; Maurer, J.J. Bacterial Composition of a Competitive Exclusion Product and Its Correlation with Product Efficacy at Reducing Salmonella in Poultry. Front. Physiol. 2023, 13, 1043383. [Google Scholar] [CrossRef] [PubMed]
- Melo-Bolívar, J.F.; Ruiz Pardo, R.Y.; Junca, H.; Sidjabat, H.E.; Cano-Lozano, J.A.; Villamil Díaz, L.M. Competitive Exclusion Bacterial Culture Derived from the Gut Microbiome of Nile Tilapia (Oreochromis niloticus) as a Resource to Efficiently Recover Probiotic Strains: Taxonomic, Genomic, and Functional Proof of Concept. Microorganisms 2022, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.; Pires, E. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Cakebread, J.; Wallace, O.A.; Henderson, H.; Jauregui, R.; Young, W.; Hodgkinson, A. The Impacts of Bovine Milk, Soy Beverage, or Almond Beverage on the Growing Rat Microbiome. PeerJ 2022, 10, e13415. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In Vitro and in Vivo Evaluation of the Prebiotic Effect of Raw and Roasted Almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Pi, Y.; Yue, X. Effects of Almond (Armeniaca sibirica L. Lam) Polysaccharides on Gut Microbiota and Anti-Inflammatory Effects on LPS-Induced RAW264. 7 Cells. Int. J. Biol. Macromol. 2024, 263, 130098. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, C.; Yao, H.; Williams, B.A.; Flanagan, B.M.; Gidley, M.J.; Mikkelsen, D. Biomass Attachment and Microbiota Shifts during Porcine Faecal in Vitro Fermentation of Almond and Macadamia Nuts Differing in Particle Sizes. Food Funct. 2024, 15, 2406–2421. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Josse, A.R.; Nguyen, T.H.; Faulkner, D.A.; Lapsley, K.G.; Singer, W. Effect of Almonds on Insulin Secretion and Insulin Resistance in Nondiabetic Hyperlipidemic Subjects: A Randomized Controlled Crossover Trial. Metabolism 2008, 57, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.R.; Considine, R.V.; Mattes, R.D. Almond Consumption Decreases Android Fat Mass Percentage in Adults with High Android Subcutaneous Adiposity but Does Not Change HBA1C in a Randomised Controlled Trial. Br. J. Nutr. 2022, 127, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]

| Per 100 g Portion | Almond | Brazil Nut | Cashew | Hazelnut | Macadamia | Pecan | Pistachio | Walnut |
|---|---|---|---|---|---|---|---|---|
| Energy, kcal | 579 | 659 | 553 | 628 | 718 | 691 | 560 | 654 |
| Protein, g * | 21.2 | 14.3 | 18.2 | 15 | 7.91 | 9.17 | 20.2 | 15.2 |
| Carbohydrate, g | 21.6 | 11.7 | 30.2 | 16.7 | 13.8 | 13.9 | 27.2 | 13.7 |
| Total Dietary Fiber, g * | 12.5 | 7.5 | 3.3 | 9.7 | 8.6 | 9.6 | 10.6 | 6.7 |
| Total Fat, g | 49.9 | 67.1 | 43.8 | 60.8 | 75.8 | 72 | 45.3 | 65.2 |
| Saturated FAs, g * | 3.8 | 16.1 | 7.78 | 4.46 | 12.1 | 6.18 | 5.91 | 6.13 |
| Monounsaturated FAs, g | 31.6 | 23.9 | 23.8 | 45.7 | 58.9 | 40.8 | 23.3 | 8.93 |
| Polyunsaturated FAs, g | 12.3 | 24.9 | 7.84 | 7.92 | 1.5 | 21.6 | 14.4 | 47.2 |
| Minerals | ||||||||
| Calcium, mg * | 269 | 160 | 37 | 114 | 85 | 70 | 105 | 98 |
| Iron, mg | 3.71 | 2.43 | 6.68 | 4.7 | 3.69 | 2.53 | 3.92 | 2.91 |
| Magnesium, mg | 270 | 376 | 292 | 163 | 130 | 121 | 121 | 158 |
| Phosphorus, mg | 481 | 725 | 593 | 290 | 188 | 277 | 490 | 346 |
| Potassium, mg | 733 | 659 | 660 | 680 | 368 | 410 | 1020 | 441 |
| Sodium, mg | 1 | 3 | 12 | 0 | 5 | 0 | 1 | 2 |
| Zinc, mg | 3.12 | 4.06 | 5.78 | 2.45 | 1.3 | 4.53 | 2.2 | 3.09 |
| Copper, mg | 1.03 | 1.74 | 2.2 | 1.72 | 0.756 | 1.2 | 1.3 | 1.59 |
| Manganese, mg | 2.18 | 1.22 | 1.66 | 6.18 | 4.13 | 4.5 | 1.2 | 3.41 |
| Selenium, µg | 4.1 | 1920 | 19.9 | 2.4 | 3.6 | 3.8 | 7 | 4.9 |
| Vitamins | ||||||||
| Vitamin A (RAE), µg | 0 | 0 | 0 | 1 | 0 | 3 | 26 | 1 |
| Thiamin, mg * | 0.205 | 0.617 | 0.423 | 0.643 | 1.2 | 0.66 | 0.87 | 0.341 |
| Riboflavin, mg * | 1.14 | 0.035 | 0.058 | 0.113 | 0.162 | 0.13 | 0.16 | 0.15 |
| Niacin, mg * | 3.62 | 0.295 | 1.06 | 1.8 | 2.47 | 1.17 | 1.3 | 1.12 |
| Pantothenic Acid, mg | 0.471 | 0.184 | 0.864 | 0.918 | 0.758 | 0.863 | 0.52 | 0.57 |
| Pyridoxine, mg | 0.137 | 0.101 | 0.417 | 0.563 | 0.275 | 0.21 | 1.7 | 0.537 |
| Biotin, µg | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Folate (DFE), µg | 44 | 22 | 25 | 113 | 11 | 22 | 51 | 98 |
| Cobalamin, µg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin C, mg * | 0 | 0.7 | 0.5 | 6.3 | 1.2 | 1.1 | 5.6 | 1.3 |
| Vitamin D (D2 + D3), µg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin E, mg * | 25.6 | 5.62 | 0.9 | 15 | 0.54 | 1.4 | 2.86 | 0.7 |
| Phylloquinone, µg | 0 | 0 | 34.1 | 14.2 | N/A | 3.5 | N/A | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singar, S.; Kadyan, S.; Patoine, C.; Park, G.; Arjmandi, B.; Nagpal, R. The Effects of Almond Consumption on Cardiovascular Health and Gut Microbiome: A Comprehensive Review. Nutrients 2024, 16, 1964. https://doi.org/10.3390/nu16121964
Singar S, Kadyan S, Patoine C, Park G, Arjmandi B, Nagpal R. The Effects of Almond Consumption on Cardiovascular Health and Gut Microbiome: A Comprehensive Review. Nutrients. 2024; 16(12):1964. https://doi.org/10.3390/nu16121964
Chicago/Turabian StyleSingar, Saiful, Saurabh Kadyan, Cole Patoine, Gwoncheol Park, Bahram Arjmandi, and Ravinder Nagpal. 2024. "The Effects of Almond Consumption on Cardiovascular Health and Gut Microbiome: A Comprehensive Review" Nutrients 16, no. 12: 1964. https://doi.org/10.3390/nu16121964









