Personalized Nutrition with Banked Human Milk for Early Gut Microbiota Development: In Pursuit of the Perfect Match
Abstract
1. Introduction
2. Gut Microbiome Development: Early Health Foundation
3. Early Life Nutrition: The Game Changer
4. Bank Milks and Hospital Practices: Policy Regarding Donor Human Milk
- Donor screening should include an oral interview and completion of a health questionnaire.
- They will be required to undergo serological testing.
- Donors should inform the HMBs if there are any changes in their behavior or health status.
- Before accepting a donor’s milk, written informed consent for its use is obtained in accordance with the HMB protocols.
- Exclude donors if they smoke cigarettes; use recreational drugs; are known or found to be infected with HIV, hepatitis B or C, syphilis or human T-lymphotropic virus; use medications not on the EMBA approved medication list; have had a recent blood transfusion, tattoo, or piercing; follow a vegan diet without vitamin B12 supplementation; or have a sexual partner who has or is at risk of acquiring sexually transmitted infections.
- Train all new donors in handwashing and hygiene requirements for expressing, handling, storing, cooling, freezing, and transporting human milk.
- Provide appropriate ongoing support for all donors.
- Premature infants, especially those with a birth weight below 1500 g, due to their high risk of infection and NEC.
- Infants with gastrointestinal anomalies undergoing gastrointestinal surgery.
- Newborns at risk of intestinal ischemia.
5. Donor Human Milk Processing: Safety and Quality
6. Microbiological and Health Outcomes in Infants: Good but Not Yet Equal
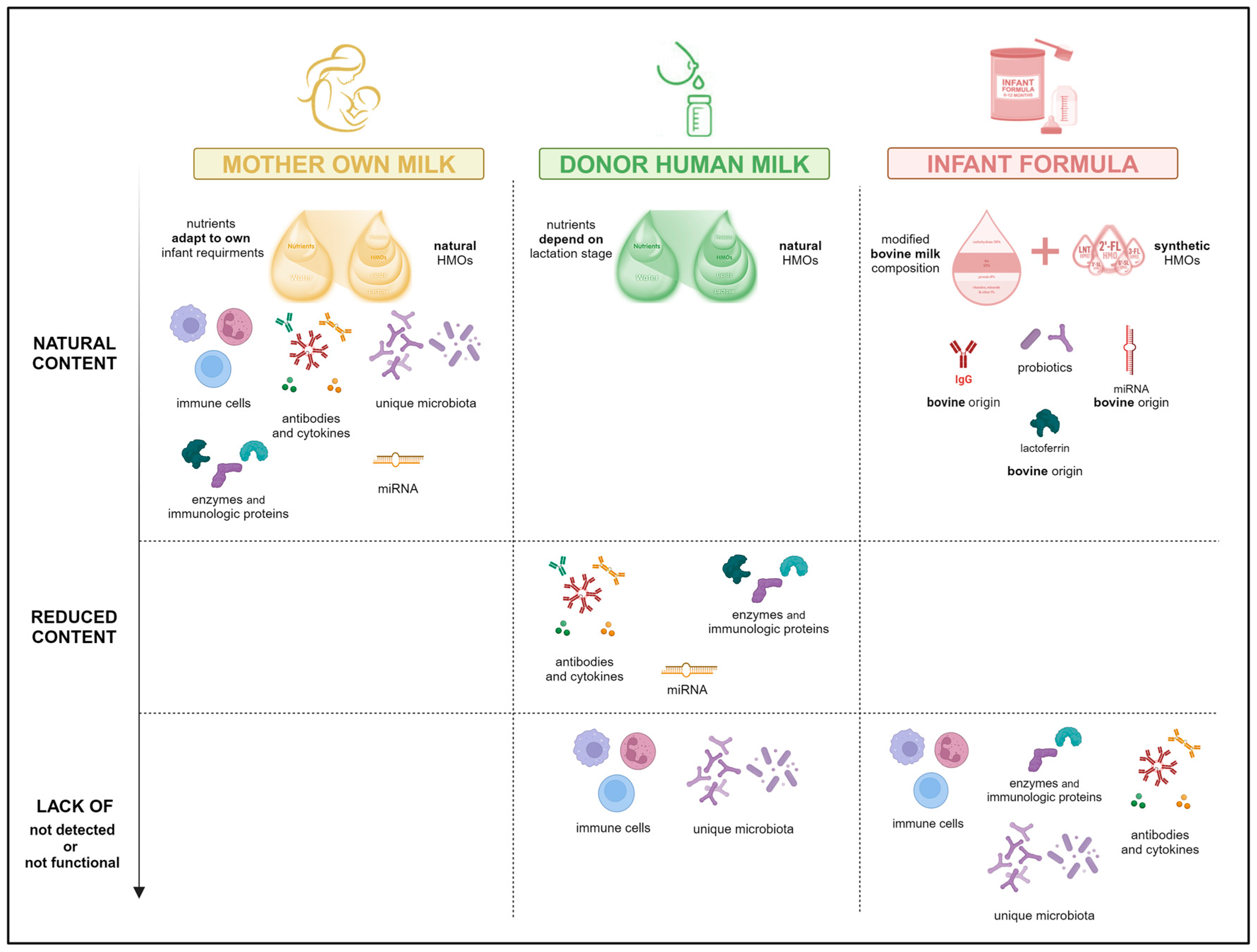
7. Personalization of Pasteurized Human Donor Milk: The Next Opportunity
8. Conclusions, Perspectives and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Park, H.; Park, N.Y.; Koh, A. Scarring the early-life microbiome: Its potential life-long effects on human health and diseases. BMB Rep. 2023, 56, 469–481. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on Optimal Feeding of Low Birthweight Infants in Low and Middle-Income Countries; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Hård, A.L.; Nilsson, A.K.; Lund, A.M.; Hansen-Pupp, I.; Smith, L.E.H.; Hellström, A. Review shows that donor milk does not promote the growth and development of preterm infants as well as maternal milk. Acta Paediatr. 2019, 108, 998–1007. [Google Scholar] [CrossRef]
- Parra-Llorca, A.; Gormaz, M.; Alcántara, C.; Cernada, M.; Nuñez-Ramiro, A.; Vento, M.; Collado, M.C. Preterm gut microbiome depending on feeding type: Significance of donor human milk. Front. Microbiol. 2018, 9, 1376. [Google Scholar] [CrossRef]
- Arboleya, S.; Saturio, S.; Suárez, M.; Fernández, N.; Mancabelli, L.; de Los Reyes-Gavilán, C.G.; Ventura, M.; Solís, G.; Gueimonde, M. Donated human milk as a determinant factor for the gut bifidobacterial ecology in premature babies. Microorganisms 2020, 8, 760. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Gosalbes, M.J.; Llop, S.; Vallès, Y.; Moya, A.; Ballester, F.; Francino, M.P. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 2013, 43, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.H.; Malatos, S.; Kennea, N.; Edwards, A.D.; Miles, L.; Duggan, P.; Reynolds, P.R.; Feldman, R.G.; Sullivan, M.H. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr. Res. 2005, 57, 404–411. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. Critical assessment of the “sterile womb” and “in utero” colonization hypothesis: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Segata, N. No bacteria found in healthy placentas. Nature 2019, 572, 317–318. [Google Scholar] [CrossRef]
- Bushman, F.D. De-Discovery of the Placenta Microbiome. Am. J. Obstet. Gynecol. 2019, 220, 213–214. [Google Scholar] [CrossRef]
- Walter, J.; Hornef, M.W. A philosophical perspective on the prenatal in utero microbiome debate. Microbiome 2021, 9, 5. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, K.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Slusher, N.A.; Cabana, M.D.; Lynch, S.V. Role of the gut microbiota in defining human health. Expert Rev. Anti Infect. Ther. 2010, 8, 435–454. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Alan Harris, R.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3839. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- Gonzalez-Perez, G.; Hicks, A.L.; Tekieli, T.M.; Radens, C.M.; Williams, B.L.; Lamousé-Smith, E.S. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J. Immunol. 2016, 196, 3768–3779. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis in maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Kumbhare, S.V.; Patangia, D.V.; Patil, R.H.; Shouche, Y.S.; Patil, N.P. Factors influencing the gut microbiome in children: From infancy to childhood. J. Biosci. 2019, 44, 49. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.A.T.M.; Man, W.H.; Chu, M.L.J.N.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef] [PubMed]
- Hesla, H.M.; Stenius, F.; Jäderlund, L.; Nelson, R.; Engstrand, L.; Alm, J.; Dicksved, J. Impact of lifestyle on the gut microbiota of healthy infants and their mothers- the ALADDIN birth cohort. FEMS Microbiol. Ecol. 2014, 90, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Chari, R.S.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L.; et al. Gut microbiota of healthy Canadian infants: Profiles by mode of delivery and infant diet at 4 months. CMAJ 2013, 185, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wampach, M.; Heintz-Buschart, A.; Fritz, J.V.; Ramiro-Garcia, J.; Habier, J.; Herold, M.; Narayanasamy, S.; Kaysen, A.; Hogan, A.H.; Bindl, L.; et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 2018, 9, 5091. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Gibson, G.R.; McCartney, A.L.; Isolauri, E. Influence of mode of delivery on gut microbiota composition in seven yearold children. Gut 2004, 53, 1388. [Google Scholar] [CrossRef]
- Korpela, K.; Zijlmans, M.A.; Kuitunen, M.; Kukkonen, K.; Savilahti, E.; Salonen, A.; de Weerth, C.; de Vos, W.M. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 2017, 5, 26. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 252, 539–544. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Persaud, R.R.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Sears, M.R.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: A prospective cohort study. BJOG 2016, 123, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernández-Barranco, A.; Margolles, A.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Micobiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.J. Necrotizing Enterocolitis: Pathogenesis, Diagnosis and Treatment; CRC Press: Boca Raton, FL, USA, 2021; p. 302. [Google Scholar]
- Claud, E.C.; Keegan, K.P.; Brulc, J.M.; Lu, L.; Bartels, D.; Glass, E.; Chang, E.B.; Meyer, F.; Antonopoulos, D.A. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome 2013, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shan, G.; Sodergren, E.; Weinstock, G.; Walker, W.A.; Gregory, K.E. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: A case-control study. PLoS ONE 2015, 10, e011863. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Li, N.; Shuster, J.; Sharma, R.; Hudak, M.L.; Neu, J. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS ONE 2013, 8, e52876. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Maldonado-Gomez, M.X.; Gomes-Neto, J.C.; Kittana, H.; Ding, H.; Schmaltz, R.; Joglekar, P.; Cardona, R.J.; Marsteller, N.L.; Kembel, S.W.; et al. Experimental evaluation of the importance of colonization history in early-life gut microbiota assembly. eLife 2018, 7, e36521. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Feliciano, I.G.; Clemente, J.C.; Costello, E.K.; Perez, M.E.; Blaser, M.J.; Knight, R.; Dominguez-Bello, M.G. Biphasic assembly of the murine intestinal microbiota during early development. ISME J. 2013, 7, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Laursen, M.F.; Andersen, L.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. Msphere 2016, 1, e00069-15. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Suárez, M.; Fernández, N.; Mantecón, L.; Mancabelli, L.; Milani, C.; Ventura, M.; de Los Reyes-Gavilán, C.G.; Solís, G.; et al. Early-Life Development of the Bifidobacterial Community in the Infant Gut. Int. J. Mol. Sci. 2021, 22, 3382. [Google Scholar] [CrossRef] [PubMed]
- Adlerberth, I.; Wold, A. Establishment of the gut microbiota in Western infants. Acta Paediatr. 2009, 98, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martín, M.; Saturio, S.; Arboleya, S.; Herrero-Morín, D.; Calzón, M.; López, T.; González, S.; Gueimonde, M. Association between diet and fecal microbiota along the first year of life. Food Res. Int. 2022, 162, 111994. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; de Vos, W.M. Early life colonization of the human gut: Microbes matter everywhere. Curr. Opin. Microbiol. 2018, 44, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Planer, J.D.; Peng, Y.; Kau, A.L.; Blanton, L.V.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Gordon, J.I. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 2016, 534, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Penders, J.; Gerhold, K.; Thijs, C.; Zimmerman, K.; Wahn, U.; Lau, S.; Hamelmann, E. New insights into the hygiene hypothesis in allergic diseases: Mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes 2014, 5, 239–244. [Google Scholar] [CrossRef]
- Martin, R.; Makino, H.; Cetinyurek Yavuz, A.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-life events, include mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.A.; McGuire, M.K.; McGuire, M.A.; Williams, J.E.; Lackey, K.A.; Hagen, E.H.; Kaul, A.; Gindola, D.; Gebeyehu, D.; Flores, K.E.; et al. Household composition and the infant fecal microbiome: The INSPIRE study. Am. J. Phys. Anthropol. 2019, 169, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Blumberg, R.S. Life at the beginning: Perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat. Immunol. 2014, 15, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the human microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Wu, R.K.S.; Oremus, M. The association between antibiotic use in infancy and childhood overweight or obesity: A systematic review and metaanalysis. Obes. Rev. 2018, 19, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Gerhold, K.; Stobberingh, E.E.; Thijs, C.; Zimmermann, K.; Lau, S.; Hamelmann, E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 2013, 132, 601–607.e8. [Google Scholar] [CrossRef]
- Ta, L.D.H.; Chan, J.C.Y.; Yap, G.C.; Purbojati, R.W.; Drautz-Moses, D.I.; Koh, Y.M.; Tay, C.J.X.; Huang, C.H.; Kioh, D.Y.Q.; Woon, J.Y.; et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes 2020, 12, 1801964. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef]
- Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast Milk Microbiota Is Shaped by Mode of Delivery and Intrapartum Antibiotic Exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- García-Ricobaraza, M.; García-Santos, J.A.; Escudero-Marín, M.; Diéguez, E.; Cerdó, T.; Campoy, C. Short- and Long-Term Implications of Human Milk Microbiota on Maternal and Child Health. Int. J. Mol. Sci. 2021, 22, 11866. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. Global Nutrition Targets 2025: Breastfeeding Policy Brief (WHO/NMH/NHD/14.7); World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr. Dev. Nutr. 2020, 4, nzaa027. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Spooner, M.; Dallas, D.C. Differences in Maternal Immunoglobulins within Mother’s Own Breast Milk and Donor Breast Milk and across Digestion in Preterm Infants. Nutrients 2019, 11, 920. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides: Structure and Functions. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 115–123. [Google Scholar] [CrossRef]
- Masi, A.C.; Stewart, C.J. Untangling human milk oligosaccharides and infant gut microbiome. iScience 2022, 25, 103542. [Google Scholar] [CrossRef]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive compounds in infant formula and their effects on infant nutrition and health: A systematic literature review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: Personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M. Section on breatfeeding, Committee on nutrition, committee on fetus and newborn. Promoting human milk and breastfeeding for the very low birth weight infant. Pediatrics 2021, 145, e2021054272. [Google Scholar] [CrossRef]
- Kashyap, V.; Choudhari, S.G. Unlocking the Potential: A Systematic Literature Review on the Impact of Donor Human Milk on Infant Health Outcomes. Cureus 2024, 16, e57440. [Google Scholar] [CrossRef]
- Gutierrez dos Santos, B.; Perrin, M.T. What is known about human milk bank donors around the world: A systematic scoping review. Public Health Nutr. 2022, 25, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Luna, M.; Martin, S.C.; Gómez de Orgaz, C.S. Human milk bank and personalized nutrition in the NICU: A narrative review. Eur. J. Pediatr. 2021, 180, 1327–1333. [Google Scholar] [CrossRef]
- Kapourchali, F.R.; Cresci, G.A.M. Early-Life Gut Microbiome—The Importance of Maternal and Infant Factors in Its Establishment. Nutr. Clin. Pract. 2020, 35, 386–405. [Google Scholar] [CrossRef]
- Tyebally Fang, M.; Grummer-Strawn, L.; Maryuningsih, Y.; Biller-Andorno, N. Human milk banks: A need for further evidence and guidance. Lancet Glob. Health 2021, 9, e104–e105. [Google Scholar] [CrossRef]
- Quitadamo, P.A.; Palumbo, G.; Cianti, L.; Lurdo, P.; Gentile, M.A.; Villani, A. The Revolution of Breast Milk: The Multiple Role of Human Milk Banking between Evidence and Experience—A Narrative Review. Int. J. Pediatr. 2021, 1, 6682516. [Google Scholar] [CrossRef] [PubMed]
- Haiden, N.; Ziegler, E.E. Human Milk Banking. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 8–15. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Berenz, A.; Wicks, J.; Esquerra-Zwiers, A.; Sulo, K.S.; Gross, M.E.; Szotek, J.; Meier, P.; Patel, A.L. The Economic Impact of Donor Milk in the Neonatal Intensive Care Unit. J. Pediatr. 2020, 224, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the establishment and operation of human milk banks in Europe: A consensus statement from the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Román, S.; Bustos-Lozano, G.; López-Maestro, M.; Rodríguez-López, J.; Orbea-Gallardo, C.; Samaniego-Fernández, M.; Pallás-Alonso, C.R. Clinical impact of opening a human milk bank in a neonatal unit. An. Pediatría 2014, 81, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.E.; Billeaud, C.; Rachel, B.; Calvo, J.; Cavallarin, L.; Christen, L.; Escuder-Vieco, D.; Gaya, A.; Lembo, D.; Wesolowska, A.; et al. Processing of Donor Human Milk: Update and Recommendations From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Kontopodi, E.; Hettinga, K.; Stahl, B.; van Goudoever, J.B.; van Elburg, R.M. Testing the effects of processing on donor human Milk: Analytical methods. Food Chem. 2022, 373 Pt A, 131413. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.A.; Beggs, M.R.; O’Connor, D.L.; Doyen, A.; Pouliot, Y.; Sergius-Ronot, M.; Unger, S. Donor human milk processing and its impact on infant digestion: A systematic scoping review of in vitro and in vivo studies. Adv. Nutr. 2023, 14, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.K.; Pitino, M.A.; Mahmood, R.; Yihang Zhu, I.; Stone, D.; O’Connor, D.L.; Unger, S.; Chan, T.C.Y. Predicting Protein and Fat Content in Human Donor Milk Using Machine Learning. J. Nutr. 2021, 151, 2075–2083. [Google Scholar] [CrossRef]
- Peila, C.; Emmerik, N.E.; Giribaldi, M.; Stahl, B.; Ruitenberg, J.E.; van Elburg, R.M.; Moro, G.E.; Bertino, E.; Coscia, A.; Cavallarin, L. Human Milk Processing. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 353–361. [Google Scholar] [CrossRef]
- Escuder-Vieco, D.; Espinosa-Martos, I.; Rodríguez, J.M.; Corzo, N.; Montilla, A.; Siegfried, P.; Pallás-Alonso, C.R.; Fernández, L. High-Temperature Short-Time Pasteurization System for Donor Milk in a Human Milk Bank Setting. Front. Microbiol. 2018, 9, 926. [Google Scholar] [CrossRef]
- Tran, H.; Nguyen, T.; Mathisen, R. The use of human donor milk. BMJ 2020, 371, m4243. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Xie, J.; Zhang, D.; Pi, C.; Zhou, L.; Yang, W. Gold standard for nutrition: A review of human milk oligosaccharide and its effects on infant gut microbiota. Microb. Cell Fact. 2021, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Roy, N.C.; Guo, Y.; Jia, H.; Ryan, L.; Samuelsson, L.; Thomas, A.; Plowman, J.; Clerens, S.; Day, L.; et al. Human breast milk and infant formulas differentially modify the intestinal microbiota in human infants and host physiology in rats. J. Nutr. 2015, 146, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2019, 172, e181161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Neupane, A.; Vo, R.; White, J.; Wang, X.; Marzano, S.L. Comparing gut microbiome in mothers’ own breast milk- and formula-fed moderate-late preterm infants. Front. Microbiol. 2020, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Sisk, P.M.; Lambeth, T.M.; Rojas, M.A.; Lightbourne, T.; Barahona, M.; Anthony, E.; Auringer, S.T. Necrotizing enterocolitis and growth in preterm infants fed predominantly maternal milk, pasteurized donor milk, or preterm formula: A retrospective study. Am. J. Perinatol. 2017, 34, 676–683. [Google Scholar] [CrossRef]
- Zamrik, S.; Giachero, F.; Heldmann, M.; Hensel, K.O.; Wirth, S.; Jenke, A.C. Impact of an in-house pediatric surgery unit and human milk centered enteral nutrition on necrotizing enterocolitis. BioMed Res. Int. 2018, 2018, 5042707. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.; Carroll, K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed. Med. 2014, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Collins, C.; Ratliff, M.; Xie, B.; Wang, Y. Breastfeeding reduces childhood obesity risks. Child. Obes. 2017, 13, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, M.; Lee, J.; Kim, Y.J.; Ha, E.; Kim, H.S. The protective effect of exclusive breastfeeding on overweight/obesity in children with high birth weight. J. Korean Med. Sci. 2019, 34, e85. [Google Scholar] [CrossRef] [PubMed]
- McCory, C.; Layte, R. Breastfeeding and risk of overweight and obesity at nine-years of age. Soc. Sci. Med. 2012, 75, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Ma, R.M.; Huang, Y.K.; Liang, K.; Ding, Z.B. Effect of breastfeeding on childhood overweight in the offspring of mothers with gestational diabetes mellitus. Zhongguo Dang Dai Er Ke Za Zhi 2013, 15, 56–61. [Google Scholar]
- Novaes, J.F.; Lamounier, J.A.; Colosimo, E.A.; Franceschini, S.C.; Priore, S.E. Breastfeeding and obesity in Brazilian children. Eur. J. Public Health. 2012, 22, 383–389. [Google Scholar] [CrossRef][Green Version]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Guthrie, L.; Vilchuck, K.; Bogdanovich, N.; Sergeichick, N.; Gusina, N.; Foo, Y.; Palmer, T.; et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: A randomized trial. JAMA 2013, 309, 1005–1013. [Google Scholar] [CrossRef]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up for a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef]
- Escribano, J.; Luque, V.; Ferre, N.; Mendez-Riera, G.; Koletzko, B.; Grote, V.; Demmelmair, H.; Bluck, L.; Wright, A.; Closa-Monasterolo, R.; et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: The EU Childhood Obesity Programme. Int. J. Obes. 2012, 36, 548–553. [Google Scholar] [CrossRef]
- Salgin, B.; Norris, S.A.; Prentice, P.; Pettifor, J.M.; Richter, L.M.; Ong, K.K.; Dunger, D.B. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int. J. Obes. 2015, 39, 939–944. [Google Scholar] [CrossRef]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; CHILD Study Investigators; Azad, M.B. Modes of infant feeding and the risk of childhood asthma: A prospective birth cohort study. J. Pediatr. 2019, 190, 192–199.e2. [Google Scholar] [CrossRef]
- Chu, S.; Chen, Q.; Chen, Y.; Bao, Y.; Wu, M.; Zhang, J. Cesarean section without medical indication and risk of childhood asthma, and attenuation by breastfeeding. PLoS ONE 2017, 12, e0184920. [Google Scholar] [CrossRef]
- Elbert, N.; van Meel, E.R.; den Dekker, H.T.; de Jong, N.W.; Nijsten, T.E.C.; Jaddoe, V.W.V.; de Jongste, J.C.; Pasmans, S.G.M.A.; Duijts, L. Duration and exclusiveness of breastfeeding and risk of childhood atopic diseases. Allergy 2017, 72, 1936–1943. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolized formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef]
- Lee, S.A.; Lim, J.Y.; Kim, B.S.; Cho, S.J.; Kim, N.Y.; Kim, O.B.; Kim, Y. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr. Res. Pract. 2015, 9, 242. [Google Scholar] [CrossRef]
- Praveen, P.; Jordan, F.; Priami, C.; Morine, M.J. The role of breast-feeding in infant immune-system: A systems perspective on the intestinal microbiome. Microbiome 2015, 3, 41. [Google Scholar] [CrossRef]
- Rodriguez-Herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Muñoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-Berkeveld, M.; et al. Gastrointestinal tolerance, growth and safety or a partly fermented formula with specific prebiotics in healthy infants: A double-blind, randomized controlled trial. Nutrients 2019, 11, 1530. [Google Scholar] [CrossRef]
- Haaman, M.; Knol, J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 2005, 71, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Salvini, F.J.; Riva, E.; Salvatici, E.; Boehm, G.; Jelinek, J.; Banderali, G.; Giovannini, M. A specific probiotic mixture added to starting infant formula has long-lasting bifidogenic effects. J. Nutr. 2011, 141, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Widger, J.; O’Connell, N.H.; Stack, T. Breast milk causing neonatal sepsis and death. Clin. Microbiol. Infect. 2010, 16, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotton, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e1. [Google Scholar] [CrossRef] [PubMed]
- Gnawali, A. Prematurity and the risk of development of childhood obesity: Piecing together the pathophysiological puzzle. A literature review. Cureus 2021, 13, e20518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Q.; Wang, J.; Wang, H.; Li, Q.; Zhu, B.; Ji, C.; Xu, X.; Johnston, L. The impact of breast milk feeding on early brain development in preterm infants in China: An observational study. PLoS ONE 2022, 17, e0272125. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Ma, J.; Lai, C.T.; Rea, A.; Perrella, S.L.; Geddes, D.T. Milk microbiome transplantation: Recolonizing donor milk with mother’s own milk microbiota. Appl. Microbiol. Biotechnol. 2024, 108, 74. [Google Scholar] [CrossRef]
- Cacho, N.T.; Harrison, N.A.; Parker, L.A.; Padgett, K.A.; Lemas, D.J.; Marcial, G.E.; Li, N.; Carr, L.E.; Neu, J.; Lorca, G.L. Personalization of the Microbiota of Donor Human Milk with Mother’s Own Milk. Front. Microbiol. 2017, 8, 1470. [Google Scholar] [CrossRef]
- Mallardi, D.; Tabasso, C.; Piemontese, P.; Morandi, S.; Silvetti, T.; Biscarini, F.; Cremonesi, P.; Castiglioni, B.; Pica, V.; Stuknyte, M.; et al. Inoculation of mother’s own milk could personalize pasteurized donor human milk used for feeding preterm infants. J. Transl. Med. 2021, 19, 420. [Google Scholar] [CrossRef]
- Torrez Lamberti, M.F.; Harrison, N.A.; Bendixen, M.M.; DeBose-Scarlett, E.M.; Thompson, S.C.; Neu, J.; Parker, L.A.; Lorca, G.L. Frozen Mother’s Own Milk Can Be Used Effectively to Personalize Donor Human Milk. Front. Microbiol. 2021, 12, 656889. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Pan, L.; Peng, C.; Dong, L.; Cao, S.; Cheng, H.; Wang, Y.; Zhang, C.; Gu, R.; Wang, J.; et al. Isolation and characterization of lactic acid bacteria from human milk. J. Dairy Sci. 2020, 103, 9980–9991. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, L.; Hu, M.; Zhu, J. 2′-Fucosyllactose (2′-FL) changes infants gut microbiota composition and their metabolism in a host-free human colonic model. Food Res. Int. 2023, 173, 113293. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.; Hirvonen, J.; Siitonen, J.; Ahonen, I.; Anglenius, H.; Maukonen, J. Selective Utilization of the Human Milk Oligosaccharides 2′-Fucosyllactose, 3-Fucosyllactose, and Difucosyllactose by Various Probiotic and Pathogenic Bacteria. J. Agric. Food Chem. 2021, 69, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Nogacka, A.M.; Arboleya, S.; Nikpoor, N.; Auger, J.; Salazar, N.; Cuesta, I.; Mantecón, L.; Solís, G.; Gueimonde, M.; Tompkins, T.A.; et al. Influence of 2′-Fucosyllactose on the Microbiota Composition and Metabolic Activity of Fecal Cultures from Breastfed and Formula-Fed Infants at Two Months of Age. Microorganisms 2021, 9, 1478. [Google Scholar] [CrossRef]
- Zabel, B.E.; Gerdes, S.; Evans, K.C.; Nedveck, D.; Koch Singles, S.; Volk, B.; Budinoff, C. Strain-specific strategies of 2′-fucosyllactose, 3-fucosyllactose, and difucosyllactose assimilation by Bifidobacterium longum subsp. infantis Bi-26 and ATCC 15697. Sci. Rep. 2020, 10, 15919. [Google Scholar] [CrossRef]
- Laursen, M.F.; Pekmez, C.T.; Wange Larsson, M.; Vendelbo Lind, M.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Bode, L.; Ove Dragsted, L.; Michaelsen, K.F.; et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021, 1, 21. [Google Scholar] [CrossRef]
| Items | Recommendations |
|---|---|
| Labeled | All donated human milk and containers should be labeled at each stage to ensure traceability and tracking of the milk. |
| Thawing | Donated human milk containers should be thawed in a refrigerator (4 °C) for 24 h. In urgent and exceptional cases, containers can be thawed at room temperature. A microwave oven has never to be used to thaw or heat the milk. Thawed donated human milk should be used within 24 h and should not be refrozen. |
| Administration | Donated human milk can be administered using a cup, spoon, or through small tubes that the baby sucks along with a pacifier, or through gastric tubes according to clinical guidelines. |
| Volumes | The required volume of donated human milk is based on the newborn’s age, gestational age and weight, as well as feeding tolerance. This volume represents the difference between recommended intake and the quantity of mother´s own milk available. |
| Consent | Before administration of donated human milk, informed consent is required from recipient´s parents. |
| Duration | Decisions about the need to continue with donated human milk are regularly reviewed, taking into account the baby’s growth and nutritional requirements. The hospital should record how donated milk is used, including in the baby’s hospital notes. |
| Type of Feeding | Characteristics of Gut Microbiota | Health Outcomes |
|---|---|---|
| Breastfeeding | ↑ Bifidobacterium, Veillonella, Propionibacterium | Breastfeeding |
| Formula | ↑ Clostridioides difficile | Increased risk of NEC, respiratory infections, asthma, obesity, diabetes, and inflammatory bowel disease |
| Donor human milk | ↓ Bifidobacterium ↑ Staphylococcaceae, Clostridiaceae and Pasteurellaceae ↑ Alpha-diversity in bifidobacterial species | Short- and long-term effects not yet evaluated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hick, E.; Suárez, M.; Rey, A.; Mantecón, L.; Fernández, N.; Solís, G.; Gueimonde, M.; Arboleya, S. Personalized Nutrition with Banked Human Milk for Early Gut Microbiota Development: In Pursuit of the Perfect Match. Nutrients 2024, 16, 1976. https://doi.org/10.3390/nu16131976
Hick E, Suárez M, Rey A, Mantecón L, Fernández N, Solís G, Gueimonde M, Arboleya S. Personalized Nutrition with Banked Human Milk for Early Gut Microbiota Development: In Pursuit of the Perfect Match. Nutrients. 2024; 16(13):1976. https://doi.org/10.3390/nu16131976
Chicago/Turabian StyleHick, Emilia, Marta Suárez, Alejandra Rey, Laura Mantecón, Nuria Fernández, Gonzalo Solís, Miguel Gueimonde, and Silvia Arboleya. 2024. "Personalized Nutrition with Banked Human Milk for Early Gut Microbiota Development: In Pursuit of the Perfect Match" Nutrients 16, no. 13: 1976. https://doi.org/10.3390/nu16131976
APA StyleHick, E., Suárez, M., Rey, A., Mantecón, L., Fernández, N., Solís, G., Gueimonde, M., & Arboleya, S. (2024). Personalized Nutrition with Banked Human Milk for Early Gut Microbiota Development: In Pursuit of the Perfect Match. Nutrients, 16(13), 1976. https://doi.org/10.3390/nu16131976







