Revealing the Potency of Growth Factors in Bovine Colostrum
Abstract
:1. Introduction
| Growth Factor | Concentration in BC (ng/mL) | Concentration in Bovine Milk(ng/mL) | Properties | Reference |
|---|---|---|---|---|
| EGF | 324.2 | 155 (pasteurized milk) | Cell proliferation, survival, and differentiation | [10,11] |
| IGF-1 | 870 | 150 | Cell growth and proliferation | [6,12] |
| IGF-2 | 206 | 2–6 | Regulates cell proliferation and survival | [12,13,14] |
| TGF-β | 100.7 | 4.3 (pasteurized milk) | Pro-inflammatory mediator, stimulating the activation and migration of immune cells and wound healing | [15,16] |
| TGF-α | 200 | - | Cell proliferation, differentiation, and development | [17,18] |
| PDGF | - | - | Cell migration, proliferation, and survival | [19] |
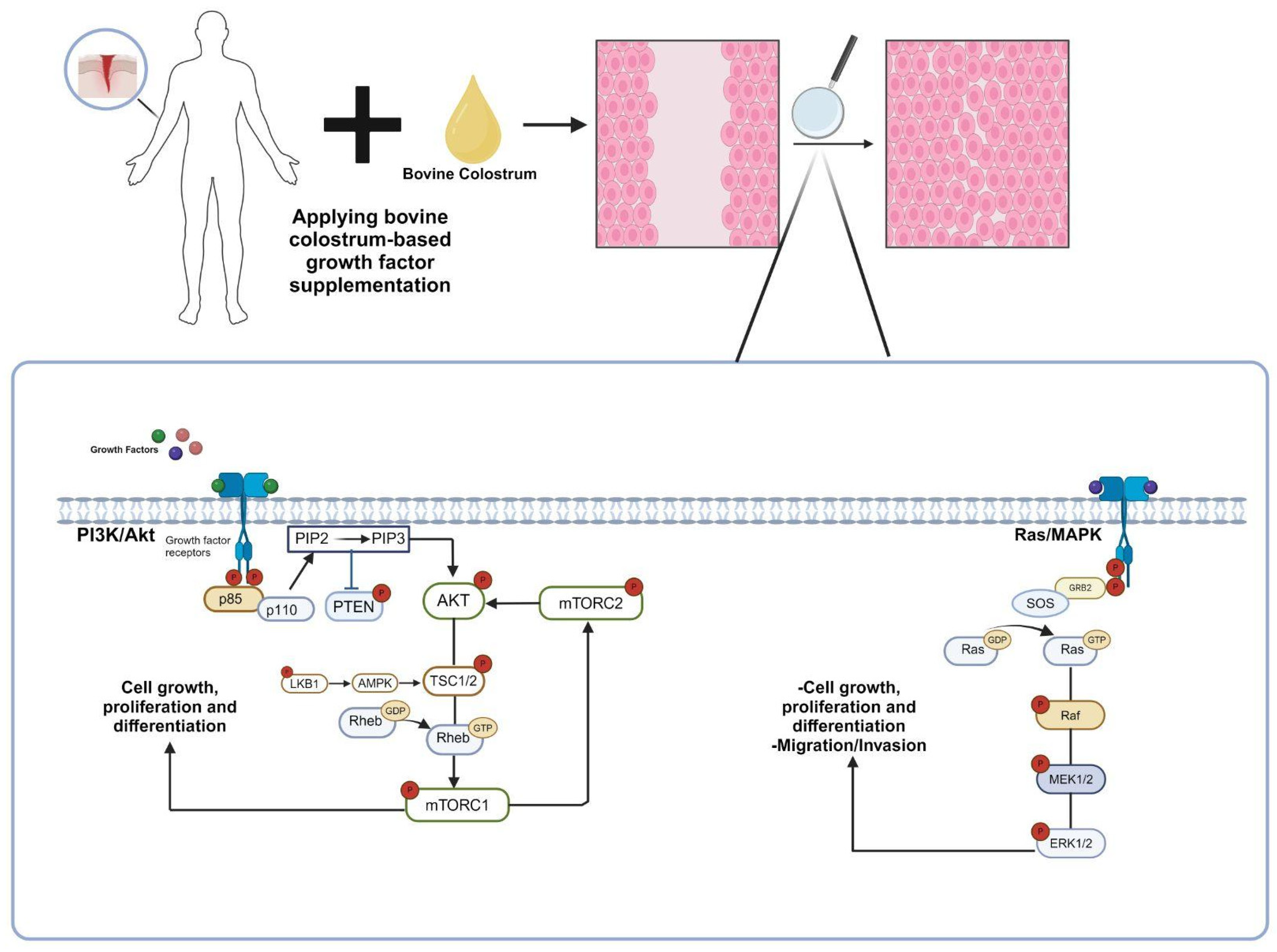
2. Exploring the Growth Factors: Types and Molecular Mechanisms
2.1. Epidermal Growth Factor (EGF)
2.2. Transforming Growth Factor Beta (TGF-β)
2.3. Platelet-Derived Growth Factor (PDGF)
2.4. Insulin-Like Growth Factors (IGFs)
3. Clinical Trials and Effects of Growth Factors on Various Diseases
3.1. Wound Healing
3.2. Gastrointestinal Health
3.3. Cancer
3.4. Bone and Muscle Health
3.5. Neurological and Mental Health
3.6. Eye Health
4. Growth Factors as Health Products
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yalçıntaş, Y.M.; Baydemir, B.; Duman, H.; Eker, F.; Bayraktar Biçen, A.; Ertürk, M.; Karav, S. Exploring the Impact of Colostrum Supplementation on Athletes: A Comprehensive Analysis of Clinical Trials and Diverse Properties. Front. Immunol. 2024, 15, 1395437. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.; Eker, F.; Kaplan, M.; Duman, H.; Arslan, A.; Saritaş, S.; Şahutoğlu, A.S.; Karav, S. Lactoferrin for COVID-19 Prevention, Treatment, and Recovery. Front. Nutr. 2022, 9, 992733. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, Growth Factors and Growth Hormone in Bovine Colostrum and the Effects of Processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Ginjala, V.; Pakkanen, R. Determination of Transforming Growth Factor-Β1 (TGF-Β1) and Insulin-Like Growth Factor 1 (IGF-1) in Bovine Colostrum Samples. J. Immunoass. 1998, 19, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S.; Gauthier, S.F.; Drouin, R.; Poubelle, P.E.; Pouliot, Y. In Vitro Digestion of Proteins and Growth Factors in a Bovine Whey Protein Extract as Determined Using a Computer-Controlled Dynamic Gastrointestinal System (TIM-1). Food Dig. 2011, 2, 13–22. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. Epidermal Growth Factor. Annu. Rev. Biochem. 1979, 48, 193–216. [Google Scholar] [CrossRef]
- Yagi, H.; Suzuki, S.; Noji, T.; Nagashima, K.; Kuroume, T. Epidermal Growth Factor in Cow’s Milk and Milk Formulas. Acta Paediatr. 1986, 75, 233–235. [Google Scholar] [CrossRef] [PubMed]
- White, M.F.; Kahn, C.R. Insulin Action at a Molecular Level—100 Years of Progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef] [PubMed]
- Skaar, T.C.; Vega, J.R.; Pyke, S.N.; Baumrucker, C.R. Changes in Insulin-like Growth Factor-Binding Proteins in Bovine Mammary Secretions Associated with Pregnancy and Parturition. J. Endocrinol. 1991, 131, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.R.; Gibson, C.A.; Skaar, T.C.; Hadsell, D.L.; Baumrucker, C.R. Insulin-like Growth Factor (IGF)-I and -II and IGF Binding Proteins in Serum and Mammary Secretions during the Dry Period and Early Lactation in Dairy Cows. J. Anim. Sci. 1991, 69, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.A.; Borchers, A.T.; Li, M.; Flavell, R.A.; Bowlus, C.L.; Ansari, A.A.; Gershwin, M.E. Transforming Growth Factor β (TGF-β) and Autoimmunity. Autoimmun. Rev. 2005, 4, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.-L.; Goddard, C.; Regester, G.O.; Ballard, F.J.; Belford, D.A. Transforming Growth Factor β in Bovine Milk: Concentration, Stability and Molecular Mass Forms. J. Endocrinol. 1996, 151, 77–86. [Google Scholar] [CrossRef]
- Okada, M.; Ohmura, E.; Kamiya, Y.; Murakami, H.; Onoda, N.; Iwashita, M.; Wakai, K.; Tsushima, T.; Shizume, K. Transforming Growth Factor (TGF)—α in Human Milk. Life Sci. 1991, 48, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh, A. Transforming Growth Factor α and β (TGF-α and TGF-β). In Angiogenesis in Health, Disease and Malignancy; Salajegheh, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 331–337. ISBN 978-3-319-28140-7. [Google Scholar]
- Heldin, C.-H. Targeting the PDGF Signaling Pathway in Tumor Treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef]
- Boonstra, J.; Rijken, P.; Humbel, B.; Cremers, F.; Verkleij, A.; en Henegouwen, P.v.B. The Epidermal Growth Factor. Cell Biol. Int. 1995, 19, 413–430. [Google Scholar] [CrossRef]
- Jacobi, N.; Seeboeck, R.; Hofmann, E.; Eger, A. ErbB Family Signalling: A Paradigm for Oncogene Addiction and Personalized Oncology. Cancers 2017, 9, 33. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Gavilondo-Cowley, J.; López-Saura, P.; González-López, T.; Castro-Santana, M.D.; López-Mola, E.; Guillén-Nieto, G.; Herrera-Martinez, L. Epidermal Growth Factor in Clinical Practice—A Review of Its Biological Actions, Clinical Indications and Safety Implications. Int. Wound J. 2009, 6, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Adrain, C.; Freeman, M. Regulation of Receptor Tyrosine Kinase Ligand Processing. Cold Spring Harb. Perspect. Biol. 2014, 6, a008995. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.P.J.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Zhu, H.-J.; Walker, F.; Frenkel, M.J.; Hoyne, P.A.; et al. Crystal Structure of a Truncated Epidermal Growth Factor Receptor Extracellular Domain Bound to Transforming Growth Factor Alpha. Cell 2002, 110, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F. PI3K/Akt: Getting It Right Matters. Oncogene 2008, 27, 6473–6488. [Google Scholar] [CrossRef]
- Waterman, H.; Sabanai, I.; Geiger, B.; Yarden, Y. Alternative Intracellular Routing of ErbB Receptors May Determine Signaling Potency. J. Biol. Chem. 1998, 273, 13819–13827. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β Signaling in Fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Zilberberg, L.; Todorovic, V.; Dabovic, B.; Horiguchi, M.; Couroussé, T.; Sakai, L.Y.; Rifkin, D.B. Specificity of Latent TGF-β Binding Protein (LTBP) Incorporation into Matrix: Role of Fibrillins and Fibronectin. J. Cell. Physiol. 2012, 227, 3828–3836. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-β Signaling and the Fibrotic Response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Sheppard, D. TGF-β Signaling in Health and Disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ Signalling in Context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.-Z.; Chen, Q.; Zhang, W.-Y.; Zhang, H.-H.; Ma, Y.; Zhang, S.-Z.; Fang, J.; Yu, C.-H. PDGF Signaling Pathway in Hepatic Fibrosis Pathogenesis and Therapeutics (Review). Mol. Med. Rep. 2017, 16, 7879–7889. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations. Cells 2020, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Bentov, I.; Werner, H. CHAPTER 193—Insulin-Like Growth Factor. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Academic Press: Burlington, ON, Canada, 2006; pp. 1385–1392. ISBN 978-0-12-369442-3. [Google Scholar]
- Kavran, J.M.; McCabe, J.M.; Byrne, P.O.; Connacher, M.K.; Wang, Z.; Ramek, A.; Sarabipour, S.; Shan, Y.; Shaw, D.E.; Hristova, K.; et al. How IGF-1 Activates Its Receptor. eLife 2014, 3, e03772. [Google Scholar] [CrossRef] [PubMed]
- Jadwin, J.A.; Curran, T.G.; Lafontaine, A.T.; White, F.M.; Mayer, B.J. Src Homology 2 Domains Enhance Tyrosine Phosphorylation in Vivo by Protecting Binding Sites in Their Target Proteins from Dephosphorylation. J. Biol. Chem. 2018, 293, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.A.; Engelmann, B.W.; Jablonowski, K.; Higginbotham, K.; Stergachis, A.B.; Nash, P.D. SRC Homology 2 Domain Binding Sites in Insulin, IGF-1 and FGF Receptor Mediated Signaling Networks Reveal an Extensive Potential Interactome. Cell Commun. Signal. 2012, 10, 27. [Google Scholar] [CrossRef]
- Shing, C.M.; Hunter, D.C.; Stevenson, L.M. Bovine Colostrum Supplementation and Exercise Performance. Sports Med. 2009, 39, 1033–1054. [Google Scholar] [CrossRef]
- Mero, A.; Kähkönen, J.; Nykänen, T.; Parviainen, T.; Jokinen, I.; Takala, T.; Nikula, T.; Rasi, S.; Leppäluoto, J. IGF-I, IgA, and IgG Responses to Bovine Colostrum Supplementation during Training. J. Appl. Physiol. 2002, 93, 732–739. [Google Scholar] [CrossRef]
- Andasari, V.; Lü, D.; Swat, M.; Feng, S.; Spill, F.; Chen, L.; Luo, X.; Zaman, M.; Long, M. Computational Model of Wound Healing: EGF Secreted by Fibroblasts Promotes Delayed Re-Epithelialization of Epithelial Keratinocytes. Integr. Biol. 2018, 10, 605–634. [Google Scholar] [CrossRef] [PubMed]
- Blais, M.; Pouliot, Y.; Gauthier, S.; Boutin, Y.; Lessard, M. A Gene Expression Programme Induced by Bovine Colostrum Whey Promotes Growth and Wound-Healing Processes in Intestinal Epithelial Cells. J. Nutr. Sci. 2014, 3, e57. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Gartner, M.H.; Benson, J.D.; Caldwell, M.D. Insulin-like Growth Factors I and II Expression in the Healing Wound. J. Surg. Res. 1992, 52, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Alejandro-Osorio, A.L.; Grorud, K.W.; Martinez, D.A.; Vailas, A.C.; Grindeland, R.E.; Vanderby, R. Systemic Administration of IGF-I Enhances Healing in Collagenous Extracellular Matrices: Evaluation of Loaded and Unloaded Ligaments. BMC Physiol. 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H.; et al. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102027. [Google Scholar] [CrossRef]
- Alexander, A.N.; Carey, H.V. Oral IGF-I Enhances Nutrient and Electrolyte Absorption in Neonatal Piglet Intestine. Am. J. Physiol. 1999, 277, G619–G625. [Google Scholar] [CrossRef]
- Drozdowski, L.; Thomson, A.B. Intestinal Hormones and Growth Factors: Effects on the Small Intestine. World J. Gastroenterol. 2009, 15, 385–406. [Google Scholar] [CrossRef]
- Allen, T.R.; Krueger, K.D.; Hunter, W.J.; Agrawal, D.K. Evidence That Insulin-like Growth Factor-1 Requires Protein Kinase C-Epsilon, PI3-Kinase and Mitogen-Activated Protein Kinase Pathways to Protect Human Vascular Smooth Muscle Cells from Apoptosis. Immunol. Cell Biol. 2005, 83, 651–667. [Google Scholar] [CrossRef]
- Hatamzade Esfahani, N.; Day, A.S. The Role of TGF-β, Activin and Follistatin in Inflammatory Bowel Disease. Gastrointest. Disord. 2023, 5, 167–186. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Baselga, J. The EGF Receptor Family as Targets for Cancer Therapy. Oncogene 2000, 19, 6550–6565. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.; Gongoll, S.; Langner, C.; Mengel, M.; Piso, P.; Klempnauer, J.; Rüschoff, J.; Kreipe, H.; von Wasielewski, R. IGF-1R, IGF-1 and IGF-2 Expression as Potential Prognostic and Predictive Markers in Colorectal-Cancer. Virchows Arch. 2003, 443, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Liefers-Visser, J.A.L.; Meijering, R.A.M.; Reyners, A.K.L.; van der Zee, A.G.J.; de Jong, S. IGF System Targeted Therapy: Therapeutic Opportunities for Ovarian Cancer. Cancer Treat. Rev. 2017, 60, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Y.; Shi, L.Z.; Kanneganti, T.-D.; Chi, H. Signaling by the Phosphatase MKP-1 in Dendritic Cells Imprints Distinct Effector and Regulatory T Cell Fates. Immunity 2011, 35, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Manokawinchoke, J.; Pavasant, P.; Sawangmake, C.; Limjeerajarus, N.; Limjeerajarus, C.N.; Egusa, H.; Osathanon, T. Intermittent Compressive Force Promotes Osteogenic Differentiation in Human Periodontal Ligament Cells by Regulating the Transforming Growth Factor-β Pathway. Cell Death Dis. 2019, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Feng, W.; Shi, J.; Chen, L.; Huang, J.; Lin, T. circRIP2 Accelerates Bladder Cancer Progression via miR-1305/Tgf-Β2/Smad3 Pathway. Mol. Cancer 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Le Roith, D. Insulin-Like Growth Factors. N. Engl. J. Med. 1997, 336, 633–640. [Google Scholar] [CrossRef]
- Delafontaine, P.; Song, Y.-H.; Li, Y. Expression, Regulation, and Function of IGF-1, IGF-1R, and IGF-1 Binding Proteins in Blood Vessels. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef]
- Laron, Z.; Klinger, B.; Jensen, L.T.; Erster, B. Biochemical and Hormonal Changes Induced by One Week of Administration of rIGF-I to Patients with Laron Type Dwarfism. Clin. Endocrinol. 1991, 35, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Nemet, D. Exercise and the GH-IGF-I Axis. In Endocrinology of Physical Activity and Sport; Hackney, A.C., Constantini, N.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–84. ISBN 978-3-030-33376-8. [Google Scholar]
- Ikemoto-Uezumi, M.; Uezumi, A.; Tsuchida, K.; Fukada, S.; Yamamoto, H.; Yamamoto, N.; Shiomi, K.; Hashimoto, N. Pro-Insulin-Like Growth Factor-II Ameliorates Age-Related Inefficient Regenerative Response by Orchestrating Self-Reinforcement Mechanism of Muscle Regeneration. Stem Cells 2015, 33, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; van Breda, E.; Verlaan, G.; Smeets, R. Effects of Oral Bovine Colostrum Supplementation on Serum Insulin-like Growth Factor-i Levels. Nutrition 2002, 18, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Cardinali, G.; Aspite, N.; Picardo, M. Bovine Colostrum Promotes Growth and Migration of the Human Keratinocyte HaCaT Cell Line. Growth Factors 2009, 27, 448–455. [Google Scholar] [CrossRef]
- Doillon, C.J.; Lehance, F.; Bordeleau, L.-J.; Laplante-Campbell, M.-P.; Drouin, R. Modulatory Effect of a Complex Fraction Derived from Colostrum on Fibroblast Contractibility and Consequences on Repair Tissue. Int. Wound J. 2011, 8, 280–290. [Google Scholar] [CrossRef]
- Kovacs, D.; Maresca, V.; Flori, E.; Mastrofrancesco, A.; Picardo, M.; Cardinali, G. Bovine Colostrum Induces the Differentiation of Human Primary Keratinocytes. FASEB J. 2020, 34, 6302–6321. [Google Scholar] [CrossRef] [PubMed]
- Jogi, R.; Tager, M.J.; Perez, D.; Tsapekos, M. Bovine Colostrum, Telomeres, and Skin Aging. J. Drugs Dermatol. 2021, 20, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-A.; Park, H.-J.; Han, M.-G.; Lee, R.; Kim, J.-S.; Park, J.-H.; Lee, W.-Y.; Song, H. Fermented Colostrum Whey Upregulates Aquaporin-3 Expression in, and Proliferation of, Keratinocytes via P38/c-Jun N-Terminal Kinase Activation. Food Sci. Anim. Resour. 2021, 41, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Joshi, A.; Singh, N. Natural Cocktail of Bioactive Factors Conjugated on Nanofibrous Dressing for Improved Wound Healing. Biomater. Adv. 2022, 143, 213163. [Google Scholar] [CrossRef]
- Han, G.; Kim, H.; Kim, D.E.; Ahn, Y.; Kim, J.; Jang, Y.J.; Kim, K.; Yang, Y.; Kim, S.H. The Potential of Bovine Colostrum-Derived Exosomes to Repair Aged and Damaged Skin Cells. Pharmaceutics 2022, 14, 307. [Google Scholar] [CrossRef]
- Wu, B.; Gao, M.; Bai, X. Therapeutic Effect of the Combination of Fractional Laser and Recombinant Bovine Basic Fibroblast Growth Factor in Acne Scar Patients. Trop. J. Pharm. Res. 2024, 23, 125–131. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Baumgartner, A.; Gomez, J.E.; Najafzadeh, M. The Efficacy of a Novel Biological Formulation from Bovine Milk Colostrum Exosomes and Its Growth Factors in Enhancing the Process of Wound Healing. J. Biol. 2024, 11. [Google Scholar] [CrossRef]
- Roohelhami, E.; Vahdat Shariatpanahi, Z.; Ardehali, S.H. Colostrum Supplement, IGF-1, and Diarrhea in Mechanically-Ventilated Patients: A Double-Blind, Randomized, Placebo-Controlled Study. Nutr. Clin. Métabolisme 2023, 37, 227–232. [Google Scholar] [CrossRef]
- Costa, M.A.; Wu, C.; Pham, B.V.; Chong, A.K.S.; Pham, H.M.; Chang, J. Tissue Engineering of Flexor Tendons: Optimization of Tenocyte Proliferation Using Growth Factor Supplementation. Tissue Eng. 2006, 12, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Fu, S.C.; Qin, L.; Rolf, C.; Chan, K.M. Supplementation-Time Dependence of Growth Factors in Promoting Tendon Healing. Clin. Orthop. Relat. Res. 2006, 448, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Belford, D.A.; Rogers, M.-L.; Regester, G.O.; Francis, G.L.; Smithers, G.W.; Liepe, I.J.; Priebe, I.K.; Ballard, F.J. Milk-Derived Growth Factors as Serum Supplements for the Growth of Fibroblast and Epithelial Cells. Vitr. Cell. Dev. Biol. Anim. 1995, 31, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Eremia, S.C.; de Boo, H.A.; Bloomfield, F.H.; Oliver, M.H.; Harding, J.E. Fetal and Amniotic Insulin-Like Growth Factor-I Supplements Improve Growth Rate in Intrauterine Growth Restriction Fetal Sheep. Endocrinology 2007, 148, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, L.C.; Lee, G.C.; Huang, K.L.; Mauck, R.L. Growth Factor Supplementation Improves Native and Engineered Meniscus Repair in Vitro. Acta Biomater. 2012, 8, 3687–3694. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. The Effect of Anisotropic Collagen-GAG Scaffolds and Growth Factor Supplementation on Tendon Cell Recruitment, Alignment, and Metabolic Activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Brown, G.L.; Nanney, L.B.; Griffen, J.; Cramer, A.B.; Yancey, J.M.; Curtsinger, L.J.; Holtzin, L.; Schultz, G.S.; Jurkiewicz, M.J.; Lynch, J.B. Enhancement of Wound Healing by Topical Treatment with Epidermal Growth Factor. N. Engl. J. Med. 1989, 321, 76–79. [Google Scholar] [CrossRef]
- Brown, G.L.; Curtsinger, L.; Jurkiewicz, M.J.; Nahai, F.; Schultz, G. Stimulation of Healing of Chronic Wounds by Epidermal Growth Factor. Plast. Reconstr. Surg. 1991, 88, 189–194. [Google Scholar] [CrossRef]
- Acosta, J.B.; Savigne, W.; Valdez, C.; Franco, N.; Alba, J.S.; Rio, A.D.; López-Saura, P.; Guillén, G.; Lopez, E.; Herrera, L.; et al. Epidermal Growth Factor Intralesional Infiltrations Can Prevent Amputation in Patients with Advanced Diabetic Foot Wounds. Int. Wound J. 2006, 3, 232–239. [Google Scholar] [CrossRef]
- Tabrizi, M.; Chams-Davatchi, C.; Esmaeeli, N.; Noormohammadpoor, P.; Safar, F.; Etemadzadeh, H.; Ettehadi, H.; Gorouhi, F. Accelerating Effects of Epidermal Growth Factor on Skin Lesions of Pemphigus Vulgaris: A Double-Blind, Randomized, Controlled Trial. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 79–84. [Google Scholar] [CrossRef]
- Fernández-Montequín, J.I.; Betancourt, B.Y.; Leyva-Gonzalez, G.; Mola, E.L.; Galán-Naranjo, K.; Ramírez-Navas, M.; Bermúdez-Rojas, S.; Rosales, F.; García-Iglesias, E.; Berlanga-Acosta, J.; et al. Intralesional Administration of Epidermal Growth Factor-Based Formulation (Heberprot-P) in Chronic Diabetic Foot Ulcer: Treatment up to Complete Wound Closure. Int. Wound J. 2009, 6, 67–72. [Google Scholar] [CrossRef]
- Sigalet, D.L.; Martin, G.R.; Butzner, J.D.; Buret, A.; Meddings, J.B. A Pilot Study of the Use of Epidermal Growth Factor in Pediatric Short Bowel Syndrome. J. Pediatr. Surg. 2005, 40, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Lopez-Virgen, V.; Gomez-Plascencia, J.; Ramos-Zuniga, R.; Gonzalez-Perez, O. Growth Factors as Clinical Biomarkers of Prognosis and Diagnosis in Psychiatric Disorders. Cytokine Growth Factor Rev. 2016, 32, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bergman, D.; Halje, M.; Nordin, M.; Engström, W. Insulin-Like Growth Factor 2 in Development and Disease: A Mini-Review. Gerontology 2012, 59, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2021, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, O.V.; Ordyan, N.E. Insulin-Like Growth Factor 2: New Roles for a Known Molecule. Neurosci. Behav. Physi. 2022, 52, 175–182. [Google Scholar] [CrossRef]
- Chen, D.Y.; Stern, S.A.; Garcia-Osta, A.; Saunier-Rebori, B.; Pollonini, G.; Bambah-Mukku, D.; Blitzer, R.D.; Alberini, C.M. A Critical Role for IGF-II in Memory Consolidation and Enhancement. Nature 2011, 469, 491–497. [Google Scholar] [CrossRef]
- Saika, S. TGFβ Pathobiology in the Eye. Lab. Investig. 2006, 86, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Saika, S.; Liu, C.-Y.; Azhar, M.; Sanford, L.P.; Doetschman, T.; Gendron, R.L.; Kao, C.W.-C.; Kao, W.W.-Y. TGFβ2 in Corneal Morphogenesis during Mouse Embryonic Development. Dev. Biol. 2001, 240, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Sanford, L.P.; Ormsby, I.; Groot, A.C.G.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFβ2 Knockout Mice Have Multiple Developmental Defects That Are Non-Overlapping with Other TGFβ Knockout Phenotypes. Development 1997, 124, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.A. Transforming growth factor—ß in human foetal eye. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3544. [Google Scholar]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Eskens, O.; Amin, S. Challenges and Effective Routes for Formulating and Delivery of Epidermal Growth Factors in Skin Care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.; Polak, M.; Woelfle, J.; Houchard, A.; EU IGFD Registry Study Group. Effectiveness and Safety of rhIGF-1 Therapy in Children: The European Increlex® Growth Forum Database Experience. Horm. Res. Paediatr. 2015, 83, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Hee, C.K.; Roach, S.; Snel, L.B. Safety of Recombinant Human Platelet-Derived Growth Factor-BB in Augment(®) Bone Graft. J. Tissue Eng. 2012, 3, 2041731412442668. [Google Scholar] [CrossRef] [PubMed]
- Frew, S.E.; Rezaie, R.; Sammut, S.M.; Ray, M.; Daar, A.S.; Singer, P.A. India’s Health Biotech Sector at a Crossroads. Nat. Biotechnol. 2007, 25, 403–417. [Google Scholar] [CrossRef]
- Senet, P. Becaplermin gel (Regranex gel). Ann. Dermatol. Venereol. 2004, 131, 351–358. [Google Scholar] [CrossRef]
- Aldag, C.; Teixeira, D.N.; Leventhal, P.S. Skin Rejuvenation Using Cosmetic Products Containing Growth Factors, Cytokines, and Matrikines: A Review of the Literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, J.E.; Rissanen, T.T.; Kivelä, A.; Ylä-Herttuala, S. Growth Factor-Induced Therapeutic Angiogenesis and Arteriogenesis in the Heart–Gene Therapy. Cardiovasc. Res. 2005, 65, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Rutanen, J.; Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Silvennoinen, P.; Kivelä, A.; Hedman, A.; Hedman, M.; Heikura, T.; Ordén, M.-R.; et al. Adenoviral Catheter-Mediated Intramyocardial Gene Transfer Using the Mature Form of Vascular Endothelial Growth Factor-D Induces Transmural Angiogenesis in Porcine Heart. Circulation 2004, 109, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
| Target Group | Study Design | Dose and Duration | Effect | Reference |
|---|---|---|---|---|
| Trained men | Pre-post-intervention study with repeated measures | 60 g bovine colostrum Total: 4 weeks | BC does not affect blood IGF-1 or IGF binding protein-3 levels. | [66] |
| Human keratinocytes | Prospective experimental study | Not specified | BC supplementation induces mitogenic and motogenic effects on human keratinocytes, promoting cell proliferation and migration. | [67] |
| Guinea pigs | Prospective experimental study | Daily application of liquid formulation or gel formulation of the IM fraction on wounds Total: 23 days | Colostrum-derived whey immune fraction (IM fraction) inhibits collagen gel contraction in vitro, delays wound closure in full-thickness wounds, and minimizes residual scar formation in scar tissues. | [68] |
| Individuals with skin diseases | Cellular and molecular approaches on keratinocytes | Not specified | Promotes keratinocyte differentiation. | [69] |
| Human fibroblasts in vitro | Experimental study | BC at three concentrations (0.125%, 0.25%, 0.50%) for 8 weeks | Liposomal BC protects against telomere length erosion in fibroblasts under normal and oxidative stress conditions. | [70] |
| Keratinocyte cells in vitro | Experimental study | Fermented colostrum whey at concentrations of 100–400 μg/mL for observed effects | BC increased AQP-3 expression and cell proliferation via JNK and p38 MAPK activation. | [71] |
| Individuals with wounds | Pre-post-intervention study | Not specified | Enhanced wound healing properties. | [72] |
| Keratinocytes, melanocytes, and fibroblasts | Experimental study | Not specified | Effect of colostrum-derived exosomes: Prevented UV-induced damage, reduced melanin production, suppressed matrix metalloproteinase expression, increased cell proliferation, enhanced collagen production. | [73] |
| Patients with acne scars | Retrospective study | Fractional laser treatment: 120 days; recombinant bovine basic fibroblast growth factor(rbFGF): 300 IU/cm2 Total: 7 days | Improved skin barrier function, reduced lactic acid-induced stinging, enhanced stratum corneum integrity. | [74] |
| Fibroblasts, immortalized keratinocytes, Human vein umbilical endothelial cells | Experimental study | Rigemed D (BC containing 20 different growth factors and exosomes) doses: 1%, 1.5%, and 2% v/v; duration not specified | Rigemed D supplementation promotes cell proliferation, migration, and regeneration; exhibits antioxidant effects; and enhances angiogenesis | [75] |
| Critically ill, mechanically ventilated patients | Randomized controlled trial | 30 g of bovine colostrum daily for 10 days | Increased serum levels of IGF-1; reduced incidence of diarrhea. | [76] |
| Rabbit flexor tendon cell populations | Experimental study | IGF-1: 10, 50, and 100 ng/mL PDGF-BB: 1, 10, and 50 ng/mL bFGF: 0.5, 1, and 5 ng/mL Duration not specified | Maximized tenocyte proliferation. | [77] |
| Injured rats | Prospective experimental study | 0, 10, 100, or 1000 ng PDGF isoform B Total: 7 days | PDGF supplementation enhances tendon healing. | [78] |
| Epithelial and fibroblastic cells | Experimental study | 2.5 mg/mL bovine milk for 48 h | Mitogenic extract from bovine milk promotes growth of mesodermal-derived cells. | [79] |
| Sheep fetuses with growth restriction | Randomized controlled trial in sheep | IGF-1: 360 μg Total: 128 days | Intrauterine administration of IGF-1 significantly increased fetal growth rate. | [80] |
| Meniscus | Experimental study | 50 ng/mL bFGF and TGF-β3 Total: 8 weeks. | bFGF and TGF-β3 improved integration strength of meniscus repair constructs and electrospun PCL scaffolds. TGF-β3 increased proteoglycan content in the explants. | [81] |
| Tendon tissue | Experimental study | PDGF-BB and IGF-1 supplementation. Dose and duration not specified. | Enhanced cell attachment, alignment, viability, and metabolic activity. | [82] |
| Patients with skin burns | Prospective, randomized, double-blind clinical trial | EGF: 10 μg Total: 1.5 days | Accelerated rate of epidermal regeneration. | [83] |
| Patients with chronic wounds | Prospective, open-label, crossover trial | EGF: 10 μg Total: 6 months | Stimulation of wound healing. | [84] |
| Diabetic neuropathic or ischemic patients with high amputation risk | Non-controlled pilot study | EGF: 25 μg Total: 8 weeks | Reduction in diabetic lower limb amputation. | [85] |
| Hospitalized patients | Randomized, double-blind, within-patient, left/right, controlled trial | EGF: 10 μg in 0.1% silver sulfadiazine cream | Reduction of healing time of skin lesions in patients with pemphigus vulgaris. | [86] |
| Diabetic patients | Pilot study | Heberprot-P (EGF Based): 75 μg. | Exploration of clinical effects up to complete wound closure | [87] |
| Pediatric patients | Prospective clinical trial | EGF: 100 μg/kg Total: 6 weeks. | Improved carbohydrate absorption, increased tolerance to enteral feeds, reduced infection rates. | [88] |
| Mice with sarcopenia | Experimental study | 12 µg pro-IGF-2/day Total: 7 days | pro-IGF-2 improves muscle regeneration by promoting satellite cell proliferation, angiogenesis, and inhibiting adipogenesis of PDGFRα+ cells | [65] |
| Format | Growth Factor | Effect | Reference |
|---|---|---|---|
| Gel | EGF | Wound healing | [99] |
| Injection | EGF | Wound healing | [99] |
| Capsule | EGF | Wound healing | [99] |
| Injection | IGF-1 | Growth disorders | [100] |
| Gel | PDGF | Bone repair and regenerative procedures | [101] |
| Topical Gel | EGF | Diabetic foot ulcer | [102] |
| Topical Gel | PDGF | Chronic wound healing (diabetic) | [103] |
| Collagen | TGF-β1 | Anti-aging | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçıntaş, Y.M.; Duman, H.; López, J.M.M.; Portocarrero, A.C.M.; Lombardo, M.; Khallouki, F.; Koch, W.; Bordiga, M.; El-Seedi, H.; Raposo, A.; et al. Revealing the Potency of Growth Factors in Bovine Colostrum. Nutrients 2024, 16, 2359. https://doi.org/10.3390/nu16142359
Yalçıntaş YM, Duman H, López JMM, Portocarrero ACM, Lombardo M, Khallouki F, Koch W, Bordiga M, El-Seedi H, Raposo A, et al. Revealing the Potency of Growth Factors in Bovine Colostrum. Nutrients. 2024; 16(14):2359. https://doi.org/10.3390/nu16142359
Chicago/Turabian StyleYalçıntaş, Yalçın Mert, Hatice Duman, Jose M. Miranda López, Alicia C. Mondragón Portocarrero, Mauro Lombardo, Farid Khallouki, Wojciech Koch, Matteo Bordiga, Hesham El-Seedi, António Raposo, and et al. 2024. "Revealing the Potency of Growth Factors in Bovine Colostrum" Nutrients 16, no. 14: 2359. https://doi.org/10.3390/nu16142359









